Abstract
Introduction
Procalcitonin concentrations are considered as a component of the inflammatory response and as an acute-phase marker, after shock or tissue injury (i.e. burn, trauma, surgery) or infections and sepsis. No data are so far available on the dynamics of procalcitonin levels in patients with cardiogenic shock following ST-elevation myocardial infarction, with no clinical or laboratory sign of infection.
Methods
We evaluated procalcitonin values every day during intensive cardiac care staying in ten cardiogenic shock patients admitted to our intensive cardiac care unit. NT-pro Brain Natriuretic Peptide, C Reactive Protein and APACHE II score were also assessed.
Results
Six patients survived, whereas 4 patients died. A progressive reduction in procalcitonin values was observed in cardiogenic shock patients who survived, whereas the lack of changes in procalcitonin concentrations was documented in cardiogenic shock patients who died (survivors: slope = -3.76; dead: slope = -0.81, p=0.004). Furthermore, higher values of glycemia, NT-pro Brain Natriuretic Peptide and C Reactive Protein (as well as higher APACHE II scores) were detectable in dead patients in respect to survivors.
Conclusions
In our preliminary study we observed that in patients with cardiogenic shock and no sign of infections a reduction of procalcitonin levels was detectable only in survivors. Moreover, higher values of NT- Brain Natriuretic Peptide, a marked systemic inflammation (higher values of C Reactive Protein) and higher severity score (as depicted by APACHE II) are associated with an ominous prognosis in cardiogenic shock patients.
Keywords: procalcitonin, cardiogenic shock, prognosis
Introduction
Procalcitonin (PCT) is a 14-kd protein encoded by the Calc-1 gene and synthesized physiologically by thyroid C-cells. Under normal conditions, serum PCT levels are negligible [1].
After shock or tissue injury (i.e. burn, trauma, surgery) or infections and sepsis, PCT mRNA expression has been documented in human extra-thyroidal tissues. Thus, systemic PCT concentrations are considered as a component of the inflammatory response and as an acute-phase marker [2]. In cardiac acute patients, data on PCT are scarce and controversial. Some studies [3, 4] reported that PCT levels were increased in ACS patients on admission, whereas other investigations [5, 6] documented that plasma PCT concentrations were in the normal range in patients with uncomplicated acute myocardial infarction. Elevated concentrations of PCT have been reported in patients with cardiogenic shock [7]. In a more recent retrospective study [8], it was observed that CS patients showed high PCT concentrations, especially in the presence of multiorgan failure (MOF) and in absence of signs of infections (cultures and clinical findings). We recently [9] observed that the degree of myocardial ischemia (clinically indicated by the whole spectrum of ACS, from unstable angina to cardiogenic shock ST-elevation following myocardial infarction) and the related inflammatory-induced response are better reflected by CRP (which was positive in most acute cardiac care patients of all our subgroups) than by PCT which seems more sensible to a higher extent of inflammatory activation, being positive only in all CS patients. In these patients, the clinical interpretation of absolute PCT values (both in diagnostic and prognostic terms), represent a major challenge since they may be influenced by several factors, such as the degree of systemic inflammatory response, the coexistence of multiorgan dysfunction, the presence/absence of infections and finally by the time of measurements during hospital course (i.e. the dynamics of PCT levels). No data are so far available on the dynamics of PCT levels in patients with cardiogenic shock. The aim of this preliminary investigation was therefore to evaluate the serum evolution of PCT during intensive cardiac care unit (ICCU) staying in a group of patients with cardiogenic shock (CS) following ST-elevation myocardial infarction (STEMI) submitted to primary percutaneous intervention (PCI) with no laboratory or clinical sign of infection.
Methods
Ten consecutive patients with cardiogenic shock following STEMI were submitted to PCI and then admitted to our 12-bed ICCU in Florence, a tertiary center, from 1 September 2008 to 31th March 2009. To be eligible for the present study, all patients had to be free of infection at the time of blood sampling, as evidenced by both clinical and microbiological examinations, including urinary cultures and microbiological examinations of tracheal aspirate in mechanical ventilated patients and blood cultures [10].
A clinical diagnosis of cardiogenic shock was made if all the following criteria were present:
1. systolic blood pressure persistently less than 90 mmHg or vasopressors required to maintain a systolic blood pressure of more than 90 mmHg;
2. signs of hypoperfusion (e.g. urine output less than 30 ml/hour or cold/diaphoretic extremities or altered mental status);
3. clinical evidence of elevated left ventricular filling pressure (e.g. pulmonary congestion on physical examination or chest x-ray) [11,12,13,14,15].
Pulmonary artery catheterization was not required when all clinical criteria and echocardiographic evidence of left ventricular dysfunction without mechanical complications were present.
The day after ICCU admission blood samples were obtained for cardiac biomarkers (TnI <0.15 ng/mL), leucocytes count (4000 - 10000/μl), CRP (<9 mg/dL), uric acid (<6.5 mg/dl), NT-pro Brain Natriuretic Peptide (NT-proBNP, in males 0-50 yrs: <88 pg/ml, >50 yrs: <227 pg/ml; in females: 0 -50 yrs: <153 pg/ml; >50 yrs: <334 pg/ml) [16] and procalcitonin measurements (normal values <0.5 ng/ml) [9]. PCT values were evaluated daily during ICCU staying. Lactate was measured on admission.
Transthoracic 2-dimensional echocardiography was performed on admission in order to evaluate left ventricular ejection fraction (LVEF). APACHE II (Acute Physiology And Chronic Health Evaluation II) score was also assessed [17]. The study design was approved by the local Ethic Committee and inform written consent was obtained for each patient.
Statistical analysis
Statistical analysis was performed by means of SPSS 13.0 package (SPSS Inc, Chicago, IL). Data are reported as frequencies and percentages or means ± S.D. and analyzed with Fisher’s exact test and Student’s t test, respectively. Since PCT was determined once a day, its mean value for each day of hospital stay in either group of patients has been calculated and graphically plotted in order to obtain two linear regression lines, whose slopes have been subsequently compared by means of an F test.
In all analyses, a p value <0.05 was considered statistically significant.
Results
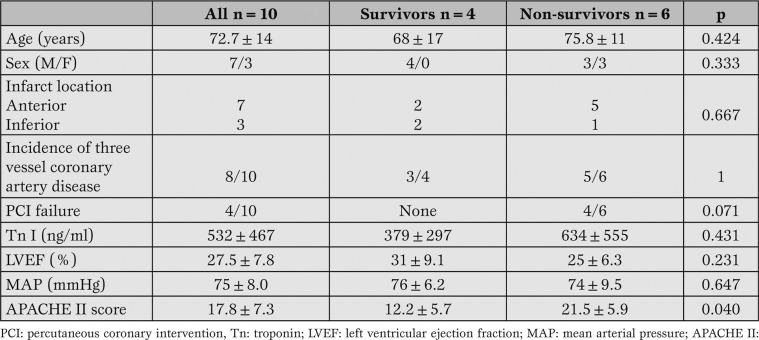
Table 1 shows the clinical characteristics of CS patients following STEMI included in the study after PCI. Six patients died during ICCU, while four patients survived.
Table 1.
Clinical characteristics of patients included in the study.
Compared with survivors, dead patients exhibited higher values of APACHE II score, as well as trend towards higher serum concentrations of troponin I, though it did not reach statistical significance. All patients who experienced PCI failure died.
No significant differences were detectable in regard to age, sex, infarct location, left ventricular ejection fraction, and mean arterial pressure between the two subgroups.
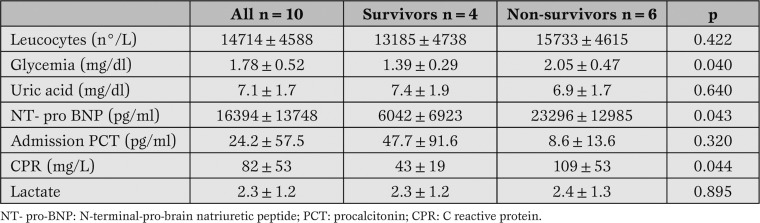
As depicted in Table 2, higher values of glycemia, NT-proBNP and CRP were detectable in dead patients in respect to survivors.
Table 2.
Biochemical data of all patients included in the study.
Basal serum concentrations of procalcitonin were higher in (dead) survivors patients, though this difference did not reach statistical significance due to high values of standard deviation. Lactate values in basal conditions were comparable between the two subgroups.
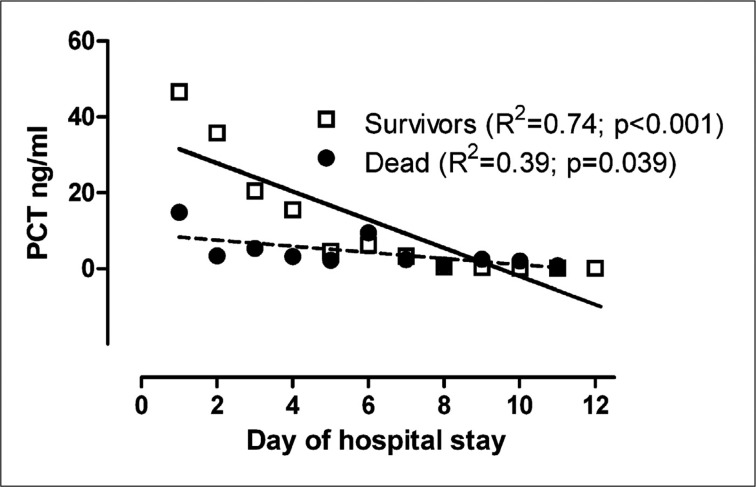
As depicted in Figure 1, the pattern of PCT variations during ICCU course was significantly different in CS patients who survived in respect to those who died (survivors: slope = -3.76±0.71 standard error; dead: slope = -0.81±0.35 standard error, p=0.004).
Figure 1.
Patterns of procalcitonin variations in survivors and dead patients.
Discussion
The main finding of the present investigation, though preliminary and performed in a small subset of patients, is that the patterns of temporal PCT variations throughout ICCU course were heterogeneous in patients with CS and no clinical or laboratory signs of infection.
A progressive reduction in PCT values was observed in CS patients who survived, whereas the lack of changes in PCT concentrations was documented in CS patients who died.
Conversely, basal absolute PCT values were not significantly different between the two subgroups, since they exhibit a wide range of values in the overall population.
Few studies investigated the dynamics of PCT in cardiac acute patients. Sponholz et al. [18] described the evolution of serum procalcitonin levels after uncomplicated cardiac surgery and observed a progressive return to normal levels within the first week. Peak PCT levels were reached within 24 hours postoperatively and this increase seemed to be dependent on the surgical procedure, being more invasive procedures associated with higher PCT levels, and on intraoperative events, including aortic cross-clamping time, duration of cardiopulmonary bypass and duration of surgery.
In infected patients, PCT levels were elevated throughout the first week postoperatively [19, 20, 21], with a more pronounced trend in bacterial and fungal infections than in viral infections of SIRS. [22, 23].
The Authors concluded that the dynamics of PCT levels, rather than absolute values, may be more important for identifying patients with infectious complications after cardiac surgery.
More recently Prat et al. [24] confirmed a slight increase in PCT values in the first postoperative day after cardiac surgery, in agreement with previous results [25, 26, 27] and with Adamik et al. [28], who showed that the development of postoperative complications after cardiac surgery with cardiopulmonary bypass was associated with increased postoperative neopterin and PCT levels.
Similarly, after heart transplantation, serum PCT levels display a rise in response to surgery, with a peak on day two, whereas high peak levels with delayed return to normal values should lead to a search for inflammatory processes, as they are often associated with increased morbidity and mortality. [29]
Likewise, in patients with cardiogenic shock and no sign of infections we documented a reduction of PCT levels only in survivors CS patients.
This time course of procalcitonin can probably be explained, both in postsurgical and in CS patients, by normal PCT kinetic. In healthy subjects the injection of endotoxin is followed by a rise in PCT, reaching a maximum 24 hours thereafter. [30] The return of PCT levels to normality within a few days in surgical patients (after an uncomplicated postoperative course) and in CS survivors patients can be explained by half-life of PCT (18 to 24 hours) [31], in absence of a further insult that might induce PCT production.
Our findings, along with those in cardiac surgery [26,27,28] strongly support the contention that the “dynamic” approach [32] may be more reliable that the static one (that is the absolute single PCT value) especially in the challenging conditions characterized by a systemic inflammatory response, such as cardiac surgery and cardiogenic shock.
Indeed, in a cohort of unselected critically ill patients, Jensen et al. [33] observed that a PCT increase was an independent predictor of 90-day survival and that the PCT day-by-day changes was able to identify critically ill patients at a higher risk of ICU mortality.
On the other hand, the initial CT level did not predict mortality, even though many patients were admitted with a PCT 1.0 ng/mL.
This suggests that several PCT measurements should be made consecutively to assess the critically ill patient’s infection-related mortality risk (to monitor treatment of infection day-by-day).
In our investigation we further confirmed that higher values of NT-proBNP are associated with increased mortality in CS patients [14,16,34] and that a more marked systemic inflammation (as inferred by higher values of CRP) and higher severity score (as indicated by APACHE II) were associated with an ominous prognosis.
The main limitation of the present investigation is represented by the small number of patients.
However, the population is homogeneous, comprising patients with cardiogenic shock following STEMI all submitted to PCI, with no clinical and laboratory sign of infections. It is interesting to note that, despite the small number of subjects, the behavior of PCT was clearly detectable.
Conclusions
According to our preliminary findings, patterns of temporal PCT variations throughout ICCU course were heterogeneous in patients with CS following STEMI submitted to PCI and no clinical or laboratory signs of infection.
A progressive reduction in PCT values was observed in CS patients who survived, whereas a lack of changes in PCT concentrations was documented in CS patients who died. Our findings strongly support the contention that the “dynamic” approach may be more reliable that the static one (that is the absolute single PCT value) especially in the challenging conditions characterized by a systemic inflammatory response, such as cardiogenic shock. We further confirmed that higher values of NT-proBNP are associated with increased mortality in CS patients and that a more marked systemic inflammation (as inferred by higher values of CRP) and higher severity score (as indicated by APACHE II) were associated with an ominous prognosis.
Footnotes
Conflict of interest No conflict of interest is acknowledged by the authors.
References
- Whicher J, Bienvenu J, Monneret G. Procalcitonin as an acute phase marker. Ann Clin Biochem. 2001;38: 483–493. doi: 10.1177/000456320103800505. [DOI] [PubMed] [Google Scholar]
- Schneider HG, Lam QT. Procalcitonin for the clinical laboratory: a review. Pathology. 2007;39:383–390. doi: 10.1080/00313020701444564. [DOI] [PubMed] [Google Scholar]
- Kafkas N, Venetsanou K, Patsilinakos S. et al. Procalcitonin in acute myocardial infarction. Acute Card Care. 2008;10:30–36. doi: 10.1080/17482940701534800. [DOI] [PubMed] [Google Scholar]
- Geppert A, Steiner A, Delle-Karth G. et al. Usefulness of procalcitonin for diagnosing complicating sepsis in patients with cardiogenic shock. Intensive Care Med. 2003;29:1384–1389. doi: 10.1007/s00134-003-1827-7. [DOI] [PubMed] [Google Scholar]
- Buratti T, Ricevuti G, Pechlaner C. et al. Plasma levels of procalcitonin and interleukin-6 in acute myocardial infarction. Inflammation. 2001;25:97–100. doi: 10.1023/a:1007166521791. [DOI] [PubMed] [Google Scholar]
- Remskar M, Horvat M, Hojker S, Noc M. Procalcitonin in patients with acute myocardial infarction. Wien Klin Wochenschr. 2002;114:205–210. [PubMed] [Google Scholar]
- Brunkhorst FM, Clark AL, Forycki ZF, Anker SD. Pyrexia, procalcitonin, immune activation and survival in cardiogenic shock: the potential importance of bacterial translocation. Int J Cardiol. 1999;72:3–10. doi: 10.1016/s0167-5273(99)00118-7. [DOI] [PubMed] [Google Scholar]
- Geppert A, Steiner A, Delle-Karth G. et al. Usefulness of procalcitonin for diagnosing complicating sepsis in patients with cardiogenic shock. Intensive Care Med. 2003;29:1384–1389. doi: 10.1007/s00134-003-1827-7. [DOI] [PubMed] [Google Scholar]
- Picariello C, Lazzeri C, Chiostri M. et al. Procalcitonin in patients with acute coronary syndromes and cardiogenic shock submitted to percutaneous coronary intervention. Intern Emerg Med. 2009 doi: 10.1007/s11739-009-0277-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Califf RM, Bengtson JR. Cardiogenic shock. N Engl J Med. 1994;330:1724–1730. doi: 10.1056/NEJM199406163302406. [DOI] [PubMed] [Google Scholar]
- Holmes DR Jr. Cardiogenic shock: a lethal complication of acute myocardial infarction. Rev Cardiovasc Med. 2003;4:131–135. [PubMed] [Google Scholar]
- Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107:2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2. [DOI] [PubMed] [Google Scholar]
- Webb JG, Lowe AM, Sanborn TA. et al. SHOCK Investigators. Percutaneous coronary intervention for cardiogenic shock in the SHOCK trial. J Am Coll Cardiol. 2003;42:1380–1386. doi: 10.1016/s0735-1097(03)01050-7. [DOI] [PubMed] [Google Scholar]
- Valente S, Lazzeri C, Vecchio S. et al. Predictors of in-hospital mortality after percutaneous coronary intervention for cardiogenic shock. Int J Cardiol. 2007;114:176–182. doi: 10.1016/j.ijcard.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Valente S, Lazzeri C, Chiostri M. et al. Time of onset and outcome of cardiogenic shock in acute coronary syndromes. J Cardiovasc Med. 2008;9:1235–1240. doi: 10.2459/JCM.0b013e3283168a27. [DOI] [PubMed] [Google Scholar]
- Valente S, Lazzeri C, Chiostri M. et al. NT-proBNP on admission for early risk stratification in STEMI patients submitted to PCI. Relation with extension of STEMI and inflammatory markers. Int J Cardiol. 2009;132:84–89. doi: 10.1016/j.ijcard.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Brunkhorst F, Sponholz C, Sakr Y, Reinhart K. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature. Crit Care Med. 2006;10:145–145. doi: 10.1186/cc5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykut D, Schulte-Herbrüggen J, Krian A. The value of procalcitonin as an infection marker in cardiac surgery. Eur J Med Res. 2000;5:530–536. [PubMed] [Google Scholar]
- Boeken U, Feindt P, Feindt P, Micek M. et al. Procalcitonin (PCT) in cardiac surgery: diagnostic value in systemic inflammatory response syndrome (SIRS), sepsis and after heart transplantation (HTX). Cardiovasc Surg. 2000;8:550–554. doi: 10.1016/s0967-2109(00)00070-3. [DOI] [PubMed] [Google Scholar]
- Rothenburger M, Markewitz A, Lenz T. et al. Detection of acute phase response and infection. The role of procalcitonin and C-reactive protein. Clin Chem Lab Med. 1999;37:275–279. doi: 10.1515/CCLM.1999.048. [DOI] [PubMed] [Google Scholar]
- Boeken U, Feindt P, Petzold T. et al. Diagnostic value of procalcitonin: the influence of cardiopulmonary bypass, aprotinin, SIRS, and sepsis. Thorac Cardiovasc Surg. 1998;46:348–351. doi: 10.1055/s-2007-1010251. [DOI] [PubMed] [Google Scholar]
- Boeken U, Feindt P, Micek M. et al. Procalcitonin (PCT) in cardiac surgery: diagnostic value in systemic inflammatory response syndrome (SIRS), sepsis and after heart transplantation (HTX) Cardiovasc Surg. 2000;8:550–554. doi: 10.1016/s0967-2109(00)00070-3. [DOI] [PubMed] [Google Scholar]
- Prat C, Ricart P, Ruyra X. et al. Serum concentration of procalcitonin after cardiac surgery. J Card Surg. 2008;23:627–632. doi: 10.1111/j.1540-8191.2008.00658.x. [DOI] [PubMed] [Google Scholar]
- Aouifi A, Piriou V, Blanc P. et al. Effect of cardiopulmonary bypass on serum procalcitonin and C-reactive protein concentrations. Br J Anaesth. 1999;83:602–607. doi: 10.1093/bja/83.4.602. [DOI] [PubMed] [Google Scholar]
- Meisner M, Rauschmayer C, Schmidt J. et al. Early increase of procalcitonin after cardiovascular surgery in patients with postoperativecomplications. Intensive Care Med. 2002;28:1094–1102. doi: 10.1007/s00134-002-1392-5. [DOI] [PubMed] [Google Scholar]
- Aouifi A, Piriou V, Bastien O. et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med. 2000;28:3171–3176. doi: 10.1097/00003246-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Adamik B, Kübler-Kielb J, Golebiowska B. et al. Effect of sepsis and cardiac surgery with cardiopulmonary bypass on plasma level of nitric oxide metabolites, neopterin, and procalcitonin: Correlation with mortality and postoperative complications. Intensive Care Med. 2000;26:1259–1567. doi: 10.1007/s001340000610. [DOI] [PubMed] [Google Scholar]
- Madershahian N, Wittwer T, Strauch J. et al. Kinetic of procalcitonin in the early postoperative course following heart transplantation. J Card Surg. 2008;23:468–473. doi: 10.1111/j.1540-8191.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- Dandona P, Nix D, Wilson MF. et al. lcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57–S61. [PubMed] [Google Scholar]
- Molnár Z, Bogár L. Let\'s go dynamic with procalcitonin! Crit Care Med. 2006;34:2687–2688. doi: 10.1097/01.CCM.0000240788.00292.F1. [DOI] [PubMed] [Google Scholar]
- Jensen JU, Heslet L, Jensen TH. et al. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596–2602. doi: 10.1097/01.CCM.0000239116.01855.61. [DOI] [PubMed] [Google Scholar]
- Jarai R, Fellner B, Haoula D. et al. Early assessment of outcome in cardiogenic shock: relevance of plasma N-terminal pro-B-type natriuretic peptide and interleukin-6 levels. Crit Care Med. 2009;37:1837–1844. doi: 10.1097/CCM.0b013e31819fe896. [DOI] [PubMed] [Google Scholar]