Abstract
We describe the development and current applications of cerebral oximetry (based on near-infrared reflectance spectroscopy) that can be used during cardiac and major vascular surgery to determined brain tissue oxygen saturation. There are presently three cerebral oximetry devices with FDA approval in the United States to measure and monitor cerebral tissue oxygen saturation. 1. INVOS (Somanetics Corporation, Troy, MI - recently COVIDIEN, Boulder, CO); FORE-SIGHT (CAS Medical Systems, Inc. Branford, CT); EQUANOX (Nonin Medical Inc.Minnesota, MN). All devices are portable, non-invasive and easy to use in operating room and intensive care unit. The data provided in these communication may provided information for improvement of perioperative neuromonitoring techniques, and may be crucial in the design of future clinical trials.
Keywords: cerebral oximetry, detection of cerebral hypoperfusion or ischemia
Introduction
Cardiac and major vascular surgery (CMVS) have entered their sixth decade of existence. During this time period, great strives have occurred towards increasing patient safety by developing and employing reliable hemodynamic and respiratory monitors. Interestingly, the monitoring of cerebral function was slow to evolve and has only recently been able to keep pace with these other advances. The problem has not been lack of the interest, but rather the logistics of not having reliable technology for continuous monitoring of the central nervous system (CNS) in the operating room and intensive care unit.
Adverse CNS outcomes following CMVS are classified into two categories: Type I (cerebral death, non-fatal stroke, focal injury, stupor, encephalopathy, coma and new transient ischemic attack); Type II (deterioration in cognitive function, deficit in memory or seizures). The incidence of these complications varies according to type of surgery, co-morbidites and age. CNS complications are associated with increased mortality, length of hospitalization, and use of long-term facilities with substantial estimated cost per year. Multiple approaches have been utilized to address neurological complications, though definitive therapeutic strategies are lacking.
The etiology of these neurological complications is multifactorial. In operations without cardio pulmonary bypass (CPB): Hypotension, anemia, low oxygen saturation, genetic factors, anesthetic agents and previous neurological pathology, are the main culprits. Operations utilizing CPB are associated with embolisation of gaseous and particulate emboli (arterial atheroma) from the surgical site as well as from placement and removal of the aortic cross clamp. Additionally, hypoperfusion resulting from loss of cerebral autoregulation, hypotension or anemia can lead to water shed cerebral hypoperfusion.
In order to recognize cerebral ischemia in a timely manner, different monitoring modalities have been introduced into clinical practice over the course of the last two decades. Electroencephalographic monitoring (EEG), serial measurements of jugular bulb saturations (jvSO2) and cerebral oximetry based on near infrared spectroscopy have all been reported to successfully identify cerebral ischemia [1,2,3].
An institutional bias leads to heterogeneity in regards on how to correctly monitor these patients in order to identify the onset of cerebral ischemia. Isoelectricity caused by deep hypothermia or excessive dosage of volatile anesthetic agents renders EEG monitoring useless. JvSO2 measurements require the invasive placement of a jugular bulb catheter. Cerebral oximetry on the other hand is non-invasive, user friendly and not influenced by the depth of anesthesia. It can even be utilized as a monitor to detect ischemia in real-time during a circulatory arrest period [4].
This communication will review the development and current applications of cerebral oximetry that can be used during CMVS to improve peri-operative outcomes.
The evolution of cerebral oximetry
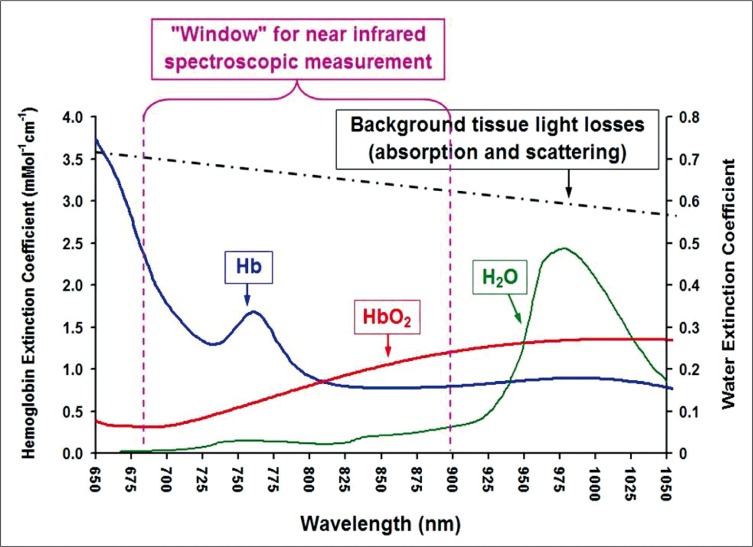
Jobsis first introduced the idea of using near infrared spectroscopy (NIRS) to noninvasively measure cerebral tissue oxygenation in 1977 [5]. The principal of NIRS is based on the fact that near-infrared light passes through skin and skull readily and is absorbed by certain biological molecules in the brain [6, 7]. A “biological spectroscopic window” exists at the wavelength range 660-940 nm because only a few chromophores like Hb and HbO2 strongly absorb light in this spectra range, allowing light to penetrate tissue to a great distance. In this wavelength range, absorption of light due to other biological compounds and tissues such as water, lipids, skin, and bone is lower in magnitude, and these biological compounds generally have a flat absorption spectra when compared to Hb and HbO2. (Figure 1).
Figure 1.
Near-infrared light passes through skin and skull readily and is absorbed by certain biological molecules in the brain. A "biological spectroscopic window" exists at the wavelength range 660 - 940 nm.
Cerebral oximetry, based on near-infrared spectroscopy (NIRS) technology, provides information on the availability of oxygen in brain tissue. Cerebral oximetry measures regional cerebral tissue oxygen saturation (SctO2) at the microvascular level. Complimentary to the arterial oxygen saturation (SaO2) measured by pulse oximetry, SctO2 reflects regional cerebral metabolism and the balance of local cerebral oxygen supply/demand, leading to the following clinical advantages:
it provides SctO2 values continuously and noninvasively;
SctO2 is a sensitive index of cerebral hypoperfusion, hypoxia and/or cerebral ischemia, which is one of the main cause of brain injury during CMVS procedures;
cerebral oximeter is portable, easy to use in operating room or at the bedside.
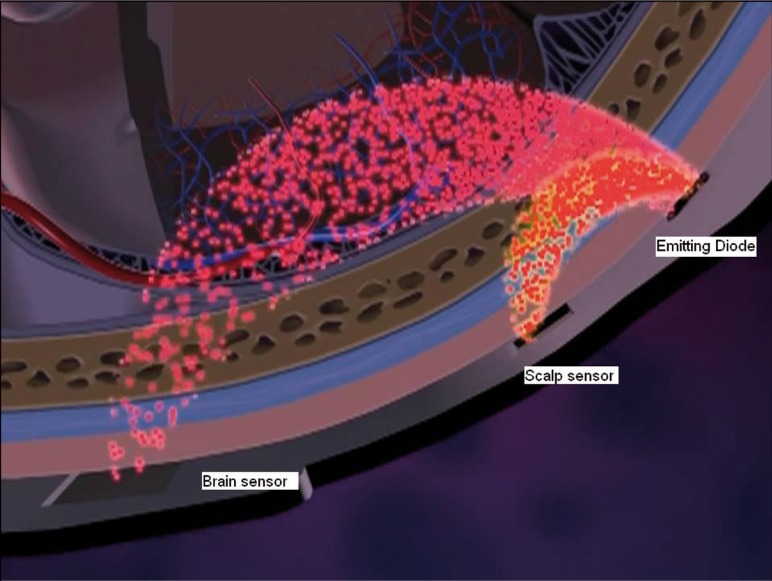
In order to guarantee that only cerebral oxygen saturation is being measured most commercially available oximeters minimize extracerebral contamination by equipping the sensors with 2 light detectors located at fixed distances from the light source. The mean penetration depth of the photons is proportional to the distance between the emitting source and receiving detector. Consequently the detector (scalp or near detector) located closer to the light source measures saturations within the scalp, whereas the detector located further away measures both cerebral as well as scalp saturations (brain or far detector) (Figure 2).
Figure 2.
By subtracting the measurements obtained from the brain detector from the scalp detector, extracererbal contamination can be minimized.
By simply subtracting the measurements obtained from the brain detector from the scalp detector, extracerebral contamination can be minimized.
There are presently three non-invasive cerebral oximetry devices with FDA approval to measure and monitor cerebral tissue oxygen saturation during the perioperative period. 1. INVOS cerebral oximeter (Somanetics Corporation, Troy, MI). 2. FORE-SIGHT absolute cerebral oximeter (CAS Medical Systems, Branford, CT). 3. NONIN regional oximeter (Nonin Medical Inc. Minnesota, MN). A fourth device, the NIRO series near-infrared spectrophotometer (Hamamatsu, Photonic. Hamamatsu, Japan), is available in the Japanese and European markets.
INVOS® (Somanetics Corporation, Troy, MI) The INVOS Cerebral Oximeter (Somanetics Corporation, Troy, MI - from July 2010 COVIDIEN, Boulder, CO) (Figure 3) has been commercially available since 1993 and the sixth generation model is the only oximeter to display four channels simultaneously, enabling the clinician to track cerebral as well as somatic (body) tissue oxygen saturations.
Figure 3.
INVOS.
The INVOS uses two wavelengths of infrared light (730 and 810 nm) from light-emitting diodes (LEDs) [8]. The INVOS has disposable sensors, which contain LEDs and two light detectors at fixed distances from the light source. The device calculates and displays the value of regional cerebral oxygen saturation (rSO2). Most publications showing the benefit of using the Invos to monitor cerebral oxygenation during cardiac surgery have used it as a trend monitorErrore: sorgente del riferimento non trovata. This means that clinical interventions were based on changes of rSO2 from the initial baseline value, which was obtained at pre-induction of anesthesia [9].
FORE-SIGHT® (CAS Medical Systems, Inc. Branford, CT)
The FORE-SIGHT® Absolute Cerebral Oximeter (Figure 4) has been commercially available since 2007 [10].
Figure 4.
FORE-SIGHT.
The FORE-SIGHT monitor is a continuous wave (CW) spatially resolved near infrared spectrometer that measures absolute cerebral tissue oxygen saturation (SctO2%). This device has two channels for bilateral brain monitoring. In contrast to the other devices currently available, the FORE-SIGHT uses Laser light at a FDA deemed safe level (Class I laser) to project four precise (bandwidth <1nm) wavelengths (690, 780, 805, & 850 nm) into the brain to capture information needed for the algorithm to calculate the absolute value of cerebral tissue oxygen saturation (SctO2). The advantage of adding additional wavelengths makes it possible to compensate for scattering losses, and to account for interference from other background light absorbers [11] (such as fluid, tissue and skin pigmentation). Consequently, the FORE-SIGHT is capable of measuring cerebral tissue oxygen saturation more precicely precisely than the INVOS [12]. This advance in technology is the basis for enabling the device to reliably measure absolute values of SctO2. This, in turn, eliminates the need to establish pre-induction baseline measurements and enables the use of threshold values to guide clinical interventions.
Figure 5.
EQUANOX.
EQUANOX® (Nonin Medical Inc. Minnesota, MN)
The most recent device to emerge onto the market in the United States is the EQUANOX (Figure 5). FDA approval was granted in the summer of 2009. The EQUANOX uses LED technology to transmit three-wavelengths (730 nm, 810 nm and 880 nm) [13]. The EQUANOX sensor differs from the INVOS and FORE-SIGHT sensors because it is equipped with dual emitter sites, enabling a crisscrossing in the transmission of the photons. According to the company this technology eliminates the challenges of surface variability by having the LEDs illuminate alternately leading to improved accuracy. Due to the novelty of the device studies confirming reliability and efficacy are not yet available. A calibration and validation study from MacLeod and colleagues shows that the EQUANOX provides, similar to the INVOS, an accurate measure of trends in cerebral oxygen saturation, however falls short of obtaining absolute measurements [14].
Applications of cerebral oximetry monitoring
As previously described, commercially available cerebral oximetry devices have been available since the early 1990s, however, most reports of the utility of cerebral oximetry during the early years focused on their ability to recognize catastrophic events. The literature is filled with such case reports [15,16,17,18]. While the importance of a monitor that can alert the clinician to an otherwise unrecognized catastrophic event cannot be underscored the true scientific advances in the field started to take place during the last 5 years. Attempts at utilizing cerebral oximetry to improve clinical outcomes and shorten hospital length of stay are now the focus of most investigators within the field.
Goldman and colleagues were the first to report that by optimizing cerebral oxygenation to maintain saturations around the pre-induction baseline that the treatment group demonstrated a significantly lower incidence of permanent stroke [19]. This study however was not without undeniable weaknesses. The study was non-randomized and retrospective in design. The control group consisted of a historical cohort. Patients that underwent cardiac surgery 18 months prior to the introduction of cerebral oximetry at the author’s institution were subsequently compared with the treatment group which consisted of patients enrolled during the following 18 months. Additionally, the authors acknowledge that they were unable to determine how close rSO2 values were maintained to baseline values during the course of surgery. Despite these shortcomings one could argue, as the authors pointed out, that better compliance with a predefined target value would translate into potentially better outcomes. The fact that the study group consisted of patients with more co-morbidities, yet had a lower incidence of permanent stroke, decreased need for prolonged ventilation and shorter length of hospital stay is impressive. To date the best trial supporting the efficacy of monitoring and optimizing cerebral oxygenation was published by Murkin et al. in 2007. This randomized, blinded study of 200 CABG patients demonstrated prospectively that rSO2 (INVOS® Somanetics) monitoring is associated with a significant improvement in overall outcome after cardiac surgery [20]. Murkin utilized an intraoperative management protocol designed to maintain rSO2 values at or above 75% of the preinduction baseline. The results were associated with a significant improvement in overall organ function and decreased postoperative length of stay. While the study was underpowered to show significant difference in stroke rates between the intervention and control groups, the idea that cerebral oximetry could be used as a surrogate marker for overall organism well being was introduced. Slater and colleagues showed that an increase in post operative neurocognitive dysfunction (POCD) was seen in patients with an increase in rSO2 desaturation score of greater than 3,000%-second [21]. They also were able to associate a near threefold increase in risk of prolonged hospital stay. Unfortunately due to flawed study design no difference was seen between the intervention and control groups regarding outcome difference.
In another randomized prospective study of elderly patients undergoing major abdominal surgery, Casati et al. [22] reported on improved mini mental scores (MMS), more rapid PACU discharge, and shortened length of hospital stay in 122 geriatric patients after major abdominal surgery in which cerebral oximetry was monitored and optimized. While the utilization of the MMS to address postoperative cognitive impairment is crude, this study did demonstrate that 20% of all patients experienced a decrease in rSO2 (INVOS® Somanetics) below 75% of baseline without any change in systemic oxygenation (SpO2).
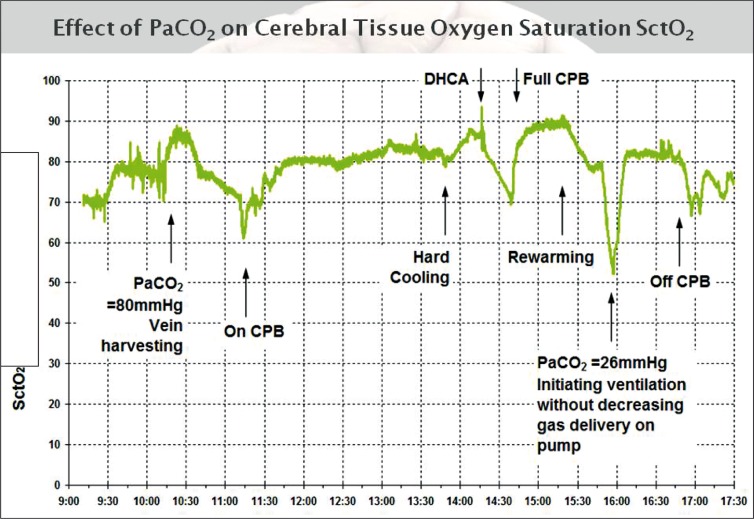
In a recently published observational study of patients undergoing aortic arch surgery with hypothermic circulatory arrest (HCA) (Figure 6), Fischer and colleagues were able to support similar findings that other investigators found in the CABG population [23].
Figure 6.
Intraoperative SctO2 course of a patient undergoing aortic arch surgery with hypothermic circulatory arrest. Note dependency of SctO2 to CO2. Insufflation of CO2 during endoscopic vein harvesting leading to increase in SctO2 as well as decline in SctO2 once mechanical ventilation was started. CPB = cardiopulmonary bypass; DHCA = deep hypothermic circulatory arrest.
Time spent beneath threshold values as well as the area under the threshold (AUT) were significantly associated with adverse outcomes as well as time on ventilator, intensive care unit and hospital length of stay. In line with Casati’s findings, Fischer found that the risk of obtaining an adverse event doubled with ever decade of life. a finding that supports the overall belief that the geriatric population is more susceptible and prone to the effects of cerebral ischemia. Additionally, the investigators found that patients who spent more than 30 minutes under the threshold value of 60% had an increased cost of care of $8,300. Mathematical models, such as the one described by Fischer et al. [24] could potentially make the technique of circulatory arrest safer and more predictable. Knowledge of the rate of cerebral desaturation can be used, in conjunction with the knowledge obtained from the threshold paper [23], to predict allowable duration of HCA prior to the occurrence of adverse events. In summary, cerebral oximetry is a non-invasive technology that has the potential to provide the clinician with information to tailor management during the perioperative period. Studies are emerging linking optimization of cerebral saturation with improved outcomes, shortened length of stay and reduction in cost.
Footnotes
Conflict of interest No conflict of interest is acknowledged by the authors.
References
- Leggat CS, Fischer GW. Early detection of an acute cerebral event during cardiopulmonary bypass using a bispectral index monitor. Semin Cardiothorac Vasc Anesth. 2008;12:80–82. doi: 10.1177/1089253208316912. [DOI] [PubMed] [Google Scholar]
- Reich DL, Horn LM, Hossain S, Uysal S. Using jugular bulb oxyhemoglobin saturation to guide onset of deep hypothermic circulatory arrest does not affect post-operative neuropsychological function. Eur J Cardiothorac Surg. 2004;25:401–406. doi: 10.1016/j.ejcts.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Fischer GW. Recent advances in application of cerebral oximetry in adult cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2008;12:60–69. doi: 10.1177/1089253208316443. [DOI] [PubMed] [Google Scholar]
- Fischer GW, Benni PB, Lin HM. et al. Mathematical model for describing cerebral oxygen desaturation in patients undergoing deep hypothermic circulatory arrest. Br J Anaesth. 2010;104:59–66. doi: 10.1093/bja/aep335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiencyand circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Silvay G, Weinreich A, Owitz S. et al. The cerebral function monitoring during open-heart surgery. Herz. 1978;3:270–275. [PubMed] [Google Scholar]
- McCormick PW, Stewart M, Ray P. et al. Measurement of regional cerebrovascular haemoglobin oxygen saturation in cats using optical spectroscopy. Neurological Res. 1991;13:65–70. doi: 10.1080/01616412.1991.11739967. [DOI] [PubMed] [Google Scholar]
- Thavasothy M, Broadhead M, Elwell C. et al. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 Near-Infrared Spectrophotometers. Anaesthesia. 2002;57:999–1006. doi: 10.1046/j.1365-2044.2002.02826.x. [DOI] [PubMed] [Google Scholar]
- Schell RM, Cole DJ. Cerebral monitoring: jugular venous oximetry. Anesthesia & Analgesia. 2000;90:559–566. doi: 10.1097/00000539-200003000-00012. [DOI] [PubMed] [Google Scholar]
- www.casmed.com. www.casmed.com
- Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Soc of Biol Psych. 2002;52:679–693. doi: 10.1016/s0006-3223(02)01550-0. [Strangman G, Boas DA, Sutton JP. ] [DOI] [PubMed] [Google Scholar]
- MacLeod D, Ikeda K. Simultaneous comparison of FORE-SIGHT and INVOS cerebral oximeters to jugular bulb and arterial co-oximetry measurements in healthy volunteers. (SCA Suppl): SCA 56. Anesth Analg. 2009;108(SC:SCA 56. [MacLeod D, Ikeda K, Vacchiano C. ] [Google Scholar]
- www.nonin.com. www.nonin.com [Google Scholar]
- MacLeod D, Ikeda K, Vacchiano C. Simultaneous Comparison of FORE-SIGHT and INVOS Cerebral Oximeters to Jugular Bulb and Arterial Co-Oximetry Measurements in Healthy Volunteers. ANESTH ANALG. 2009;108(SC:1–104. [Google Scholar]
- Han SH, Kim CS, Lim C, Kim WH. Obstruction of the Superior Vena Cava Cannula Detected by Desaturation of the Cerebral Oximeter. J Cardiothorac Vasc Anesth. 2005;19:420–421. doi: 10.1053/j.jvca.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Orihashi K, Sueda T, Okada K, Imai K. Malposition of selective cerebral perfusion catheter is not a rare event. Eur J Cardiothorac Surg. 2005;27:644–648. doi: 10.1016/j.ejcts.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Rodriguez RA, Cornel G, Semelhago L. et al. Cerebral effects in superior vena caval cannula obstruction: the role of brain monitoring. Ann Thorac Surg. 1997;64:1820–1822. doi: 10.1016/s0003-4975(97)01066-7. [DOI] [PubMed] [Google Scholar]
- Fischer GW, Stone ME. Cerebral air embolism recognized by cerebral oximetry. Semin Cardiothorac Vasc Anesth. 2009;13:56–59. doi: 10.1177/1089253208330710. [DOI] [PubMed] [Google Scholar]
- Goldman S, Sutter F, Ferdinand F, Trace C. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. Heart Surg Forum. 2004;7:376–381. doi: 10.1532/HSF98.20041062. [DOI] [PubMed] [Google Scholar]
- Murkin JM, Adams SJ, Novick RJ. et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104:51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- Slater JP, Guarino T, Stack J. et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44. doi: 10.1016/j.athoracsur.2008.08.070. [DOI] [PubMed] [Google Scholar]
- Casati A, Fanelli G, Pietropaoli P. et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. 2005;101:740–747. doi: 10.1213/01.ane.0000166974.96219.cd. [DOI] [PubMed] [Google Scholar]
- Fischer GW, Lin HM, Krol M. et al. Noninvasive cerebral oxygenation may predict outcome in patients undergoing aortic arch surgery. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2010.05.017. [Epub ahead of print. PMID: 20579669.] [DOI] [PubMed] [Google Scholar]
- Fischer GW, Benni PB, Lin HM. et al. Mathematical model for describing cerebral oxygen desaturation in patients undergoing deep hypothermic circulatory arrest. Br J Anaesth. 2010;104:59–66. doi: 10.1093/bja/aep335. [DOI] [PMC free article] [PubMed] [Google Scholar]