Abstract
The role of acid secretion in regulating short-term changes in growth rate and wall extensibility was investigated in emerging first leaves of intact, water-stressed maize (Zea mays L.) seedlings. A novel approach was used to measure leaf responses to injection of water or solutions containing potential regulators of growth. Both leaf elongation and wall extensibility, as measured with a whole-plant creep extensiometer, increased dramatically within minutes of injecting water, 0.5 mm phosphate, or strong (50 mm) buffer solutions with pH ≤ 5.0 into the cell-elongation zone of water-stressed leaves. In contrast, injecting buffer solutions at pH ≥ 5.5 inhibited these fast responses. Solutions containing 0.5 mm orthovanadate or erythrosin B to inhibit wall acidification by plasma membrane H+-ATPases were also inhibitory. Thus, cell wall extensibility and leaf growth in water-stressed plants remained inhibited, despite the increased availability of (injected) water when accompanying increases in acid-induced wall loosening were prevented. However, growth was stimulated when pH 4.5 buffers were included with the vanadate injections. These findings suggest that increasing the availability of water to expanding cells in water-stressed leaves signals rapid increases in outward proton pumping by plasma membrane H+-ATPases. Resultant increases in cell wall extensibility participate in the regulation of water uptake, cell expansion, and leaf growth.
A primary response of developing plants to suboptimal water availability is the inhibition of leaf growth, which can lead to reductions in the final size and yields of crop plants (Hsiao, 1973; Boyer, 1982; Blum, 1988). However, the cellular mechanisms underlying the regulation of leaf or other growth responses to decreased water availability are still far from clear (Close and Bray, 1993; Bohnert et al., 1995; Neumann, 1995, 1997).
In the uninhibited state leaf growth is dependent on the massive and irreversible expansion of new daughter cells that are produced by meristematic divisions. Imposition of moderate water stress, defined here as suboptimal water availability for growth, can result in rapid (within minutes) decreases in growth, presumably via inhibition of cell expansion (Serpe and Matthews, 1992; Chazen and Neumann, 1994). Additional decreases in rates of cell production can subsequently contribute to longer-term inhibition of growth (Silk, 1992). Water stress may rapidly limit cell expansion by decreasing the water-potential gradients driving water uptake and the turgor pressure that drives the expansion of the cell walls. Subsequently, the hydraulic conductivity of the water-uptake pathway may be affected (Evlagon et al., 1990; Nonami and Boyer, 1990; Nonami et al., 1997). Stress-induced reductions in the extensibility characteristics of the growing cell walls can also contribute to growth inhibition (Passioura, 1994; Neumann, 1995).
For example, in intact maize (Zea mays L.) seedlings, the early inhibition of leaf growth by water stress applied to the root involved rapid decreases in the extensibility of cell walls in the elongation zone (i.e. measured decreases in the irreversible extension caused by a 3-min application of a small force in the direction of leaf growth; Chazen and Neumann, 1994). Reductions in wall-extensibility characteristics hours or days after imposing growth-inhibitory water stresses have also been found in a range of species (Nonami and Boyer, 1990; Cramer, 1992; Schultz and Matthews, 1993). Moreover, both the extensibility of leaf cell walls and leaf growth in maize were increased within 20 min of removing water stress (Neumann, 1993; Chazen and Neumann, 1994). Intact, water-stressed algal cells and other species and cultivars of higher plants have also been shown to respond to relief of water stress with rapid increases in growth rate, which may be followed by feedback inhibition of growth. Such changes have been related to changes in turgor pressure and wall-extensibility traits (Green et al., 1971; Serpe and Matthews, 1992; Passioura, 1994; Hsiao et al., 1998).
Since cell wall extensibility and leaf growth in maize seedlings changed within minutes of imposing or removing root water deficits, it seemed unlikely that gene activation and de novo synthesis of growth-essential proteins could be rapid enough to regulate these initial responses (Neumann, 1993; Chazen and Neumann, 1994). This report concerns an investigation of alternative regulatory mechanisms that might underlie the rapid transduction of changes in water availability to intact maize plants into changes in leaf-growth rates and extensibility of expanding leaf cell walls. Short-term responses were assayed so that the data could be interpreted without complications due to longer-term feedback or developmental changes (Blum, 1988; Serpe and Matthews, 1994). Intact plants were assayed to avoid artifacts that may be associated with the use of excised segments of growing organs (Cosgrove, 1997).
The working hypothesis was that rapid changes in rates of acid secretion into the cell walls, and therefore wall pH, might be involved in the regulation of early leaf-growth responses to changes in water availability, as suggested by the acid-growth hypothesis of auxin action (Rayle and Cleland, 1970, 1992; Hager et al., 1971; Jacobs and Taiz, 1980; Kutschera, 1994; Frias et al., 1996). According to this hypothesis, auxin can stimulate growth in auxin-depleted tissues by inducing increases in the amount or activity of the outwardly directed proton-pumping ATPases on the PMs of expanding cells. Consequent lowering of cell wall pH can then regulate spontaneous or enzymatic hydrolysis (and possible reformation) of covalent or ionic bonds between load-bearing wall polymers. This loosens the cell wall, i.e. increases its ability to expand in response to applied force. Changes in wall pH may also lead to changes in the activity of wall proteins such as expansins, which can loosen cell walls by affecting hydrogen bonding between load-bearing wall polymers. Conversely, cessation of wall acidification could be a factor leading to hardening of expanding cell walls and growth inhibition (Carpita and Gibeaut, 1993; Cosgrove, 1997).
A few reports indicated that inhibition of leaf growth by water-stress episodes may also be associated with decreases in cell wall acidification: Long-term water stress was associated with small decreases in the acidity of the apoplastic sap that could be expressed from the leaves (Hartung et al., 1988; Wilkinson and Davies, 1997). Both H+ excretion and the elongation of peeled coleoptile segments held in an auxin solution were inhibited by exposure to negative water potentials for 2.5 h (Cleland, 1975). Finally, the surface-acidification capacity of abraded leaf segments excised from plants exposed to drying soil for 3 to 5 d and in vitro wall extensibility in killed segments were both reduced in comparison with segments from watered controls (Van Volkenburgh and Boyer, 1985). However, the possibility that changes in the wall-acidifying activity of PM H+-ATPase are involved in regulating the very rapid changes in wall extensibility and leaf growth that are induced by increasing water availability to waterstressed plants does not appear to have been previously investigated.
We report here the use of a novel technique to assay early cell wall and growth responses to direct injection of water, strong pH buffers, or PM H+-ATPase inhibitors into the leaf-expansion zone of intact, water-stressed maize seedlings.
MATERIALS AND METHODS
Plant Growth
Seedlings of maize (Zea mays L. cv 643, Galilee Seeds, Israel) were germinated on filter paper and then grown hydroponically for 2 d under controlled conditions: a 12-h photoperiod at 35 W m−2 PAR from mixed incandescent and fluorescent light sources and a temperature of 27°C ± 2°C, as previously described (Chazen and Neumann, 1994). Seedlings were assayed when the length of the primary roots was about 10 cm and the tip of the still-curled, first true leaf was 0.5 to 0.8 cm above the surrounding coleoptile. The elongating leaf tissues were located within a 1-cm section above the node, at the base of the leaf (Neumann, 1993; Snir and Neumann, 1997).
Short-term rates of leaf elongation were assayed using a computerized whole-plant extensiometer (Neumann, 1993; Snir and Neumann, 1997). Each seedling was firmly fitted into a plastic holder so that the roots alone were bathed in the appropriate aerated nutrient solution. The caryopsis was fixed above the solution by the holder, and the shoot (coleoptile and protruding primary leaf) extended vertically above it. The leaf tip was stuck to an aluminum foil tab connected to a small alligator clip connected to a thread looped over a low-resistance pulley wheel and joined to the core of a LVDT (Instruments and Control Inc., Haifa, Israel). Small-range (±2 mm), high-sensitivity transducers (ST-3, 950 mV/mm) were used. A 0.4-g weight was continuously applied in the direction of leaf growth to overcome frictional resistance. Up to 16 plants could be monitored simultaneously.
The electrical output of the LVDTs varied linearly with changes in the position of the tip of the growing leaves and was sampled at 1-s intervals. Output from the LVDTs was amplified and fed into an A/D card (Das 1401, Keithley Metrabyte, Taunton, MA) in a 486 PC. Stored data were displayed graphically using data acquisition and graphical display software (Viewdac 2.1, Keithley Metrabyte). The software was programmed to directly produce graphic plots of changes in individual leaf-tip positions against time. A moving average based on seven data points reduced background noise and caused an insignificant delay of about 4 s in the graphical display of the data. The accuracy of leaf-position measurements was within ±2 μm.
In Vivo Extensibility
The whole-plant extensiometer system used here for measuring comparative extensibility traits in maize leaves is a modification of previous approaches (Nonami and Boyer, 1990; Cramer, 1992) and has been intensively characterized. Moreover, several previous reports have shown a close correlation between changes in growth (defined as an irreversible increase in size) and the apparently irreversible component of maize leaf extensibility as assayed in vivo and in vitro (Neumann, 1993, 1995; Chazen and Neumann, 1994; Snir and Neumann, 1997). In vivo extensibility in elongating leaves was assayed by following the changes in the position of leaf tips induced by careful application of a small additional force (a weight of 2 g) in the direction of growth and then removing it after 3 min. The irreversible extension produced by the weight could be measured on the graphic display of leaf position against time and used to calculate a comparative in vivo measure of leaf extensibility in units of micrometers per application of a 2-g weight for 3 min (μm [2 g 3 min)]−1). The reversible extension gave a comparative measure of elastic extensibility in the same units. Means were always determined for the simultaneous assay of three or more plants to compensate for variability between individuals.
In Vivo Extensibility of Mature Leaf Tissue
To estimate the relative contributions of growing and mature leaf tissues to in vivo extensibility of whole leaves, mature tissues were excised from the leaf and carefully separated from the coleoptile sheath. The excision was carried out in a humidity chamber and the exposed leaf was coated with petroleum jelly to minimize dehydration. The leaf base and tip were attached to aluminum foil tabs. The basal tab was clamped in a fixed position and the apical tab was attached to the extensiometer for assay as described above. As previously reported (Neumann, 1993), the mature tissues of the first leaves contributed no more than 15% of the extensibility measured in intact leaves.
In Vitro Assay of Cell Wall Extensibility
For the in vitro assay of leaf cell wall extensibility, the growing tissues in the basal 1-cm section of the first leaf of treated or control seedlings, prepositioned in the extensiometer as for in vivo assays, were locally frozen with a gas jet and then thawed as previously described (Neumann, 1993; Chazen and Neumann, 1994; Snir and Neumann, 1997). Complete inhibition of leaf elongation and subsequent tissue necrosis confirmed the effectiveness of the freeze-thaw treatment. This treatment disrupts membrane hydraulic resistance, turgor, and growth in the cell-expansion zone and results in a cell wall preparation. At 15 min after freezing, when leaf contraction due to loss of turgor had declined, in vitro extensibility of the walls was assayed using the procedure used for the in vivo assay. The leaf-tip position attained 1.5 min after weight removal was used as the end point of the assay. Determination of the apparently irreversible component of cell wall extensibility by prolonged in vitro assays of freeze-killed plant segments may generate artifacts and provide an insufficient model for understanding in vivo regulation (Cosgrove, 1997; Ding and Schopfer, 1997). However, these in vitro assays can confirm that alterations in cell wall rheology are involved in any extensibility changes detected by in vivo assays.
Leaf Injection
Aqueous solutions of buffers or inhibitors were injected directly into the basal growing zone of intact leaves attached to position transducers so that treatment effects on leaf elongation could be continuously observed and compared with the effects of appropriate control solutions injected into other plants. A microsyringe fitted with a fine needle (o.d. 250 μm) and a stop device that limited needle penetration to 1 mm was used for the leaf injections. The needle was inserted horizontally through the surrounding coleoptile and into the leaf at a point 3 mm above the node; 12 μL of solution was injected into the leaf tissues, which were supported from behind. Any effects on growth and in vivo or in vitro extensibility were then determined. A moderate water deficit was imposed prior to leaf injection at time 0 by first exposing the roots to aerated nutrient solution containing nonpenetrating osmolyte PEG 6000 at a water potential of −0.4 MPa for 1 h. Water potentials of PEG solutions were calculated as previously described (Chazen and Neumann, 1994).
Chemicals
pH buffers (succinic acid, pK1 5.6 and pK2 4.2; Mes, pK 6.1; acetic acid, pK 4.7) and inhibitors of proton-pumping ATPases in the PM (vanadate and erythrosin B [Jacobs and Taiz, 1980; Amodeo et al., 1992; Cowan et al., 1993]) were of analytical grade or equivalent purity. Solutions were brought to the desired pH by the addition of NaOH or HCl. Clear vanadate solutions were obtained by diluting freshly prepared, boiled, and cooled stock solutions of sodium orthovanadate (5 mm adjusted to pH 6.5) into water or buffer solutions at the desired pH. Osmotic potentials of injected solutions were determined using a semi-micro freezing point osmometer (Knauer, Berlin, Germany).
All experiments were repeated one or more times with similar results.
RESULTS
Stimulatory Effects of Water Injection on Leaf Growth and Extensibility of Stressed Plants
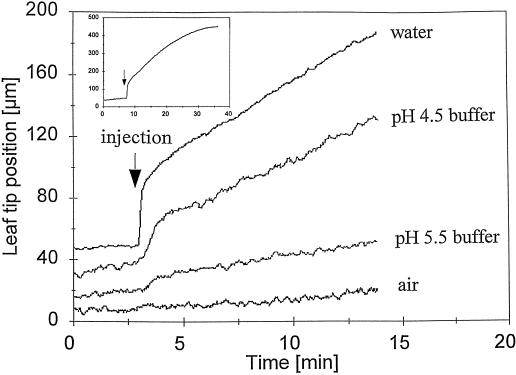
Water (12 μL) was injected into the elongation zone of the emerging first leaves of intact, water-stressed maize seedlings to characterize short-term growth responses. The seedlings had been water stressed by incubation for 1 h with their roots in an aerated nutrient solution containing the nonpenetrating osmolyte PEG 6000 (−0.4 MPa water potential). This stress treatment caused leaf-growth rates to decline to low values (Table I). Injections of air into the elongation zone had no significant effects on the leaf-growth rates of these water-stressed plants. However, injections of water caused rapid increases in leaf-growth rates (Table I). The kinetics of leaf-growth responses to water injection are shown in Figure 1. Following a 60-s burst of rapid expansion, a quasilinear period of slower but still accelerated growth was established. Growth began to decline progressively after about 15 min and returned to the slower rates characteristic of noninjected plants about 25 min after injection (Fig. 1). Subsequent measurements of treatment effects on leaf-growth rates and extensibility were confined to the quasilinear period of accelerated growth.
Table I.
Rapid stimulatory effects of water injection on rate of leaf growth and extensibility in water-stressed maize seedlings
| Treatment | Growth Rate | Extensibility
|
|
|---|---|---|---|
| in vivo | in vitro | ||
| μm min−1 | μm (2 g 3 min)−1 | ||
| Nonstressed | 15 ± 1 | 32 ± 4 | 26 ± 1 |
| Water stressed | 2 ± 1 | 8 ± 1 | 12 ± 2 |
| +Air injection (12) | 1 ± 0 | 11 ± 0 | 13 ± 2 |
| +Water injection (4) | 11 ± 1 | 25 ± 2 | 27 ± 2 |
| +Water injection (12) | 10 ± 1 | 19 ± 1 | 25 ± 3 |
Leaf-growth rates were determined from changes in leaf position over 3 min just prior to assay of extensibility. The nonstressed treatment shows data for leaves of plants without PEG addition to the root medium. In water-stress treatments, plant roots were exposed to PEG 6000 (−0.4 MPa water potential) for 1 h prior to injection. Values in parentheses are times (in minutes) between injection and commencement of the comparative assays of leaf or cell wall extensibility (in vivo or in vitro, respectively). Results are means ± se; n = 6 for growth rates; n = 3 for extensibility measurements. Other details are as in Figure 1.
Figure 1.
Rapid effects of water and buffer injections on elongation kinetics of the first leaves of water-stressed maize seedlings. A microsyringe fitted with a fine needle (o.d. 250 μm) was used to inject 12-μL aliquots into the elongation zone of the emerging first leaf of water-stressed maize seedlings. Water stress was applied 1 h before the injection by adding PEG 6000 (−0.4 MPa) to the aerated root medium. Changes in leaf position were determined with LVDTs at 1-s intervals. Each plot shows computer printouts of growth responses for single leaves. The inset shows longer-term (30-min) responses. Average leaf-growth responses are shown in Tables I–IV.
The acceleration of growth following injection of water into stressed leaves was presumably associated with water uptake and accelerated cell expansion. Osmotic potentials in the expanding leaf tissues of unstressed maize plants were −0.8 MPa, and the relaxed water potentials were −0.4 MPa (Z. Lu and P.M. Neumann, unpublished data). Moreover, osmotic potentials in expanding maize leaf tissues became more negative shortly after imposing water stress (Chazen and Neumann, 1994). Negative water potentials would promote the rapid uptake of injected water at 0 MPa (Fig. 1). Tissue uptake of water from injected solutions (e.g. of buffers or inhibitors, see below) should also be favored, although to a lesser extent, since a smaller water-potential gradient would be established. The dissolved solutes would presumably be cotransported with the injected water to the wall-membrane interfaces of the expanding leaf cells.
The decreases in leaf growth induced by the addition of PEG to the roots of unstressed plants were accompanied by decreases in the irreversible component of leaf and cell wall extensibility, as measured in vivo and in vitro, respectively (Table I). More importantly in the present context, the increases in leaf-growth rates induced by water injection were accompanied by rapid increases in leaf extensibility. The elastic (i.e. reversible) component of extensibility, as assayed 12 min after injection of water into treated plants, did not increase significantly compared with air-injected controls: 17 ± 2 μm (2 g 3 min)−1 after air injection and 21 ± 3 μm (2 g 3 min)−1 after water injection (means ± se; n = 3). Thus, the increase in leaf growth induced by water injection into stressed plants was associated with increases in the irreversible component of extensibility but was not associated with significant increases in the elastic component.
Effects of pH Buffers
The possibility that rates of wall acidification might rapidly increase after water injection, thus facilitating the increases in wall extensibility and growth, was investigated by assaying the short-term effects on leaf growth of injecting aqueous buffer solutions at different pHs (Fig. 1; Table II). Injection of a control solution of strong (50 mm) succinate buffer at pH 4.5 resulted in a clear stimulation of leaf growth, although to a lesser extent than water alone, perhaps because the additional solutes in the buffer solution resulted in a lower (more negative) osmotic potential (−0.3 MPa) than that of water (0 MPa). Accumulation of these solutes in the apoplast of expanding cells could then decrease the water-potential gradient that drives water uptake for cell expansion.
Table II.
Inhibitory effect of pH-5.5 buffer on water-induced increases in leaf growth rate and extensibility
| Treatment | Growth Rate | Extensibility
|
|
|---|---|---|---|
| in vivo | in vitro | ||
| pH | μm min−1 | μm (2 g 3 min)−1 | |
| 4.5 | 7 ± 1 | 22 ± 3 | 23 ± 3 |
| 5.5 | 3 ± 0 | 13 ± 2 | 12 ± 1 |
Isoosmotic solutions containing 50 mm succinate buffer at pH 4.5 or 5.5 were injected into the leaf-elongation zone as in Figure 1. Growth rate and extensibility were assayed 9 and 12 min after injection, respectively. Results are means ± se, n = 10 for growth rates and n = 5 for the extensibility measurements.
More importantly, treatments with 50 mm succinate buffer adjusted to pH 5.5 instead of 4.5 had little stimulatory effect on growth (Fig. 1; Table II). The original water potential of the pH-4.5 buffer (approximately −0.25 MPa) was slightly less negative than that of the pH 5.5 buffer (−0.3 MPa). Succinate was therefore added to the pH 4.5 buffer so as to make it isoosmotic with the pH 5.5 buffer. Thus, the comparatively inhibitory effect of pH 5.5 buffer compared with pH 4.5 buffer was not due to any difference in their water potentials. Similar effects on leaf growth were obtained when Mes buffer at pH 4.5 and 5.5 was used. In this case, the leaf-growth rate 12 min after injection with Mes at pH 5.5 remained low (1 ± 1 μm min−1), whereas the leaf-growth rate 12 min after injection with Mes at pH 4.5 was increased (9 ± 2 μm min−1; means ± se; n = 5). Mes was adjusted to lower pH values with HCl; therefore, the inhibitory effect on leaf growth of Mes buffer at pH 5.5 occurred even though it had a lower Cl− level (and a less negative osmotic potential) than the more acidic Mes solution at pH 4.5. The fact that solutions of both succinate and Mes buffers at pH 5.5 inhibited the increases in leaf-growth rate induced by the same solutions at pH 4.5 makes it unlikely that the growth-inhibitory effect of the pH 5.5 buffers was an artifact of pH-specific and rapid toxic effects on a growth-essential metabolic reaction in the expanding cells.
Early differences in leaf-growth responses to injection with buffer solutions at pH 4.5 or 5.5 were also associated with differences in cell wall responses. For example, the mean in vivo extensibility of intact leaves injected with pH 5.5 buffer remained at the low values typical of water-stressed plants. In contrast, injection with pH 4.5 buffer induced significant increases in extensibility. Measurements of in vitro extensibility of the leaf-elongation zone revealed similar response patterns (Table II). Note that the in vitro measurements were made on the cell walls of killed tissues with zero turgor pressure. Thus, the treatment-induced differences in wall extensibility measured by in vitro assay were clearly independent of any effects of growth rate, wall metabolism, or turgor pressure during the assay. We conclude that injection of water or pH 4.5 buffer into the cell-elongation zone of intact, water-stressed maize leaves rapidly induces wall acidification and increases wall extensibility (i.e. wall loosening), which can then facilitate the acceleration of leaf growth. Conversely, pH 5.5 buffers appear to inhibit increases in wall acidification and loosening, thus preventing the usual water-induced acceleration of leaf growth.
Effects of Inhibitors of PM H+-ATPase
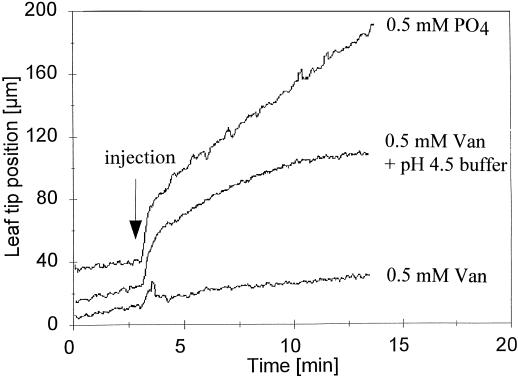
The aim of these experiments was to determine whether inclusion of 0.5 mm sodium vanadate with water injections could also inhibit the rapid growth recovery usually initiated by increasing the availability of water to stressed leaves. Vanadate is an inhibitor of the PM-bound proton-pumping ATPases that are thought to participate in regulating cell wall acidification. Injections of aqueous solutions containing 0.5 mm vanadate (or 2 mm vanadate, not shown) effectively prevented the usual water-induced acceleration of leaf growth (Fig. 2; Table III). Injected solutions of an organic proton-pump inhibitor, 0.5 mm erythrosin B, also prevented the usual water-induced acceleration of leaf growth (leaf-growth rates before and 12 min after erythrosin B injection were 2 ± 1 and 3 ± 1 μm min−1, respectively; means ± se; n = 5). However, 0.5 mm phosphate controls at the same pH as vanadate and erythrosin B solutions (i.e. pH 6.5) had clear stimulatory effects on growth (Fig. 2; Table III). Thus, the weak buffer effect of 0.5 mm phosphate at pH 6.5 did not prevent growth stimulation. Moreover, the pH-buffer capacity of dilute phosphate solution is greater than that of the vanadate solution (Jacobs and Taiz, 1980). Thus, the greater growth-inhibitory effect of vanadate appeared to be associated with its inhibitory effect on PM H+-ATPases rather than with any weak-buffer effects on cell wall acidification. In addition, it is important to note that vanadate also inhibited the usual water-induced increases in extensibility, whereas equimolar phosphate solution did not (Table III).
Figure 2.
Inhibitory effect of vanadate on rapid leaf-growth responses to water injection and its reversal by pH 4.5 buffer. Unbuffered solutions of sodium vanadate (Van) and sodium phosphate controls (PO4) were at 0.5 mm and pH 6.5; 50 mm sodium succinate was used to strongly buffer vanadate to pH 4.5 (0.5 mm Van + pH 4.5 buffer). Other details are as in Figure 1.
Table III.
Inhibitory effects of vanadate on water-induced increases in leaf-growth rate and extensibility
| Treatment | Growth Rate | Extensibility
|
|
|---|---|---|---|
| in vivo | in vitro | ||
| μm min−1 | μm (2 g 3 min)−1 | ||
| Phosphate | 8 ± 1 | 20 ± 2 | 24 ± 3 |
| Vanadate | 3 ± 0 | 13 ± 2 | 16 ± 1 |
Solutions of sodium phosphate (control) or sodium vanadate, both at 0.5 mm and pH 6.5, were injected as in Figure 1. The relatively weak pH-buffer capacity of dilute phosphate solution is greater than that of vanadate solution (Jacobs and Taiz, 1980). Thus, the phosphate buffer control shows that direct, weak-buffer effects on cell wall pH did not account for the inhibitory effects of vanadate. Results are means ± se, n = 10 for growth rates and n = 5 for extensibility measurements. Other details are as in Table II.
Reversal of the Inhibitory Effect of Vanadate on Water-Induced Recovery of Leaf Growth and Extensibility by Strong pH 4.5 Buffers
It seemed probable that the inhibitory effect of vanadate on water-induced recovery of wall extensibility and leaf growth was associated with its inhibition of renewed cell wall acidification by PM H+-ATPase. The effects of injecting vanadate solution were therefore compared with the effects of injecting vanadate solution strongly buffered to pH 4.5 with 50 mm succinate. The inclusion of the acidic buffer initially reversed the inhibitory effect of vanadate on water-induced recovery of leaf growth (Fig. 2). Similar results were obtained using acetate buffer to minimize possible effects on the activity of Ca2+ in the cell walls (not shown). However, the curvilinear acceleration of leaf growth induced by vanadate plus pH 4.5 buffer was relatively short-lived and had tapered off after about 10 min. It differed from the longer-term and more linear acceleration of growth induced by injection of pH 4.5 buffer without vanadate (compare Fig. 1).
The addition of pH 4.5 buffer also reversed the inhibitory effect of vanadate on the induction of cell wall loosening. Thus, both in vivo and in vitro extensibility were significantly increased 4 min after injection with vanadate plus pH 4.5 buffer, as compared with vanadate alone (Table IV). However, as with leaf growth, the pH-associated reversal of effects of vanadate on recovery of extensibility were relatively short-lived; they were no longer detectable 12 min after injection (not shown). One possible explanation is that a time-dependent passage of buffer and protons from the apoplast into the less-acidic cytoplasm led to a gradual loss of effect of the acidic buffer in the cell wall. At the same time, vanadate would continue to inhibit the reacidification of the cell walls by the proton-pumping ATPases. In all events, the inhibitory effects of vanadate on water-induced wall loosening and leaf-growth stimulation were clearly postponed by buffering vanadate to pH 4.5.
Table IV.
Reversal of early growth-inhibitory effects of vanadate injection by inclusion of pH 4.5 buffer
| Treatment | Growth Rate | Extensibility
|
|
|---|---|---|---|
| in vivo | in vitro | ||
| μm min−1 | μm (2 g 3 min)−1 | ||
| Vanadate | 3 ± 1 | 17 ± 2 | 10 ± 4 |
| Van + pH 4.5 | 8 ± 1 | 24 ± 1 | 22 ± 2 |
Nonbuffered controls contained vanadate alone. Succinate (50 mm) was added to buffer vanadate solution to pH 4.5 (Van + pH 4.5). Assays of growth rate and extensibility were started 1 and 4 min after injection. Results are means ± se; n = 10 for growth rates and n = 5 for extensibility measurements.
DISCUSSION
The results presented here show that imposition of water stress (via PEG addition to the root solution for 1 h) inhibited leaf growth and induced a relative hardening (decrease in extensibility) of expanding leaf cell walls. Similar findings were reported previously (Neumann, 1993; Chazen and Neumann, 1994). However, Hsiao et al. (1998) reported apparently opposite wall responses. They pressurized the roots of control or previously water-stressed maize seedlings for 15 min to calculate volumetric extensibility values for the leaves. Root pressurization increased water availability to the leaves of stressed plants and significantly increased their average elongation rate, as assayed for 15 min after root pressurization. Unstressed plants showed less of a leaf-growth response to root pressurization and therefore gave lower volumetric extensibility values. Thus, volumetric extensibility values appeared to be higher in the water-stressed plants.
An alternative interpretation of these findings that is more consistent with our results is that the comparatively larger increase in growth rate shown by the leaves of stressed plants upon pressurization was associated with pressure-induced increases in water availability, which caused increases in wall extensibility. The relatively higher volumetric extensibility in the leaves of stressed plants would then reflect the rapid leaf responses to pressurization rather than pretreatment values.
Our results showed clearly that leaf injection with water, 0.5 mm sodium phosphate, and 50 mm succinate or Mes buffers at pH 4.5 caused an initial spurt of rapid elongation (approximately 60 s), which is typical of viscoelastic extension. This was followed by a fairly linear period of accelerated leaf growth and, after about 15 min, by a progressive decrease to slower growth rates typical of stressed plants before injection.
Several other reports have shown that the growth of algal cells (Green et al., 1971) or shoot tissues of intact plants (Serpe and Matthews, 1992; Neumann, 1993; Chazen and Neumann, 1994; Passioura, 1994; Hsiao et al., 1998) accelerate rapidly following reversal of water stress. Despite overall similarities, the kinetics and duration of leaf responses induced by the novel water-injection technique can differ from previously reported growth responses to reducing the level of osmoticum in the root medium or pressurization of the root medium. Some of these differences may be due to differences in methodology. For example, the early, 60-s burst of rapid growth we observed may be associated with the fact that our leaf-length data were collected at 1-s intervals and could therefore reveal more detail of initial responses than studies based on collecting average growth-rate data at intervals of several minutes.
Differences in the duration of leaf-growth responses may be partly due to the fact that the 12 μL of injected water represents a finite resource, whereas continuous pressurization or replacement of the root solution can clearly provide a more prolonged increase in water availability. An approximate calculation based on total leaf-volume increase following injection indicated that only a small percentage (approximately 4.5%) of injected water was actually used for accelerating leaf growth. Some of the remaining water may have been taken up by surrounding tissues and lost by evaporation. The slowdown in accelerated leaf growth that occurred about 15 min after leaf injection may have reflected the onset of cellular feedback responses similar to those that have often been observed previously (compare Green et al., 1971; Serpe and Matthews, 1992; Chazen and Neumann, 1994; Passioura, 1994). However, in the present case, feedback effects on growth may have been relatively intensified by inhibitory signals from the roots that remained in an unmodified water-stress environment (Hartung et al., 1988; Wilkinson and Davies, 1997). Finally, species and even cultivar differences may also contribute to differences in patterns of leaf-growth responses to the relief of water stress.
We cannot rule out the possibility that some elastic swelling occurred in the relatively short initial burst of accelerated growth that immediately followed water injection. However, the subsequent quasilinear period of accelerated growth induced by injection of water, 50 mm buffer at pH 4.5, or 0.5 mm phosphate into the expansion zone of water-stressed maize leaves was clearly associated with measured increases in the irreversible components of extensibility, as measured 4 and 12 min after injection. These increases were not associated with significant increases in the measured elastic component of extensibility or with any signs of shrinkage once the growth stimulation ended. These facts, together with results of previous investigations in which the relationships between growth recovery and maize leaf-extensibility traits were also shown to be related to irreversible rather than elastic changes (Neumann, 1993; Chazen and Neumann, 1994; Snir and Neumann, 1997), suggest that the major effect of the injected solutions was to induce changes in irreversible growth processes.
In contrast to the growth-stimulatory effects of water or pH 4.5 buffer solutions, injections of 0.5 mm sodium vanadate (or erythrosin B) and of strong buffer solutions at pH 5.5 strongly inhibited increases in extensibility and the recovery of leaf growth. These findings support the hypothesis that inhibition of wall acidification may play an important role in inhibiting cell expansion growth in maize leaves under water-stress conditions. They also support and extend similar conclusions reached in previous investigations of growth and extensibility responses to longer-term imposition of water stress (Cleland, 1975; Van Volkenburgh and Boyer, 1985).
In addition, the present findings show that the extensibility traits directly measured with the whole-plant creep extensiometer are closely related to leaf-growth potential. For example, when wall acidification was inhibited by vanadate or 50 mm buffer at pH 5.5, wall extensibility remained at the lower level induced by water stress. Thus, despite the increase in water availability to the expanding tissues, growth recovery appeared to be prevented by the low extensibility of the cell walls. Conversely, rapid growth recovery following injections of water, pH-4.5 buffer, or dilute phosphate solution was consistently associated with rapid increases in extensibility.
Several caveats concerning the interpretation of the above findings need to be made. For example, it might be argued that additional Na+ or Cl− ions resulting from the use of NaOH or HCl to adjust succinate and Mes buffers, respectively, to desired pH values were the primary factors affecting wall extensibility and growth; however, there appears to be little evidence to suggest that either Na+ or Cl− ions are of particular importance in directly regulating cell wall extensibility traits (Bonner, 1961). In contrast, protons have long been known to have strong stimulatory effects on wall extensibility and cell expansion (Rayle and Cleland, 1970; Hager et al., 1971). Moreover, our experiments showed that Mes buffer at pH 5.5 inhibited leaf-growth recovery in the same way as succinate buffer at pH 5.5, even though no Na+ ions were used to adjust the pH of the Mes buffer. Similarly, the inhibition of leaf-growth recovery induced by injecting sodium vanadate also appeared to be independent of direct growth-inhibitory effects of Na+ ions, since sodium phosphate at the same concentration promoted growth recovery. Finally, Mes at pH 5.5 inhibited leaf-growth recovery but contained less Cl− than the growth-stimulatory Mes solution adjusted to pH 4.5 by the addition of HCl. Thus, comparatively lower proton activity in the pH-5.5 buffer, not Cl− ions, appeared to be the cause of its growth-inhibitory effect.
Another possibility that we cannot completely rule out is that injection of the buffers at pH 5.5 (but not pH 4.5) and vanadate (but not phosphate) somehow reduced the conductivity of the cells' water-uptake pathway, thereby preventing growth recovery. However, the occurrence of such rapid inhibitory effects of buffers at a specific pH or of vanadate on PM permeability to water does not appear to have been reported in the literature and must remain speculative. Moreover, even if hydraulic conductivity were to be rapidly reduced by both pH 5.5 buffers and vanadate, our finding that inhibition of wall acidification prevents loosening of the expanding cell walls of stressed plants remains valid, as does the apparent association between resupply of water or pH 4.5 buffer, wall loosening, and increases in leaf growth.
The possibility that increasing water availability to stressed plants simply accelerates leaf growth by increasing turgor pressure in the expanding cells also needs to be considered. In this scenario, H+ secretion would be constitutive and would be unaffected by water availability. The rapid (within minutes) wall-hardening and loosening responses to changes in water availability would then result somehow from the changes in growth. However, several reports have shown that the changes in the rate of plant growth associated with moderate water or salinity stress can occur independently of changes in turgor pressure, i.e. changes in growth rate need not necessarily be related to turgor changes (for review, see Neumann, 1995). Moreover, the present findings show clearly that imposition or relief of water stress can rapidly alter wall extensibility and that wall extensibility can be directly involved in the regulation of growth. For example, a large water potential gradient in favor of water uptake and accelerated leaf growth should have been established after the injection of 0.5 mm sodium vanadate solution (with no additional buffer and near zero water potential) into growing leaves of water-stressed plants. The expected acceleration of leaf growth was clearly observed when a control solution of 0.5 mm sodium phosphate was injected. However, leaf growth was not accelerated by the injection of vanadate solution. Cell expansion in this case was presumably inhibited by more than the availability of water.
It seems that vanadate inhibited the acceleration of leaf growth because it prevented an increase in wall acidification and associated wall loosening. Thus, acidification of the vanadate solution to pH 4.5 did facilitate increases in wall extensibility and a partial acceleration of leaf growth. Clearly, therefore, rapid changes in wall extensibility can be involved in regulating early leaf-growth responses to changes in water availability. This is not to say that increases in turgor pressure are not involved. The point is that possible increases in turgor pressure associated with increased water availability may not completely explain the regulation of changes in leaf growth. Changes in wall-extensibility traits and their regulation by rapid changes in cellular metabolism also appear to be involved.
Previous investigations have also suggested the possible involvement of rapid, metabolically regulated changes in wall extensibility in regulating early growth responses to removal of water stress. Such suggestions were based on direct assays of growth rate and turgor pressure and indirect estimations of wall-extensibility parameters (Green et al., 1971; Serpe and Matthews, 1992). For example, Serpe and Matthews (1992) showed that leaf growth in water-stressed begonia plants remained higher than in well-watered plants for about 45 min after rewatering. Moreover, leaf-growth rates were 3 times higher for the first 15 min after rewatering, even though epidermal turgor pressures had not yet attained prestress levels. These findings suggested the possibility that, as in maize, the early phase of accelerated growth was partially mediated by changes (i.e. increases) in wall-yielding properties. Such increases may underlie the “stored-growth” phenomenon. Subsequent onset of feedback inhibition of growth, while turgor was maintained, indicated that compensatory decreases in wall-yielding properties (through metabolic activity or strain-hardening effects) can occur at a later stage.
Nonami and coworkers (Nonami and Boyer, 1990; Nonami et al., 1997) concluded that a primary cause of ongoing growth inhibition in expanding soybean stems following the lowering of external water potentials is a decrease in the water-potential gradient that allows water uptake and cell volume increases. We suggest that, in expanding maize leaves, changing water potential gradients may also be involved in signaling the initiation of rapid metabolic responses. These then regulate appropriate changes in wall extensibility. The precise transduction mechanisms remain to be determined. However, in the case of water injection into the leaf-elongation zone of water-stressed maize plants, resultant increases in proton pumping would presumably be dependent on rapidly induced increases in the catalytic activity of preexisting PM H+-ATPase (rather than slower de novo synthesis). These increases could rapidly lead to a lowering of cell wall pH to less than pH 5.5, thus facilitating increases in wall extensibility, water uptake, and leaf expansion. The increases in wall extensibility may involve direct pH effects on bonding between load-bearing polymers or pH-associated activation of various cell wall proteins with similar effects (Cosgrove, 1997). Slower onset of hormonal and gene-regulated increases in the amount of PM H+-ATPase (Frias et al., 1996) could be involved in longer-term responses to increased water availability.
Of course, additional factors such as (a) changes in the activity of Ca2+ or other regulatory compounds at the cell wall-membrane interface (Neumann, 1971; Takahashi et al., 1997), (b) changes in rates of transmembrane electron transport (Ding and Schopfer, 1997), or (c) passive changes in levels of wall hydration (Money and Harold, 1992; Passioura, 1994; Edelman, 1995) may also participate in regulating the molecular interactions underlying rapid changes in the extensibility of expanding cell walls. However, the data in this report are primarily consistent with a large body of previous evidence indicating a central role for wall acidification in the regulation of cell wall extensibility and growth (Rayle and Cleland, 1992; Kutschera, 1994; Frias et al., 1996; Cosgrove, 1997).
In conclusion, our results strongly support the possibility that water stress inactivates PM H+-ATPase in expanding maize leaf cells, thus hardening the cell walls and contributing to the inhibition of leaf growth; resupply of water reverses these processes. Future demonstration of associations between water stress (or its relief) and directly measured changes in wall pH or PM H+-ATPase activity could strengthen this conclusion. In any case, the hypothesis that changes in the activity of a specific enzyme (PM H+-ATPase) may be closely involved in regulating leaf-growth responses to imposition or removal of water stress is an attractive one that deserves further investigation.
Abbreviations:
- LVDT
linear variable displacement transducer
- PM
plasma membrane
Footnotes
This work was supported in part by the fund for the promotion of research at Technion.
LITERATURE CITED
- Amodeo G, Srivastava A, Zeiger E. Vanadate inhibits blue light stimulated swelling of Vicia guard cell protoplasts. Plant Physiol. 1992;100:1567–1570. doi: 10.1104/pp.100.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. Plant Breeding for Stress Environments. Boca Raton, FL: CRC Press; 1988. [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7:1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J (1961) On the Mechanics of Auxin Induced Growth. Plant Growth Regulation. Iowa State University Press, Ames, pp 307–328
- Boyer J. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Chazen O, Neumann PM. Hydraulic signals from the roots and rapid cell wall hardening in growing maize (Zea mays L.) leaves are primary responses to polyethylene glycol-induced water deficits. Plant Physiol. 1994;104:1385–1392. doi: 10.1104/pp.104.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE. Auxin induced hydrogen ion excretion: correlation with growth and control by external pH and water stress. Planta. 1975;127:233–242. doi: 10.1007/BF00380720. [DOI] [PubMed] [Google Scholar]
- Close T, Bray E (1993) Plant Responses to Cellular Dehydration during Environmental Stress. Current Topics in Plant Physiology, Vol 10. American Society of Plant Physiologists, Rockville, MD
- Cosgrove DJ. Relaxation in a high stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell. 1997;9:1031–1041. doi: 10.1105/tpc.9.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan DSC, Clarkson DT, Hall JL. A comparison between the ATPase and proton pumping activities of plasma membrane isolated from the stele and cortex of Zea mays roots. J Exp Bot. 1993;44:983–989. [Google Scholar]
- Cramer GR. Kinetics of maize leaf elongation. 2. Response of a Na-excluding cultivar and a Na-including cultivar to varying Na/Ca salinities. J Exp Bot. 1992;43:857–864. [Google Scholar]
- Ding B, Schopfer P. Metabolic involvement in acid mediated extension growth of maize coleoptiles. J Exp Bot. 1997;48:721–728. [Google Scholar]
- Edelman HG. Water potential modulates extensibility of rye coleoptile cell walls. Bot Acta. 1995;105:374–380. [Google Scholar]
- Evlagon D, Ravina I, Neumann PM. Interactive effects of salinity and calcium on osmotic adjustment, hydraulic conductivity and growth in primary roots of maize seedlings. Isr J Bot. 1990;39:239–247. [Google Scholar]
- Frias I, Caldeira MT, Perez-Castineira JR, Navarro-Avinio JP, Culianez-Macia FA, Kuppinger O, Stransky H, Pages M, Hager A, Serrano R. A major isoform of the maize plasma membrane H+-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell. 1996;8:1533–1544. doi: 10.1105/tpc.8.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PB, Erickson RO, Buggy J. Plant Physiol. 1971;47:423–430. doi: 10.1104/pp.47.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Menzel H, Krauss A. Versuche und hypothese zur primarwirkung des auxins beim shtrekungswachstum. Planta. 1971;100:47–75. doi: 10.1007/BF00386886. [DOI] [PubMed] [Google Scholar]
- Hartung W, Radin JW, Hendrix DL. Abscisic acid movement into the apoplastic solution of water stressed cotton leaves. Role of apoplastic pH. Plant Physiol. 1988;86:908–913. doi: 10.1104/pp.86.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TC. Plant responses to water stress. Annu Rev Plant Physiol. 1973;24:519–570. [Google Scholar]
- Hsiao TC, Frensch J, Rojas Lara BA. The pressure jump technique shows maize leaf growth to be enhanced by increases in turgor only when water status is not too high. Plant Cell Environ. 1998;21:33–42. [Google Scholar]
- Jacobs M, Taiz L. secretion and elongation in pea epicotyls and oat coleoptiles Proc Natl Acad Sci USA. 1980;77:7242–7246. doi: 10.1073/pnas.77.12.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U. Tansley Review 66. The current status of the acid-growth hypothesis. New Phytol. 1994;126:549–569. [Google Scholar]
- Money NP, Harold FM. Extension growth of the water mold Achlya: interplay of turgor and wall strength. Proc Natl Acad Sci USA. 1992;89:4245–4249. doi: 10.1073/pnas.89.10.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann PM. Possible involvement of a glycerophosphate compound in auxin-induced growth. Planta. 1971;99:56–62. doi: 10.1007/BF00392120. [DOI] [PubMed] [Google Scholar]
- Neumann PM. Rapid and reversible modifications of extension capacity of cell walls in elongating maize leaf tissues responding to root addition and removal of NaCl. Plant Cell Environ. 1993;16:1107–1114. [Google Scholar]
- Neumann PM. The role of cell wall adjustment in plant resistance to water deficits. Crop Sci. 1995;35:1258–1266. [Google Scholar]
- Neumann PM. Salinity resistance and plant growth revisited. Plant Cell Environ. 1997;20:1193–1198. [Google Scholar]
- Nonami H, Boyer JS. Wall extensibility and cell hydraulic conductivity decrease in enlarging stem tissues at low water potentials. Plant Physiol. 1990;93:1610–1619. doi: 10.1104/pp.93.4.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonami H, Wu Y, Boyer JS. Decreased growth-induced water potential. A primary cause of growth inhibition at low water potentials. Plant Physiol. 1997;114:501–509. doi: 10.1104/pp.114.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura J. The physical chemistry of the primary cell wall: implications for the control of expansion rate. J Exp Bot. 1994;45:1675–1682. [Google Scholar]
- Rayle DL, Cleland RE. Enhancement of wall loosening and elongation by acid solution. Plant Physiol. 1970;46:250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HR, Matthews MA. Growth, osmotic adjustment, and cell wall mechanics of expanding grape leaves during water deficits. Crop Sci. 1993;33:287–294. [Google Scholar]
- Serpe MD, Matthews MA. Rapid changes in cell wall yielding of elongating Begonia argenteo-guttata L. leaves in response to changes in plant water status. Plant Physiol. 1992;100:1852–1857. doi: 10.1104/pp.100.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe MD, Matthews MA. Growth, pressure, and wall stress in epidermal cells of Begonia argenteo-guttata L. leaves during development. Int J Plant Sci. 1994;155:291–301. [Google Scholar]
- Silk WK. Steady form from changing cells (supplement) Int J Plant Sci. 1992;153:S49–S58. [Google Scholar]
- Snir N, Neumann PM. Mineral nutrient supply, cell wall adjustment and the control of leaf growth. Plant Cell Environ. 1997;20:239–246. [Google Scholar]
- Takahashi K, Isobe M, Knight MR, Trewavas AJ, Muto S. Hypoosmotic shock induces increases in cytosolic Ca2+ in tobacco suspension culture cells. Plant Physiol. 1997;113:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E, Boyer JS. Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol. 1985;77:190–194. doi: 10.1104/pp.77.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol. 1997;113:559–573. doi: 10.1104/pp.113.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]