Abstract
Obesity is known to be a major risk factor of a whole range of cardiovascular, metabolic and respiratory disorders. The pattern of regional fat distribution plays an important role in the pre-disposition of obese subjects to respiratory complications. Obesity is responsible for important changes in respiratory function both during spontaneous breathing as well as during general anesthesia and mechanical ventilation. The most characteristic abnormalities consist of decreased functional residual capacity, reduced expiratory reserve volume, decreased compliance and increased resistance of the respiratory system. Breathing at low lung volume promotes airway closure in the dependent lung zones with consequent gas exchange abnormalities even though lung carbon monoxide-diffusing capacity is normal or increased. Weight loss can reduce many of the alterations in pulmonary function related to obesity.
Keywords: lung volumes, compliance, oxygen consumption, obesity, airway resistance, diffusion capacity, ventilation, perfusion, oxygenation, pulmonary physiology
Introduction
For several decades, the global prevalence of obesity has been rising dramatically [1, 2]. The greatest increase has been noted in the United States. Compared with some European countries, the prevalence of obesity in the United States is three times higher than in France, and one and a half times higher than in the United Kingdom [3]. Between 1980 and 2004, the prevalence of obesity in the US more than doubled in adults and more than tripled in children. The greatest relative increase has been in the proportion of individuals with a body mass index (BMI) greater than 50 kg/m2. This review describes the mechanisms whereby obesity brings about the functional abnormalities on resting and exercise related respiratory physiology.
Lung mechanics
Obesity decreases total respiratory compliance by as much as two-thirds of the normal value measured in non-obese individuals [4]. The decrease in compliance was thought to result primarily from a reduced chest wall compliance associated with the deposition of fat in and around the ribs, the diaphragm and the abdomen. Subsequent investigations in healthy obese subjects revealed higher total respiratory system and chest wall elastance during voluntary muscle relaxation than during paralysis [5], suggestingthat incomplete relaxation may have contributed to lower chest wall compliance reported in earlier studies. Actually, the chest wall compliance is usually normal in obese subjects and the decrease in total respiratory compliance is that of the lung. The reduction in lung compliance in obese individuals is exponentially related to BMI [6]. This decrement is the result of increased pulmonary blood volume, closure of dependent airways [10], and increased alveolar surface tension due to the reduction in functional residual capacity (FRC) [7,8,9].
Lung volumes and spirometry
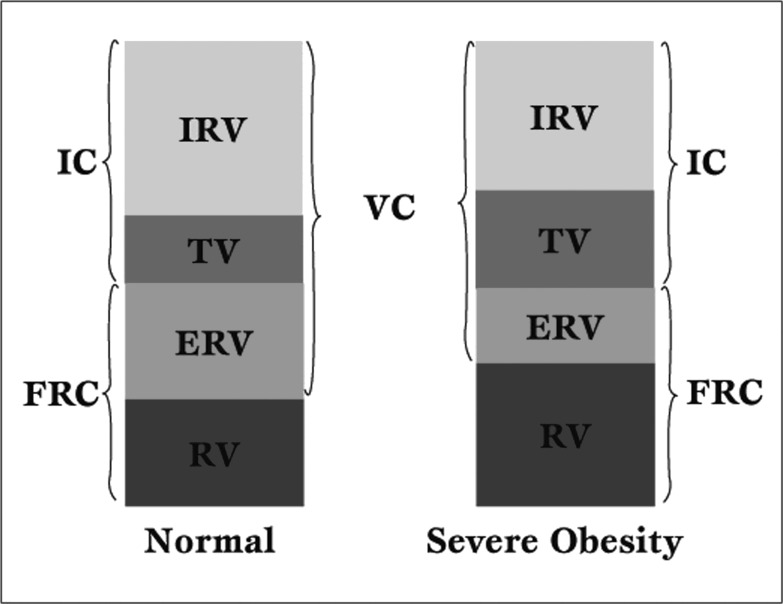
The most common and consistent characteristic of obesity on lung function is a reduction in FRC (Figure 1).
Figure 1.
Impact of obesity on lung volumes. IC = Inspiratory capacity, IRV = Inspiratory reserve volume, TV = Tidal volume, ERV = Expiratory reserve volume, RV = Residual volume, FRC = Functional residual capacity, VC = Vital capacity.
This derangement reflects the mass load of adipose tissue around the rib cage and abdomen [11].
In contrast, residual volume (RV) is usually well preserved, and the RV-to-total lung capacity (TLC) ratio remains normal or slightly increased [12]. As a result, ERV decreases exponentially with increasing BMI, even in mild obesity or overweight due to displacement of the diaphragm into the thorax and increased chest wall mass. ERV reduction is greatest in the supine position. The reduction is often so marked that FRC approaches RV. At that point, regional thoracic gas trapping may take place causing an elevated RV/TLC ratio [13].
Total lung capacity (TLC) and vital capacity (VC) decrease linearly with a rising body mass index, however, the changes are small, and TLC is usually maintained above the lower limit of normal. A marked abnormality of lung volumes in mild to moderate obesity should raise suspicion of an underlying intrinsic lung disease or neuromuscular pathology except in those with morbid obesity or those with excessive central adiposity (waist-to-hip ratio ≥ 0.95) [14].
Spirometry is normal in mild obesity. As BMI increases, there is a reduction in expiratory flow and a decrease in FEV1 and FVC [15]. The ratio of FEV1 to FVC is preserved and even increased, which is attributed to peripheral airway closure and gas trapping, hence reducing the VC. However, the reduction in FEV1 and FVC is strongly correlated with abdominal obesity. FVC, FEV1, and TLC were found to be significantly lower in subjects with upper body fat distribution or central obesity [16].
Abdominal obesity is responsible for a reduced FEV1/FVC ratio suggesting an effect of obesity on large airway caliber as well. In addition to these spirometric derangements, tidal volume is reduced in severe obesity, and breathing follows a rapid, shallow pattern [17]. This functional change is typically due to the elastic load which can be replicated in normal weight subjects with elastic strapping of the chest [18]. As FRC becomes less than the closing volume, airway closure occurs during tidal breathing. Together with alveolar collapse, this leads to decreased ventilation of the lung bases, ventilation-perfusion mismatch, and hypoxemia. For these reasons, both the PaO2 and the alveolar-arterial gradient are related to FRC. The improvement of lung function with weight loss supports the causative effects of obesity on respiratory physiology. Following bariatric surgery, restrictive pulmonary mechanics improves significantly with corresponding increase in FEV1, FVC, and in FEV25-75%.
Additionally, the obstructive lung pattern (FEV1/FVC ratio less than 0.8) tends to normalize [19]. In one program, weight loss was accompanied by an improvement of 73 ml in FEV1 and 92 ml in FVC for every 10% relative loss of pretreatment weight [20].
The effect of obesity on lung volumes and chest compliance can be worsened by anesthesia and muscular paralysis, which is manifested by decreased lung volumes, and higher lung and respiratory system elastance. This deterioration in lung function is more pronounced with abdominal surgeries [21], but it also seen in other types of non abdominal procedures. Although the post-operative spirometry of obese patients shows a decrease in FEV1, FVC and PEF, the reduction in vital capacity is the most prominent in both abdominal and non-abdominal surgery, and is greater with increasing BMI.
Various methods have been tried to compensate for the effects of obesity on lung function, and they range from positional changes, to adopting altered intra-operative ventilation strategies, to the use of prophylactic BiPAP post operatively [22].
The reverse Trendelenburg (RT) position is one measure that seems to improve the respiratory compliance and gas exchange in morbidly obese patients during bariatric surgery.
However, it is not clear yet if the beneficial effect of this position can be replicated to all abdominal and non-abdominal surgeries in obese patients. Similarly, in intubated patients with large abdomen (obesity, distended abdomen or large ascites), a 45° RT position is associated with larger tidal volume and lower respiratory rate compared with 90° position. These results suggest that the reverse Trendelenburg could be the optimal position to be used in obese patients, particularly in intubated ICU patients, or those undergoing or recovering from anesthesia and surgery [21,22,23].
In addition to body positioning, different ventilation strategies are used to improve respiratory function in obese patients.
Increasing PEEP to 10 cm H2O in anesthetized or paralyzed patients significantly reduces elastance of the respiratory system, lung, and chest wall, and improves oxygenation.
In one study that used computed tomography to assess atelectasis in morbidly obese anesthetized patients undergoing gastroplasty, recruitment maneuver with 55 cm H2O inspiratory pressure for 10 seconds, followed by 10 cm of PEEP reduced atelectasis and improved oxygenation, while recruiting maneuver alone without PEEP yielded only a transient reduction of atelectasis that was not sustained 20 minutes later. In addition, 10 cm of PEEP alone did not affect atelectasis. Repeating the recruitment maneuver every 10 minutes, in addition to PEEP of 10 cm H2, had better results in terms of improving respiratory compliance and oxygenation.
Whether such ventilation strategies can be applied to medical obese patients in the intensive care unit is to be determined [24,25,26].
The use of BiPAP as a prophylactic measure to improve pulmonary function after surgery has been studied in obese patients after undergoing gastroplasty. The prophylactic use of BiPAP System 12/4 (but not 8/4), during the first 24 hours postoperatively reduces pulmonary dysfunction after gastroplasty, and accelerates reestablishment of preoperative pulmonary function, which is reflected in improved FVC and FEV1, as well as SpO2. The BiPAP acts through enhancing the alveolar recruitment during inspiration, while preventing the expiratory alveolar collapse, and thus reducing the postoperative restrictive syndrome [27].
Respiratory muscles/work of breathing
Studies on the respiratory muscles of obese individuals are scarce. Overall, obese subjects demonstrate inefficiency of respiratory muscles, most notably the diaphragm. The maximum inspiratory and expiratory pressures at all lung volumes are lower in obese patients compared to controls, without reaching statistical significance however, except in patients with obesity hypoventilation syndrome (OHS).
The maximal voluntary ventilation, a measurement of respiratory muscle endurance, is reduced by 20% in healthy obese individuals and by 45% in patients with OHS [28]. It is suggested that the additional load causes a length-tension disadvantage for the diaphragm due to fiber overstretching placing the diaphragmatic fibers at suboptimal length.
Furthermore, analysis of the diaphragmatic electromyogram revealeda persistence of activity into early expiration, the length of which alsodepended on the degree of obesity. These findings indicate that thediaphragm’s volume-generating function in the obese is reduced, andfurthermore the persistence of its activity in expiration serves toattenuate the rate of expiratory flow [28,29,30].
On a cellular level, obesity with high intake-associated lipid accumulation in muscle interferes with cellular mitochondrial function through the generation of reactive oxygen species [31].
These compounds lead to lipid membrane peroxidative injury and disruption of mitochondrial-dependent enzymes resulting in decrease oxidative metabolism. A reduced ability to oxidize fatty acids has also been reported in skeletal muscle of obese individuals both before and after weight loss, which would support an intrinsic abnormality of fatty acid oxidation [32]. After weight loss, there is a significant increase and return to normal reference values, with regard to both the strength and endurance of respiratory muscles, with the latter showing greater increases. This improvement in respiratory muscle endurance is related to increased chest wall compliance and pulmonary volumes, as a consequence of weight reduction [33].
Airways resistance
Obese subjects have an increased total respiratory resistance due to a predominantly increased airway resistance rather than chest wall resistance. However, when airway resistance is adjusted for the lung volume at which the measurements are made, specific airway resistance is in the normal range indicating that the apparent reduction in airway caliber in the obese is attributable to the reduction in lung volumes rather than to airway obstruction [34]. However, recent investigations have suggested that the increase in resistant may not be entirely due to reduction of FRC since differences between obese and non-obese may persist after lung volume adjustment [35,36]. The mechanism by which obesity could cause increased airway resistance is not well understood. The possible hypotheses include increased atopic reaction related to an enhanced inflammatory state secondary to obesity [38]. Some in vitro studies, as well as human studies suggest that lower lung volumes secondary to obesity, lead to a reduction in peripheralairway diameter, which over time causes smoothmuscle dysfunction, and causes both airways obstruction and hyper-responsiveness. In addition, leptin has been suggested to be involved in the airway dysfunction associated with obesity, through its pro-inflammatory properties and/or via a direct effect on airways smooth muscles [37]. However, the data addressing this question have been inconclusive so far and further studies are needed to understand the mechanism of increased airway resistance and responsiveness in obesity [38,39,40,41,42,43,44,45].
The effect of obesity on airway hyper-responsiveness (AHR) has been inconsistently demonstrated. Investigators simulated obesity-related lung volume reductions in non-asthmatic subjects by externally mass loading the chest wall and abdomen and documented an augmentation of airway responsiveness to metacholine relative to that of control [46]. The relation between BMI and AHR has also been reported in The European Community Respiratory Health Survey [47]. However, the association between asthma and obesity in adults and children so far has failed to show a consistent increase in AHR [38]. In addition, weight loss programs did not result in substantial change in AHR despite documented improvements in lung function. Therefore, there is a plausible mechanism to explain how obesity is implicated in AHR but is not consistently reproducible in clinical studies.
Control of breathing
Although some studies investigating ventilatory drive in simple obesity have demonstrated that the ventilatory responses to inhalation of carbon dioxide (DVE/DPCO2) are normal, others have indicated a reduced response particularly in patients with obesity hypoventilation syndrome (OHS)[48,49]. These abnormalities were initially attributed to the mechanical limitations and decreased chest compliance preventing adequate ventilation. However the anticipated response to CO2 did not improve in OHS patients following weight loss. Further, Vd/Vt did not correlate with subjects’ resting PCO2 [50].One theory proclaims that the diminished responsiveness may represent an adaptive process sparing O2 for non-ventilatory demands. Yet there is an inherent problem with using ventilatory responses as a marker of respiratory drive because minute ventilation response to a stimulus may also be influenced by respiratory muscle function and respiratory system mechanics. The mouth occlusion pressure (P0.1) believed to reflect neurogenic drive is twice the normal value in mild obesity and increases normally with CO2 inhalation. In contrast, the P0.1 response to CO2 in patients with OHS is half that of subjects with simple obesity [49].
The fact that OHS subjects can normalize their PaCO2 by hyperventilation provides supportive evidence that ventilatory control is abnormal in OHS [51]. Hence, the cumulative data indicates that subjects with simple obesity have an enhanced respiratory drive while the respiratory drive of subjects with OHS is either depressed or inappropriately suppressed.
Oxygen cost of breathing
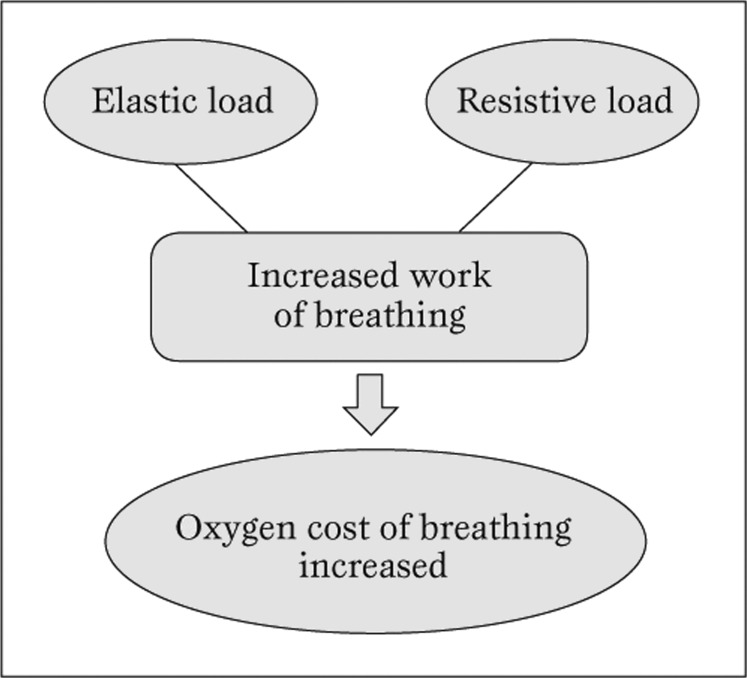
In non-obese individuals, the percentage of cardiac output and total body oxygen consumption (VO2) dedicated to respiratory muscle work during quiet breathingis very small (less than 3%). In contrast, the oxygen cost of breathing is 4 to 10 fold higher than normal among subjects with eucapneic obesity (Figure 2).
Figure 2.
Interplay of respiratory mechanics on oxygen consumption in obese patients.
In one study of obese patients undergoing bariatric surgery, a 16% reduction in mean VO2 in obese patients was observed compared to less than 1% reduction in the non-obese during the transitionfrom spontaneous breathing to positive pressure ventilation. This suggests that morbidly obese patients dedicate a disproportionate high percentage of total VO2 for respiratory work.
Of interest, the obese patients demonstrate a significantly lower VO2 when standardized by BMI that has been attributed to the lower blood flow andmetabolic rate of adipose tissue compared with lean body tissue. Nevertheless, the lower VO2 standardized to body size does not ameliorate the detrimental impact of morbid obesity on oxygen consumption.
This respiratory inefficiency results in alimited ventilatory reserve that predisposes these patients to respiratory failure in the setting of acute pulmonary or systemicillnesses [52,53].
Ventilation/Perfusion (V/Q)
Ventilation in non-obese patients is greatest in dependent lung zones and decreases toward the upper zones; however, this distribution may be reversed in obesity. When lungs ventilation and perfusion were examined in obese subjects with ERV at 21% of predicted, the normal tidal breath predominantly distributed to the upper zones, while perfusion was predominant in the lower lung zones.
In contrast, subjects who had an average ERV of 49% of predicted value had normal ventilation distribution [54]. Thus, impairment of the V/Q relationships depends on the location of the excess body weight. Individuals with central obesity seem the most affected. Similar results were reproduced in lateral decubitus position (55). This ventilation/perfusion mismatch results from airway closure in the lungs’ dependent areas of obese patients.
Diffusing capacity and gas exchange
The diffusing capacity of obese subjects is usually preserved although studies have reported increased and decreased values [56]. An increased DLCO in obese patients is probably related to increased pulmonary blood volume and flow while a decreased DLCO may result from structural changes in the interstitium from lipid deposition or decreased alveolar surface area. In either scenario, weight loss appears to have little effect on diffusing capacity as DLCO values remained unchanged following surgical or medical treatment [57,58].
Morbid obesity is associated with low arterial pressure of oxygen (PaO2) and increased alveolar-to-arterial oxygen partial pressure difference [58]. These changes are usually more prominent in men than in women secondary to gender differences in waist-to-hip ratio. Arterial pressure of carbon dioxide (PaCO2) is usually normal in obese patients who do not have obesity hypoventilation syndrome. While the gas exchanges improve with peak exercise, obese subjects have a poor compensatory hyperventilation, resulting in low exercise tolerance and premature termination of exercise [59].
The mechanism by which obesity impairs blood gas exchange and oxygenation is related to lower lung volumes and basilar atelectasis secondary to airway closure and alveolar collapse. Increased airway resistance has little role in impaired gas exchange at rest (normal PaCO2); it may however play a role in the poor exercise tolerance through increased expiratory airflow limitation and dynamic hyperinflation.
Therapeutic measures that are used to improve lung volumes and decrease atelectasis are also associated with improvement of oxygenation and blood gas exchange. In fact, both PaO2 and alveolar-arterial oxygen difference were ameliorated when PEEP of 10 cm H2O was applied to paralyzed and anesthetized postoperative obese patients after abdominal surgery, compared to PEEP of zero. Similarly, the alveolar-arterial oxygen difference was significantly reduced when morbidly obese patients undergoing bariatric surgery were placed in reverse Trendelenburg position. In addition, the application of BiPAP 12/4 improved oxygen saturation when used prophylactically in obese patients for 24 hours after undergoing gastroplasty [56,60].
Altered exercise respiratory physiology
At rest, the baseline VO2 is approximately 25% greater than the VO2 for non-obese individuals. Because adipose tissue has a lower metabolic rate than other tissues, peak VO2 uptake adjusted for true body weight is reduced, however peak VO2 is usually normal or increased when adjusted for ideal body weight [61]. Interestingly, the slope of the VO2 work-rate relationship is unchanged but it is shifted upward by approximately 6 ml/min/kg of extra body weight for a cycle ergometer. This means that an appropriate peak VO2 standard reference for an obese subject can be predicted by increasing the standard peak VO2 from the reference body weight by 6 ml/min for each kilogram greater than the reference weight. Other responses may vary depending on the exercise protocol and severity of obesity. Parameters including peak O2 pulse (VO2/heart rate (HR)), and anaerobic threshold are usually normal in mild to moderate obesity [62].
The resting HR is usually elevated, reflecting an increase in cardiac output at rest. With exercise, there is a normal HR-VO2 relationship reflected by a normal HR-VO2 slope and attainment of the predicted HR with no HR reserve.
It is unusual for obese individuals to demonstrate ventilatory limitation despite the abnormalities imposed on the respiratory system at rest. Because ventilation perfusion relationship normalizes during exercise, dead space ventilation usually responds normally with a decrease toward the normal range with exercise [63].
Pulmonary vasculature
Pulmonary artery systolic pressure (PASP) correlates echocardiographically with BMI independently of age, gender, or comorbid diseases. Using echocardiography, PASP ≥ 30 mmHg and ≥ 35 mmHg occurred in up to 66% and 36% of obese subjects, respectively. For each unit increase in BMI, the PASP increases by 0.1 to 0.4 mm Hg.
The exact mechanism of increased PASP in obesity is not clear, likely related however to increased blood volume. Obstructive sleep apnea and pulmonary capillary spasm secondary to nocturnal hypoxia are other possible contributing factors. However in the absence of right heart catheterization, echocardiographic findings should be interpreted with caution [64].
Conclusions
Obesity affects respiratory physiology in many ways, with significant clinical implications. There are few measures that are shown to improve respiratory function in obese patients undergoing medical or surgical treatment (Table 1).
Table 1.
Patients characteristics and perioperative data
They include reverse Trendelenburg position, higher PEEP with recruitment maneuvers or pressure control mode in ventilated patients, and prophylactic use of BiPAP after surgery/sedation. Increasing positive end expiratory pressure (PEEP) to 10 cm H2O significantly reduced elastance of the respiratory system, lung, and chest wall in obese patients.
Additionally,application bi-level positive airway pressure of (BiPAP) 12/4 improves oxygen saturation in obese patients for 24 hours after surgery.
Footnotes
Source of Support Authors do not have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stockonership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Conflict of interest None declared.
Cite as: Porhomayon J, Papadakos P, Singh A, Nader ND. Alteration in respiratory physiology in obesity for anesthesia- critical care physician. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2011; 3(2): 109-118
References
- Mokdad A H, Ford E S, Bowman B A. et al. Prevalence of obesity, diabetes, and obesity related health risk factors. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Ogden C, Yanovski S, Carroll M. et al. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Sturm R. Increases in morbid obesity in the USA 2000-2005. Public Health. 2007;121:492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimark A, Cherniack R M. Compliance of the respiratory system and its components in health and obesity. J Appl Phsyiol. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- Van Lith P, Johnson F N, Johnson J T. Respiratory elastance in relaxed and paralyzed states in normal and abnormal men. J Appl Physiol. 1967;23:472–486. doi: 10.1152/jappl.1967.23.4.475. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Croci M, Ravagnan I. et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. AnesthAnalg. 1998;87:654–660. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Croci M, Ravagnan I. et al. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–151. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- Hedenstierna G, Santesson J. Breathing mechanics, dead space and gas exchange in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976;20:248–254. doi: 10.1111/j.1399-6576.1976.tb05036.x. [DOI] [PubMed] [Google Scholar]
- Suratt P M, Wilhoit S C, Hsiao H S. et al. Compliance of chest wall in obese subjects. J Appl Physiol. 1984;57:403–407. doi: 10.1152/jappl.1984.57.2.403. [DOI] [PubMed] [Google Scholar]
- Effects of obesity on research function. Am Rev Respir Dis. 1983;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- Sharp J T, Henry J P, Swaeny S K. et al. Effects of mass loading the respiratory system in man. J Appl Physiol. 1964;19:959–966. doi: 10.1152/jappl.1964.19.5.959. [DOI] [PubMed] [Google Scholar]
- Watson R A, Pride N B. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005;98:512–517. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- Jones R L, Nzekwu M. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Sparrow D, Weiss S. Effects of obesity and fat distribution on ventilator function: the normative aging study. Chest. 1997;111:891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Zamel N, DuBarry L. et al. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med. 1990;112:828–832. doi: 10.7326/0003-4819-112-11-828. [DOI] [PubMed] [Google Scholar]
- Leone N, Courbon D, Thomas F. et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J RespirCrit care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- Sampson M G, Grassino A E. Load compensation in obese patients during quiet tidal breathing. J ApplPhysiol. 1983;55:1269–1276. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- Caro C G, Butler J, DuBois A B. Some effects of restriction of chest cage expansion on pulmonary function man: an experimental study. J ClinInvest. 1960;39:573–583. doi: 10.1172/JCI104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Hinojosa M, Smith B. et al. Improvement of restrictive and obstructive pulmonary mechanics following laparoscopic bariatric surgery. SurgEndosc. 2009;23:808–812. doi: 10.1007/s00464-008-0084-9. [DOI] [PubMed] [Google Scholar]
- Aaron S D, Fergusson D, Dent R. et al. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125:2046–2052. doi: 10.1378/chest.125.6.2046. [DOI] [PubMed] [Google Scholar]
- vonUngern-Sternberg B S, Regli A, Schneider M. et al. Effect of obesity and site of surgery on perioperative lung volumes. Brit J Anaesth. 2004;92:202–207. doi: 10.1093/bja/aeh046. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Ravagnan I, Giurati G. et al. Positive end-expiratory pressure improves respiratory function in obese but not in normal subjects during anesthesia and paralysis. Anesthesiology. 1999;91:1221–1231. doi: 10.1097/00000542-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Perilli V, Sollazzi L, Bozza P. et al. The effects of the reverse trendelenburg position on respiratory mechanics and blood gases in morbidly obese patients during bariatric surgery. AnesthAnalg. 2000;91:1520–1525. doi: 10.1097/00000539-200012000-00041. [DOI] [PubMed] [Google Scholar]
- Burns S M, Egloff M, Ryan B. et al. Effect of body position on spontaneous respiratory rate and tidal volume in patients with obesity, abdominal distension and ascites. Am J Crit Care. 1994;3:102–106. [PubMed] [Google Scholar]
- Reinius H, Jonsson L, Gustafson S. et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis. Anesthesiology. 2009;111:979–987. doi: 10.1097/ALN.0b013e3181b87edb. [DOI] [PubMed] [Google Scholar]
- Almarakbi W A, Fawzi H M, Alhashemi J A. Effects of four intraoperative ventilatory strategies on respiratory compliance and gas exchange during laparoscopic gastric banding in obese patients. Br J Anaesth. 2009;102:862–868. doi: 10.1093/bja/aep084. [DOI] [PubMed] [Google Scholar]
- Joris J L, Sottiaux T M, Chiche J D. et al. Effect of Bi-level positive airway pressure (BiPAP) nasal ventilation on the postoperative pulmonary restrictive syndrome in obese patients undergoing gastroplasty. Chest. 1997;111:665–670. doi: 10.1378/chest.111.3.665. [DOI] [PubMed] [Google Scholar]
- Magnami C, Cataneo A. Respiratory muscle strength in obese individuals and influence of upper body fat distribution. Sao Paulo Med. 2007;125:215–219. doi: 10.1590/S1516-31802007000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T M, Jensen R L, Elliott C G, Crapo R O. Maximum respiratory pressures in morbidly obese subjects. Respiration. 1988;54:73–77. doi: 10.1159/000195504. [DOI] [PubMed] [Google Scholar]
- Koenig S M. Pulmonary complications of obesity. Am J Med. 2001;321:249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Petersen K F, Dufour S, Befroy D. et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D, Goodpaster B, Wing R. et al. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity and weight loss. Am J Physiol. 1999;277:1130–1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Weiner P, Waizman J, Weiner M. et al. Influence of excessive weight loss after gastroplasty for morbid obesity on respiratory muscle performance. Thorax. 1998;53:39–42. doi: 10.1136/thx.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerah F, Harf A, Perlemuter L. et al. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–1476. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- King C, Brown N, Diba C. et al. The effects of body weight on airway caliber. Eur Respir J. 2005;25:896–901. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- Watson R, Pride N. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J ApplPhysiol. 2005;98:512–517. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- Gunst S J, Tang D D, Opazo Saez A. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol. 2003;137:151–168. doi: 10.1016/s1569-9048(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Schachter L, Salome C, Peat J. et al. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56:4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi H, Shoseyov D, Feigenbaum D. et al. The relationship between asthma and obesity in children: is it real or a case of over diagnosis? J Asthma. 2004;41:403–410. doi: 10.1081/jas-120026097. [DOI] [PubMed] [Google Scholar]
- Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118:1315–1321. doi: 10.1378/chest.118.5.1315. [DOI] [PubMed] [Google Scholar]
- Maniscalco M, Zedda A, Faraone S. et al. Weight loss and asthma control in severely obese asthmatic females. Respir Med. 2008;102:102–108. doi: 10.1016/j.rmed.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Huang S L, Shiao G, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy. 1999;29:323–329. doi: 10.1046/j.1365-2222.1999.00455.x. [DOI] [PubMed] [Google Scholar]
- Shore S A, Schwartzman I N, Mellema M S. et al. Effect of leptin on allergic airway responses in mice. J Allergy ClinImmunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Shore S A. Obesity and asthma: possible mechanisms. J Allergy ClinImmunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Nair P, Radford K, Fanat A. et al. The effects of leptin on airway smooth muscle responses. Am J Respir Cell Mol Biol. 2008;39:475–481. doi: 10.1165/rcmb.2007-0091OC. [DOI] [PubMed] [Google Scholar]
- Wang L, Cerny F, Kufel T. et al. Simulated obesity-related changes in lung volume increases airway responsiveness in lean, nonasthmatic subjects. Chest. 2006;130:834–840. doi: 10.1378/chest.130.3.834. [DOI] [PubMed] [Google Scholar]
- Chin S, Jarvis D, Burney P. Relation of bronchial responsiveness to body mass index in the ECRHS (European Community Respiratory Health Survey). Thorax. 2002;57:1028–1033. doi: 10.1136/thorax.57.12.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki N K, Baker R W. Ventilatory regulation in eucapnic morbid obesity. Am Rev Respir Dis. 1984;129:538–543. [PubMed] [Google Scholar]
- Lopata M, Onal E. Mass loading, sleep apnea, and the pathogenesis of obesity hypoventilation. Am Rev Respir Dis. 1982;126:640–645. doi: 10.1164/arrd.1982.126.4.640. [DOI] [PubMed] [Google Scholar]
- Kaufman B J, Ferguson M H, Cherniack R M. Hypoventilation in obesity. J Clin Invest. 1959;38:500–507. doi: 10.1172/JCI103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech J, Onal E, Aronson R. et al. Voluntary hyperventilation in obesity hypoventilation. Chest. 1991;100:1334–1338. doi: 10.1378/chest.100.5.1334. [DOI] [PubMed] [Google Scholar]
- Rochester D. In: Alpert M, Alexander J, editors. The heart and lung in obesity. 1998. Obesity and pulmonary function. pp. 108–132. [Google Scholar]
- Refsum H E, Holter P, Lovig T. et al. Pulmonary function and energy expenditure after marked weight loss in obese women: observations before and one year after gastric banding. Int J Obesity. 1990;14:175–183. [PubMed] [Google Scholar]
- Holley H S, Milic-Emili J, Becklake M R, Bates D V. Regional distribution of pulmonary ventilation and perfusion in obesity. J Clin Invest. 1967;46:475–481. doi: 10.1172/JCI105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurewitz A N, Susskind H, Harold W H. Obesity alters regional ventilation in lateral decubitus position. J Appl Physiol. 1985;59:774–783. doi: 10.1152/jappl.1985.59.3.774. [DOI] [PubMed] [Google Scholar]
- Thomas P, Cowen E, Hulands G. et al. Respiratory function in the morbidly obese before and after weight loss. Thorax. 1989;44:382–386. doi: 10.1136/thx.44.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Chan D, Wong E. et al. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88:361–363. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack C, Harris D, Katzel L. et al. Weight loss, not aerobic exercise, improves pulmonary function in older obese men. J Gerontol A Biol Sci Med Sci. 2000;55:453–457. doi: 10.1093/gerona/55.8.m453. [DOI] [PubMed] [Google Scholar]
- Zavorsky G, Kim do J, Sylvestre J. et al. Alveolar membrane diffusing capacity improves in the morbidly obese after bariatric surgery. Obes Surg. 2008;18:256–263. doi: 10.1007/s11695-007-9294-9. [DOI] [PubMed] [Google Scholar]
- Zavorsky G, Hoffman S. Pulmonary gas exchange in the morbidly obese. Obes Rev. 2008;9:326–339. doi: 10.1111/j.1467-789X.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- Buskirk E, Taylor H. Maximal oxygen intake and its relation to body composition, with special reference to chronic physical activity and obesity. J Appl Phys. 1957;11:72–78. doi: 10.1152/jappl.1957.11.1.72. [DOI] [PubMed] [Google Scholar]
- ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- Johnson B D, Weisman I M, Zeballos R J, Beck K C. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116:488–503. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- Weyman A, Davidoff R, Gardin J. et al. Echocardiographic evaluation of pulmonary artery pressure with clinical correlates in predominantly obese adults. J Am Soc Echocardiogr. 2002;15:454–462. doi: 10.1067/mje.2002.114912. [DOI] [PubMed] [Google Scholar]