Abstract
Introduction
Pulmonary stenosis remains the most frequent complication and cause of reintervention after the arterial switch operation for transposition of the great arteries We investigated the onset, incidence, and outcome of pulmonary stenosis after arterial switch operation in neonates with transposition of the great arteries and intact ventricular septum.
Methods
Arterial switch operation using Lecompte maneuver was performed in 222 neonates with transposition of great arteries and intact ventricular septum. Complete medical records with serial echocardiograms were available for 174 (73%) patients and were reviewed for incidence of postoperative pulmonary stenosis defined as a thickened and doming pulmonary valve and/or a pressure gradient of >25 mmHg.
Results
During a mean follow-up of 14.4 ± 0.54 years, 31 children developed pulmonary stenosis. Onset of significant stenosis occurred as early as 30 days and as late as 10 years after arterial switch operation. Uncomplicated interventional balloon/stent angioplasty was performed in 11 patients with supravalvular stenosis (mean pressure gradients of 65 mmHg). Severe restenosis occurred in these patients post-angioplasty (range 2-7 years). In other 10 patientseither patch enlargement of the area involved or angioplasty were performed. Freedom from intervention was 68.6±8.7% at 1 year and 42.8.1±9.5% at 15 years and onwards.
Conclusion
Over time, pulmonary stenosis developed after arterial switch operation. Balloon angioplasty for supravalvular pulmonary stenosis could be the initial treatment of choice owing to the high success rate. Surgical intervention is offered to those with pulmonary valve stenosis having pressure gradients of >50 mmHg, and for re-stenosis after intervention/stent implantation.
Keywords: cardiac surgery, pulmonary stenosis, arterial switch operation, transposition of the great arteries, children
Introduction
Arterial switch operation (ASO) is the treatment of choice for transposition of the great arteries with intact ventricular septum (TGA-IVS) in neonatal age, because of the low mortality and morbidity during the long follow-up [1]. Right ventricular outflow obstruction is the most frequent postoperative complication [2,3,4], occuring with sufficient severity to require reintervention in up to 10% of cases [1,5]. Usually the obstruction is at the level of either the pulmonary trunk or pulmonary bifurcation or, less commonly, in the right ventricular infundibulum. Conversely, right ventricular outflow obstruction resulting from pulmonary valve stenosis (previously aortic valve) is exceedingly rare [6]. Currently, there are only few reports on the timing of occurrence of pulmonary stenosis (PS) after arterial switch operation for transposition of the great arteries.
We report our series of TGA with IVS who underwent ASO using the Lecompte maneuver [7]. We aimed to evaluate the onset and development of postoperative PS over time, to assess the outcome of reintervention and to identify risk factors for development of pulmonary artery stenosis and opportunities for improvement in surgical technique.
Methods
The Institutional Review Board approved the study and waived the need for patient consent. Patient population. We conducted a retrospective review of patients with TGA-IVS from the congenital heart surgery and pediatric cardiology databases. All data were regularly transmitted to our institution and missing data were obtained by recall of the referring cardiologist. Operative and hospital discharge data from January 1987 to December 2010 were available for 222 neonates who underwent arterial switch operation using Lecompte maneuver for TGA-IVS. The cardiology database was used for the retrospective collection of postdischarge information, and the last known contact was used for the final determination of status and the generation of Kaplan-Meier curves. Patients with incomplete follow-up and inadequate echocardiographic information were excluded. Adequate follow-up data and complete serial echocardiograms done at regular intervals were available for 174 children, and were reviewed for onset and incidence of postoperative PS either at the valvular, supravalvular or branch artery level. The right ventricular outflow tract (RVOT) was evaluated by pulsed wave and continuous wave Doppler echocardiography obtained from the parasternal long- or short-axis views. Mean pressure gradient was determined by Bernoulli’s simplified equation. Pulmonary valve stenosis was considered present when pulmonary valve leaflets were seen to be doming and thickened and an echocardiographic mean pressure gradient of more than 25 mmHg across the valve was documented. The diameters of the neopulmonary valve annulus, main pulmonary artery (neo-mPA) at anastomotic sites as well as the diameters of the right and left branch pulmonary arteries (PA) were recorded. Supravalvular pulmonary artery stenosis was considered present when the region above the pulmonary valve sinuses was less than that of the pulmonary valve annulus, with or without the presence of branch PS. In children who developed neo-pulmonary stenosis, intervening serial echocardiograms were also reviewed. Peak systolic pressure gradients were assigned according to the categories given in Table 2.
Cardiac catheterization was indicated when echocardiography revealed peak pressure gradients of more than 40 mmHg or other abnormalities.
Reinterventions, consisting of balloon angioplasties and stent placement and/or reoperations, were indicated when PS was severe and progressive. Balloon angioplasty was preferred in distal or peripheral branch stenosis, and reoperations in central or proximal stenoses or after unsuccessful balloon angioplasties. The severity and localization of supravalvular PS and frequency of reinterventions on the RVOT and the pulmonary arteries were recorded.
Data analysis. All data were analyzed with the SPSS 16.0 (SPSS Inc., Chicago Il, USA) software program. Data are expressed as absolute and percentage frequency values and continuous data as mean ± standard deviation, as appropriate. Univariate analyses of continuous variables were performed with the Student t test. Univariate comparisons for categorical variables and the association of risk factors with reoperations were analyzed with Pearson’s chi-square test, two-tailed Fisher’s exact tests of the displayed proportions, and odds ratios. Cox regression analysis was used to determine the risk factors for development of pulmonary stenosis or multivariable predictors of freedom from reintervention. Every univariate parameter that reached significance with a p value of 0.05 or less was then tested in a multivariate logistic regression model. Time related events were examined by the actuarial method; analyses were done with censoring of incompletely traced patients after the time of the last follow-up and differences between groups were calculated by the log rank test. Freedom from reoperation was analyzed according to Kaplan-Meier estimates with 95% CI. Echocardiographic measurements were reported as mean pressure gradient in mmHg. Onset of pressure gradients changes was recorded and time to reintervention was plotted with the use of Kaplan-Meier estimates.
Results
Study population. The study population included 174 children (112 males, 62 females) who had an ASO in neonatal age (<30 days of life) for TGA-IVS. Their demographic profile is shown in Table 1.
Table 1.
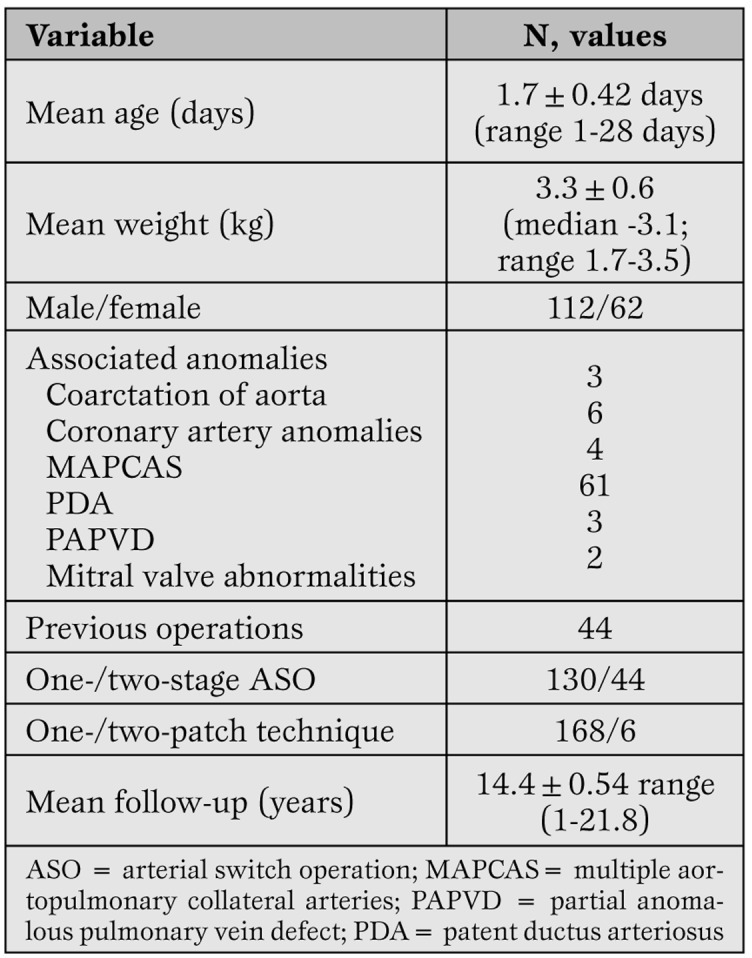
Demographic profile of 174 neonates who underwent arterial switch operation for simple transposition of great arteries.
The ASO was performed at a mean age of 1.7±0.42 days (range 1-28 days) and at body weight of 3.3 kg (range 1.7-3.5 kg). Mean follow-up interval was 18 months (range <1 month to 90 months). Seventy-nine (45.43%) had other associated cardiac anomalies, which were corrected during the same surgical procedure.
Operation. The surgical technique of ASO at the Deutsches Herzzentrum Berlin is standard. The ASO was performed with cardiopulmonary bypass without circulatory arrest using Lecompte’s maneuver with removal of coronary artery buttons and patch replacement of the valve sinus where the coronary buttons were excised. To close the defects in the sinuses, a single generous autologous pericardial patch fashioned in a pantaloon-like shape was used in all except 6 patients, in whom individual pericardial patches were used.
Follow-up evaluation. Onset and incidence of pulmonary stenosis. Mean systolic pressure gradients across the pulmonary valve of 174 children over a mean follow-up of 14.4±0.54 (range 1-21.8) years are categorized in Table 2 and distribution over subsequent follow-up years is shown in Figure 1.
Table 2.
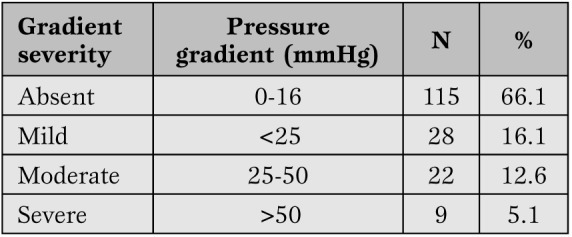
Peak systolic gradients across the right ventricular outflow tract and the pulmonary vessels.
Figure 1.
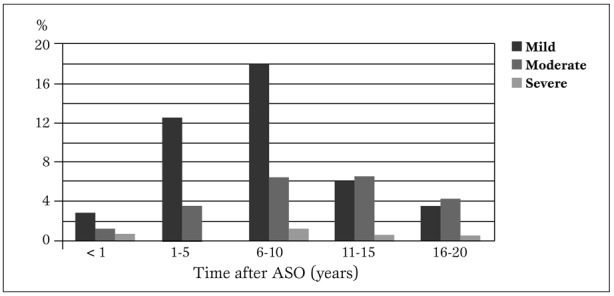
Category distribution of pulmonary stenosis after ASO in children with TGA-IVS.
ASO = arterial switch operation.
Concerning its location (Figure 2), the obstruction occurred mostly at the main pulmonary trunk (28.1%), followed by the right pulmonary branch artery (24.3%). The pulmonary bifurcation, left pulmonary branch artery and infundibulum had a prevalence of 16.54%, 18.4% and 5% respectively. Valvular stenosis had a prevalence of 7.7%.
Figure 2.
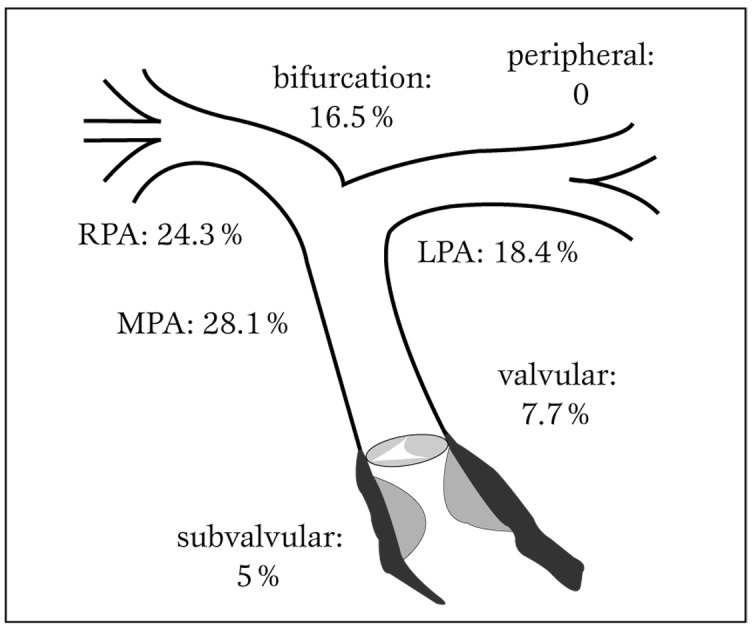
Localization of the pulmonary stenosis.
RPA = right pulmonary artery; LPA = left pulmonary artery; MPA = main pulmonary artery.
Over this period, 31 (17.8%) patients, whose demographic profile is shown in Table 3, developed significant PS warranting intervention. Serial echocardiographic studies of these particular children showed that at 1 month postoperatively, they started having pressure gradients across the valve in the range of 16-23 mm Hg, which over time increased to mean peak pressure gradient of 55±17 mmHg.
Table 3.
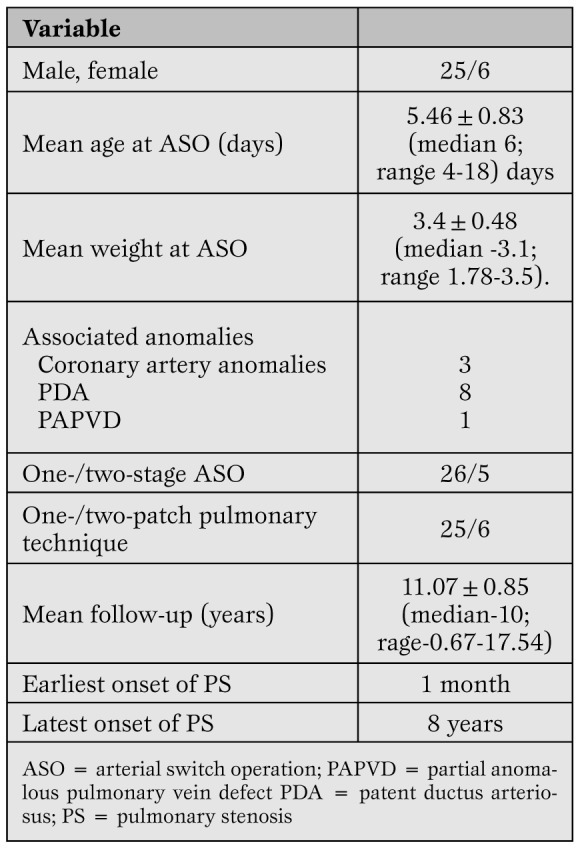
Demographic profile of 31 post-arterial switch children who developed pulmonary stenosis.
Onset of significant stenosis occurred as early as 30 days and as late as 10 years after arterial switch operation. Mean systolic pressure gradients across the pulmonary artery anastomosis at the supravalvular, right and left branch pulmonary artery levels are shown in Figures. 3a, b and c, respectively.
Figure 3.
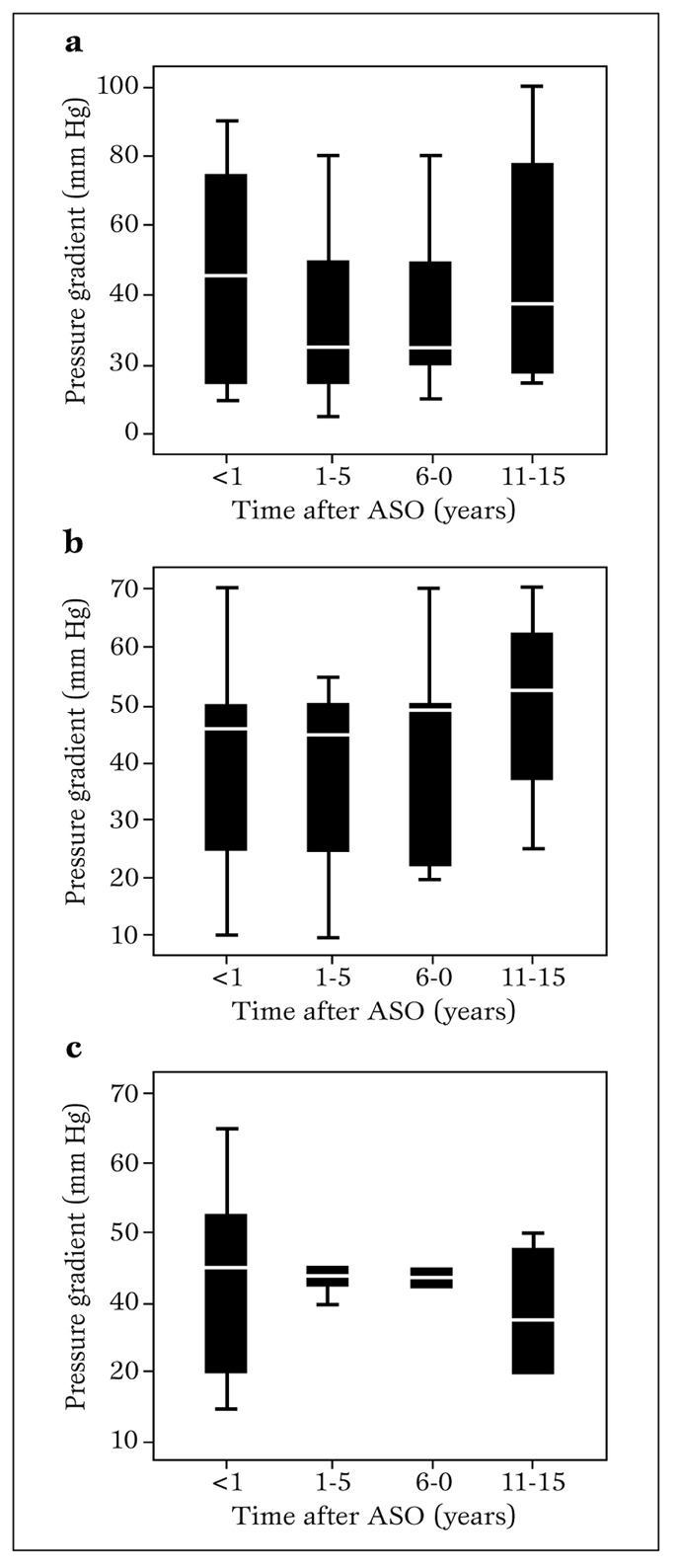
Mean pressure gradient through the pulmonary artery anastomosis at follow-up after arterial switch operation using Lecompte maneuver. a. (main pulmonary trunk level, supravalvar); b. right pulmonary artery branch level; c. left pulmonary artery branch level.
ASO = arterial switch operation.
Stenosis of main pulmonary artery combined with stenosis of both right and left branch arteries occurred in 9 (29%) patients. Stenosis of the left pulmonary artery branch occurred in 8 (25.8%), of both right and left branches in 7 (22.6%), of the right pulmonary artery branch alone in 2 (6.4%) and of the main pulmonary artery alone also in 2 (6.4%); 3 (9.7%) patients had stenosis of both main pulmonary and right artery branch.
Interventions. These 31 children, at a mean follow-up of 11.07±0.85 (median 10.9: range 0.67-22.54) years, had a total of 57 re-interventions in the form of balloon or stent implantation, patch enlargement and/or pulmonary artery valved conduit replacement or a combination, as shown in Figure 4a.
Figure 4a.
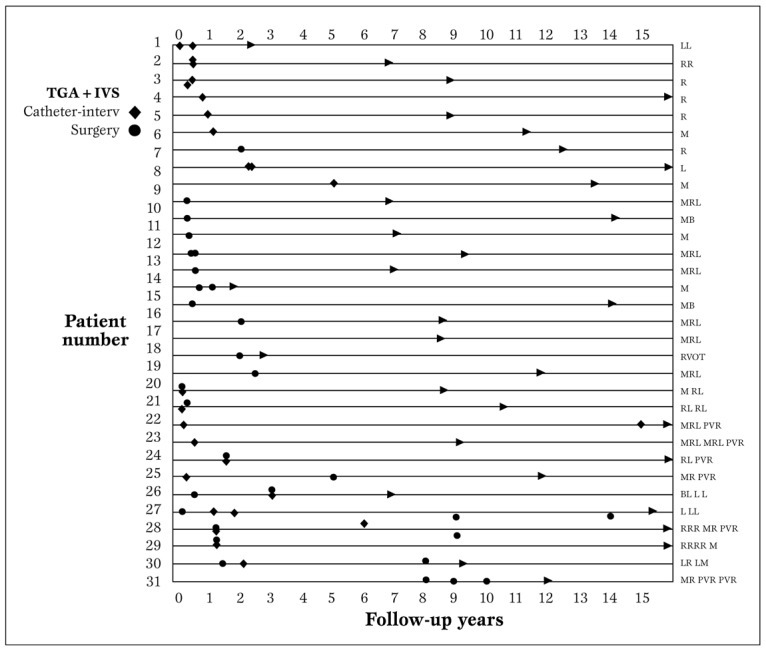
Site-related reinterventions performed during follow-up. The characters on the right show the specific site of reinterventions: B-Bifurcation, L- left pulmonary branch artery, M- main pulmonary artery, PVR- pulmonary valve replacement, R- right pulmonary branch artery, RVOT-right ventricular outflow tract.
Interventional balloon or stent implantation was performed without complications in 11 patients with supravalvular stenosis (main pulmonary artery, bifurcation, right and/or left pulmonary branches, mean pressure gradients of 65 mmHg). Post-interventional mean pressure gradients were reduced to a mean of 16 mmHg. In 10 patients severe restenosis occurred post-angioplasty - 2 had repeat balloon angioplasty, 5 underwent patch enlargement of the involved areas and and 3 had involvement of the pulmonary valve, forwhich replacement with Contegra (Medtronic Inc, Minneapolis, MN) conduit was done. In 10 patients, pulmonary stenosis was determined to have occurred at a mean of 9 months with mean pressure gradient across the valve of 25 mmHg. Over a mean of 3.4±1.8 years, mean pressure gradients increased to >50 mmHg. Hence, over a span of 5 years postoperatively, these patients (mean age 6.3±0.4 years) underwent patch enlargement of the area involved. Balloon angioplasty was performed in 2 patients who had already undergone patch enlargement of the stenosed areas. The actual frequency trend of these interventions over time is shown in Figure 4b.
Figure 4b.
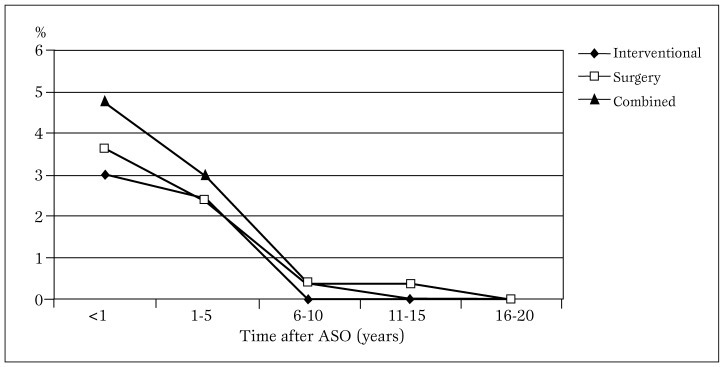
Distribution trend of interventions done on 31 post-ASO children over a 20-year follow-up period.
ASO = arterial switch operation.
Reoperations for PS were observed up to 10 years after the ASO in our population.
Kaplan-Meier estimates of freedom from intervention were 68.6±8.7% at 1 year and 42.8.1±9.5% at 5, 10 and 15 years (Figure 5).
Figure 5.
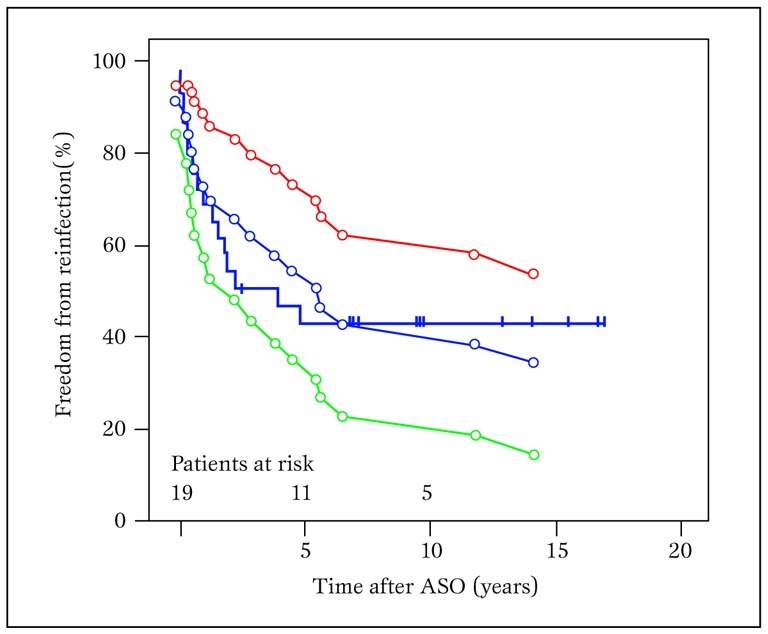
Kaplan- Meier estimates for freedom of reintervention for neo-pulmonary stenosis
Encircled lines represent 95% confidence limits.
ASO = arterial switch operation.
Median freedom from intervention was 4.03 years (0.40-7.66). Reinterventions occurred at different points throughout the follow-up period.
Latest follow-up. At the last follow-up, among the 174 children including those who had surgical repair or balloon angioplasty, PS with a mean systolic gradient >50 mmHg was observed in only 2.2%.
Risk factors for pulmonary stenosis. Pulmonary stenosis occurred in 22.3% males and 9.7% females. The mean age at ASO was 5.46±0.83 (median 6; range 4-18) days for patients with PS (p=0.240) versus 8.46±0.83 (median 7; range 5-16) days for patients without PS (p=0.240) Associated coronary anomaly patterns did not relate to the development of neopulmonary stenosis: PS developed in 28 children without coronary artery anomalies and in 3 with abnormal origins (single coronary artery) (p=0.546). Previous pulmonary banding/shunting was not correlated with development of PS (p=0.888). The pulmonary patch technique used was highly associated with the development of pulmonary stenosis (p=0.002) which occurred in all 6 patients in whom two individual pericardial patches were used (Table 4).
Table 4.
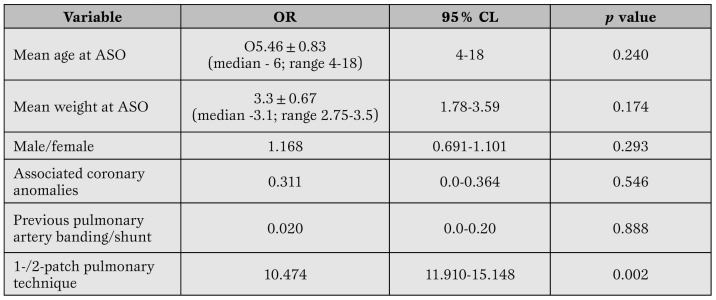
Predictor analysis for development of pulmonary stenosis (mean pressure gradient of >25 mmHg).
ASO = arterial switch operation.
Discussion
The arterial switch operation in neonatal age has become the most accepted procedure for TGA owing to the theoretical advantages over the physiologic repair of Mustard or Senning and an acceptable mortality and morbidity [7,8]. The most common complication is stenosis of the pulmonary arteries with a reported incidence of 7 to 40% [8,9]. Pulmonary artery stenosis may be related to the technique of reconstruction of the proximal pulmonary arteries with a patch to fill the defects in the posterior wall where the coronary artery buttons were excised [3] or to the Lecompte’s maneuver [7] itself. Pulmonary valve stenosis has been reported rarely in patients who have undergone either a single-stage [10] or a two-stage [11] ASO. Staging with pulmonary artery banding has been noted as a risk factor for development of neopulmonary valve stenosis [11] in contrast with our findings. However, the incidence, risk factors, and early outcome after a single-stage ASO in neonates have not been described.
In our series, the preoperative examination did not show pulmonary valve dysplasia or stenosis, and direct inspection during surgery showed a trileaflet aortic valve. No significant right ventricular outflow tract obstruction early after surgery was recorded. Thus the cause of the late onset neopulmonary valve stenosis is not well understood, although it could be presumed that, over time, negligible mild valvular dysplasia evolved into frank stenosis. It could also be speculated that the increased pulmonary trunk pressure secondary to the supravalvar stenosis may have caused turbulence and shear stress of the valve leaflets, resulting in progressive valvular stenosis. This form of right ventricular outflow tract obstruction had developed only in 3 patients who initially had stenosis of the main pulmonary trunk and right pulmonary branch artery. This was successfully treated initially by percutaneous balloon angioplasty, but later replacement with a jugular bovine valved conduit was deemed necessary.
Onset of neopulmonary stenosis: In 19 (10.95%) children, PS developed within the first year after repair, whereas in 12 (6.9%) the lesion developed beyond 1 year after operation. This observation concurs with the suggestion of other investigators that neopulmonary stenosis tends to develop primarily during the first year after repair [12]. In our series, neopulmonary stenosis has been responsible for 58% of reoperations including percutaneous catheter interventions.
Frequency of supravalvular PS and PA branch stenosis after ASO varies largely in the literature, ranging from 3.9% [13] to 41.6% [14], depending on the complexity of the patient population (TGA-VSD, Taussig-Bing anomaly included or not), on the definition of relevant supravalvular PS (>15 mmHg or >50 mmHg), the method of hemodynamic evaluation (echocardiography only or catheter), and on the duration of follow-up.
Accordingly, the incidence of surgical reinterventions ranges from 1% [15] to 22% [16] and is higher when catheter interventions are also included and the policy of reintervention was more aggressive [dilatation already at 35 mmHg [17].
Our 23-year follow-up series with 17.8% of patients receiving treatment for PS confirms the general observation that PS is the most frequent complication after ASO [13,14,16,18,19]. Though reoperation for PS was more frequent early after ASO, its incidence decreased afterward, but persisted at least 10 years after ASO in our series. Our present technique to reconstruct the pulmonary artery and avoid late PS includes previously described principles with some minor modifications. Basically, fresh autologous pericardium fashioned in pantaloon shape is used to fill the gap in the pulmonary root due to the button harvesting of coronary arteries. This pericardium is finely sutured around the edges of the pulmonary root and tailored so as to restore preoperative anatomy. Moreover, the division of the pulmonary artery before the Lecompte maneuver is performed as low as possible, namely, a very few millimeters above the pulmonary valve commissures, so that the suture of the neopulmonary trunk is away from the pulmonary bifurcation.
We assume that stenosis of the reconstructed pulmonary artery is usually due to the distortion or retraction of the pericardial patch used to cover the defect left by the coronary artery harvest. Technical refinements, especially the use of larger patch, and percutaneous dilatation have greatly reduced the incidence of severe stenosis and the need for reoperation.
In accordance with the results of other investigators [18], we found that two sites are predisposed to develop PS, i.e. the proximal and distal sites.
Supravalvular PS, as depicted in Figure 2a is undoubtedly attributable to the extensive surgery at that specific site. In addition, any suture and material used for covering the excision defects in the neo-pulmonary artery roots has only a very restricted growth potential. Because of the compensating circumferential growth of the vessel wall, the whole diameter normally may increase sufficiently [20] and asymmetrically, because of scarring.
Stenosis of the pulmonary branches has been attributed to “inadequate surgery” [20] and unfavorable flow phenomena [21,22]. Both pulmonary artery banding [24] and the type of patch technique used to correct the defects in the pulmonary artery wall7 have been suggested as technical factors that can lead to valvular and supravalvular stenosis.
In this series, ASO was performed with a fairly standardized technique for repair of the buttons (a generous single pantaloon-shaped patch of fresh or glutaraldehyde-treated autologous pericardium). Therefore, the technique for RVOT reconstruction appeared in our series to be related to the development of neo-pulmonary stenosis. The influence of varying the number of patches used was assessed because the double-patch method was used in 6 patients, who all developed pulmonary stenosis. We were able to find a correlation between the use of double-patch technique versus pantaloon patch technique and the development of pulmonary artery stenosis, as in some reports that progression of stenosis is higher in patients undergoing the double-patch technique, because this fills only the defects left by coronary artery explantation, whereas the single pantaloon patch provides additional length and tissue quantity to the proximal neopulmonary root for a complete pulmonary circumferential anastomosis. Pretre et al. [25] suggested that stenosis of the reconstructed pulmonary artery is usually due to distortion or retraction of the pericardial patch used to cover the defect left by the coronary harvest.
In our series, we did not find any correlation between age at operation and development of moderate to severe mean transpulmonary gradient, as was also the findings by several investigators [26,27].
We may only assume that there could have been inadequate growth of the pulmonary artery resulting in flattening of the reconstructed right ventricular outflow tract. Another cause may be the technique employed for PA reconstruction [28].
The tension of the anastomotic site has been implicated as a possible adjunctive factor to inadequate somatic growth at the suture line.
Thus far, after a mean follow-up of nearly 15 years, the pulmonary artery seems to withstand the hemodynamic stress of the pulmonary circulation. However, we should still be cautious as distal supravalvular stenosis can still occur over time, although its frequency has decreased because of technical refinements.
The ASO technique keeps its promise.
The present study showed good left ventricular function in the overwhelming majority of patients.
Limitations
The retrospective nature of this study imposed several inherent limitations. First, these patients were referred from various institutions; thus follow-up echocardiograms were done by various referring cardiologists; hence findings were not uniformly reported and quantification of right ventricle-pulmonary artery gradient may change with the observers and the centers.
Second, it would have been better if other parameters such as preoperative and follow-up pulmonary valve annulus and pulmonary artery (previous aorta) diameter had been measured to assess pulmonary growth.
Finally, end points and the protocol of the follow-up should have been determined prospectively.
Conclusion
This study indicates that as early as 1 month through a period of 15 years, supravalvular as well as valvular pulmonary stenosis developed after arterial switch operation for transposition of great arteries with intact ventricular septum. It is the most common cause of reintervention. Balloon angioplasty could be the initial treatment of choice owing to the high success rate, without complications.
Surgical intervention is offered for recurrent and combined stenosis after intervention or stent implantation, and for patients with pulmonary stenosis with pressure gradients of >60 mmHg.
Our data show that pulmonary stenosis is not an uncommon event after neonatal ASO for simple transposition of great arteries. Even if a uniform surgical procedure is adopted. Echocardiograms should be done at regular intervals during follow-up.
Although we found only a single risk factor for the development of pulmonary stenosis, we should be still cautious and vigilant in following up these children, bearing in mind that any patient can have pulmonary stenosis at any time after ASO.
Acknowledgments
We thank Julia Stein for tireless data management and Cornelia Hanke for conscientiously following-up patients.We also appreciate the assistance of Astrid Benhennour, Christine Detschades, Daniela Moeske-Scholz, Heike Schultz, and Carla Weber. We thank Anne M. Gale, Editor in the Life Sciences, for editorial assistance.
Footnotes
Source of Support Nil.
Conflict of interest None declared.
Cite as: Delmo Walter EM, Miera O, Nasseri B, Huebler M, Alexi-Meskishvili V, Berger F, Hetzer R. Onset of pulmonary stenosis after arterial switch operation for transposition of great arteries with intact ventricular septum. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2011; 3 (3): 177-187
References
- Kirklin JW, Barrat-Boyes BG. Complete transposition of the great arteries. In: Kirklin JW, Barrat-Boyes BG, eds. Cardiac surgery. Edinburgh: Churchhill Livingstone. 2006:1438–508. [Google Scholar]
- Wernowsky G, Hougen TJ, Walsh EP. et al. Mid-term results after arterial switch operation for transposition of the great arteries with intact ventricular septum: clinical, hemodynamic, echocardiographic and electrophysiological data. Circulation. 1988;77:1333–44. doi: 10.1161/01.cir.77.6.1333. [DOI] [PubMed] [Google Scholar]
- Lupinetti FM, Bowe EL, Minich LL. et al. Intermediate term survival and functional results after arterial repair for transposition of the great ateries. J Thorac Cardiovasc Surg. 1992;103:421–7. [PubMed] [Google Scholar]
- Yacoub MH, Bernhard A, Radley-Smith R. et al. Supravalvular pulmonary stenosis after anatomic correction of transposition of the great arteries: causes and prevention. Circulation. 1982;66:193–197. [PubMed] [Google Scholar]
- Serraf A, Roux D, Lacour-Gayet F. et al. Reoperation after arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg. 1995;110:892–899. doi: 10.1016/s0022-5223(05)80155-2. [DOI] [PubMed] [Google Scholar]
- Nakamishi T, Matsumoto Y, Seguchi M. et al. Balloon angioplasty for postoperative pulmonary artery stenosis in transposition of the great arteries. J Am Coll Cardiol. 1993;22:859–66. doi: 10.1016/0735-1097(93)90204-e. [DOI] [PubMed] [Google Scholar]
- Lecompte Y, Zannini L, Hazan E. et al. Anatomic correction of the transposition of the great arteries: new technique without use of a prosthetic conduit. J Thorac Cardiovasc Surg. 1981;82:629–31. [PubMed] [Google Scholar]
- Layman TE, Edwards JE. Anomalies of the cardiac valves associated with complete transposition of the great vessels. Am J Cardiol. 1967;19:247–55. doi: 10.1016/0002-9149(67)90541-3. [DOI] [PubMed] [Google Scholar]
- Trusler GA, Castaneda AR, Rosenthal A. et al. Current results of management in transposition of the great arteries, with special emphasis on patients with associated ventricular septal defect. J Am Coll Cardiol. 1987;10:1061–71. doi: 10.1016/s0735-1097(87)80347-9. [DOI] [PubMed] [Google Scholar]
- Gleason MM, Chin AJ, Andrews BA. et al. Two-dimensional and Doppler echocardiographic assessment of neonatal arterial repair for transposition of the great arteries. J Am Coll Cardiol. 1989;13:320–8. doi: 10.1016/0735-1097(89)90308-2. [DOI] [PubMed] [Google Scholar]
- Nakamishi T, Momoi N, Satoh M. et al. Growth of the neopulmonary valve annulus after arterial switch operation in transposition of the great arteries. Circulation. 1996;94:1127–31. [PubMed] [Google Scholar]
- Wernowsky G, Mayer JE, Jonas RA. et al. Factors influencing early and late outcome of arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg. 1995;109:289–302. doi: 10.1016/S0022-5223(95)70391-8. [DOI] [PubMed] [Google Scholar]
- Losay J, Touchot A, Serraf A. et al. Late outcome after serial switch operation for transposition fo the great arteries. Circulation. 2001;104:1121–6. doi: 10.1161/hc37t1.094716. [DOI] [PubMed] [Google Scholar]
- Hövels-Gürich HH, Seghaye MC, Ma Q. et al. Long term results of cardiac and general health status in children after neonatal switch operation. Ann Thorac Surg. 2003;75:935–43. doi: 10.1016/s0003-4975(02)04410-7. [DOI] [PubMed] [Google Scholar]
- Däbritz SH, Nollert G, Sachweh JS. et al. Anatomical risk factors for mortality and cardiac morbidity after arterial switch operation. Ann Thorac Surg. 2000;69:1880–6. doi: 10.1016/s0003-4975(00)01241-8. [DOI] [PubMed] [Google Scholar]
- Spiegelenberg SR, Hutter PA, van-de-Wal HJ. et al. Late re-interventions following arterial switch operations in transposition of the great arteries. Incidence and surgical treatment of postoperative pulmonary stenosis. Eur J Cardiothorac Surg. 1995;9:7–10. doi: 10.1016/s1010-7940(05)80041-7. [DOI] [PubMed] [Google Scholar]
- Schmaltz AA, Bein G, Grävinghoff L. et al. Balloon valvuloplasty of pulmonary stenosis in infants and children-co-operative study of the German Society of Pediatric Cardiology. Eur HJ. 1989:967–71. doi: 10.1093/oxfordjournals.eurheartj.a059421. [DOI] [PubMed] [Google Scholar]
- Haas F, Wottke M, Poppert H, Meisner H. Long-term survival and functional follow-up in patients after the arterial switch operation. Ann Thorac Surg. 1999;68:1692–7. doi: 10.1016/s0003-4975(99)01039-5. [DOI] [PubMed] [Google Scholar]
- Williams WC, Quaegebeur JM, Kirklin JW, Blackstone EH. Outflow obstruction after the arterial switch operation: a multi-institutional study. Congenital Heart Surgeons\' Society. J Thorac Cardiovas Surg. 1997;114:975–90. doi: 10.1016/s0022-5223(97)70012-6. [DOI] [PubMed] [Google Scholar]
- Massin MM, Nitsch GB, Däbritz S. et al. Growth of the pulmonary artery afterial arterial switch operation for simple transposition of the great arteries. Eur J Pediatr. 1998;157:95–100. doi: 10.1007/s004310050777. [DOI] [PubMed] [Google Scholar]
- Tang T, Chiu IS, Chen HC. et al. Comparison of pulmonary arterial flow phenomena in spiral and Lecompte models by computational fluid dynamics. J Thorac Cardiovasc Surg. 2001;122:529–34. doi: 10.1067/mtc.2001.115230. [Tang T, Chiu IS, Chen HC, et al. ] [DOI] [PubMed] [Google Scholar]
- Beierlein W, Freitag HP, Salehi-Gilani S. et al. Excision of the coronary orifices in arterial switch operations "O" like obstructive or "U" like unobstructive? Thorac Cardiov Surg. 2004;52:169–73. doi: 10.1055/s-2004-820881. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Adman FH. In: Paul MH, Wernowsky G, editors. Heart disease in infants, children and adolescents: including the fetus and young adult. 5th ed. Baltimore: Williams & Willkins. 1995. Transposition of the great arteries. pp. 1154–224. [Google Scholar]
- Serraf A, Bruniaux J, Lacour-Gayet F. et al. Anatomic correction of transposition of the great arteries with ventricular septal defect. J Thorac Cardiovasc Surg. 1991;102:140–7. [PubMed] [Google Scholar]
- Prêtre R, Tamisier D, Bonhoeffer P. et al. Results of the arterial switch operation in neonates with transposed great arteries. The Lancet. 2001;357:1826–30. doi: 10.1016/S0140-6736(00)04957-6. [DOI] [PubMed] [Google Scholar]
- Edwin F, Mamorare H, Brink J, Kinsley R. Primary arterial switch operation for transposition of the great arteries with intact ventricular septum - is it safe after three weeks of age? Interact CardioVasc Thorac Surg. 2010;11:641–4. doi: 10.1510/icvts.2010.243832. [DOI] [PubMed] [Google Scholar]
- Ismail SR, Kabbani MS, Najm HK. et al. Early outcome for the primary arterial switch operation beyond the age of three weeks. Pediatr Cardiol. 2010;31:663–67. doi: 10.1007/s00246-010-9679-8. [Ismail SR, Kabbani MS, Najm HK, et al. ] [DOI] [PubMed] [Google Scholar]
- Kuroczynski W, Kampmann C, Peivandi AA. et al. Mid-tem results of a modified arterial switch operation using the direct reconstruction technique of the pulmonary artery. Cardiology Journal. 2010;17:574–9. [PubMed] [Google Scholar]


