Abstract
Hepatitis C virus (HCV) infection is associated with a number of extrahepatic disorders. The most studied conditions associated with HCV are type II mixed cryoglobulinemia and B cell lymphoma. However, many reports suggest that HCV might also be associated with a number of autoimmune disorders, both organ-specific and not organ-specific. Although concomitant treatment of HCV infection is a confounding factor when ascertaining the actual role of HCV in inducing autoimmune disease, a considerable amount of experimental data indicates that HCV is able to subvert the immune system and consequently induce autoimmunity. In the present review, we report a series of observations which associate chronic HCV infection with the onset of autoimmune disorders.
Keywords: hepatitis C virus, immune regulation, autoimmune diseases
Introduction
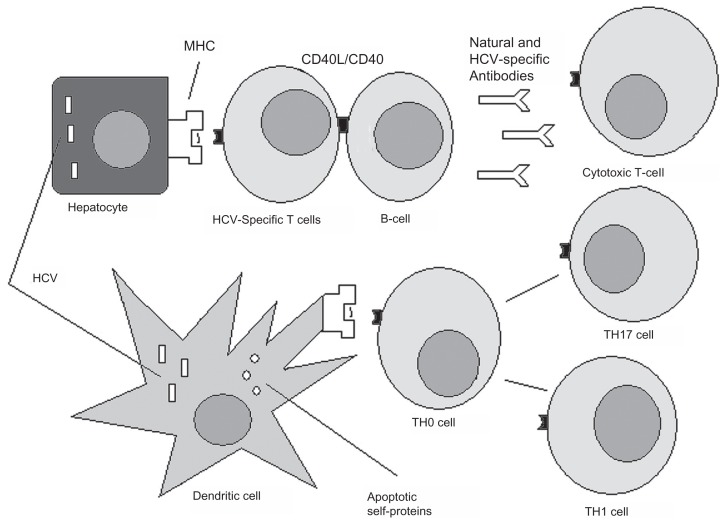
In 1987, hepatitis C virus (HCV) was discovered to be the causative agent of a type of hepatitis previously known as non-A, non-B hepatitis.1 However, it has been observed with time that presence of the virus might be also responsible for extrahepatic manifestations. 2 Therefore, the concept of systemic HCV infection has emerged.3,4 The most studied HCV-related conditions are lymphoproliferative disorders, including type II mixed cryoglobulinemia,5–7 which can evolve into frank lymphoma,8,9 and lymphomas unrelated to mixed cryoglobulinemia.10–12 Among the hematologic disorders, monoclonal gammopathies associated with HCV infection have also been described.13 This review focuses on non-lymphoproliferative disorders as a manifestation of chronic HCV infection, namely autoimmune diseases. HCV can in fact subvert the immune system in several ways, from expanding selective B cell subsets to induce breaking tolerance and by reaction of T cells against apoptosis-derived self-antigens, with consequent promotion of T helper-17 cells.14–18 The possible mechanisms by which autoimmunity can be triggered by HCV infection are shown in Figure 1. However, the causal relationship between HCV infection and the onset of clinically relevant autoimmunity is still a matter of debate. This review may help the clinician to understand whether the onset of autoimmune manifestation in the course of HCV might actually be related to the presence of the virus.
Figure 1.
Possible mechanisms of autoimmunity induced by hepatitis C virus (HCV).
Notes: HCV infects hepatocytes and interacts with B lymphocytes. HCV and hepatocyte-derived apoptotic proteins are taken up by tissue-resident dendritic cells, which mature and migrate into the draining lymph nodes. Here they activate naïve HCV-specific and self-peptide-specific T cells. These cells in turn differentiate into effector proinflammatory T helper-1, T helper-17, or cytotoxic T lymphocytes. Effector T cells can recognize either HCV-derived peptides on the hepatocyte surface or peptides self-expressed by noninfected cells, with the possible triggering of autoimmunity. Specific B cells are activated by T helper cells via CD40L/CD40 interaction and are induced to produce both anti-HCV and natural nonorgan-specific autoantibodies. These autoantibodies can react against components of self. Finally, HCV-specific T and B cells can recognize self-antigens by a mechanism of molecular mimicry between virus and host.
Abbreviation: MHC, major histocompatibility complex.
Thyroid disorders
In the course of HCV infection, both hypothyroidism and hyperthyroidism may emerge. Hashimoto’s thyroiditis is the most common thyroid disorder observed in patients with HCV infection. Treatment with interferon-alpha (IFNA) can be an additional risk factor for the development of thyroid complications. In fact, IFNA can induce autoimmunity by several molecular mechanisms, including polymorphisms in the IFNA-signaling pathways, a feed-forward loop of IFNA production, and a mutually positive regulatory feedback loop between IFNA and estrogen receptor-α. Increased levels of IFNA have numerous immunomodulatory functions, including activating both innate and adaptive immune responses.19 In a recent study, 293 patients with chronic HCV who underwent IFNA therapy for 24 or 48 weeks, were investigated for thyroid function.20 The investigators found that hypothyroidism was the most frequent thyroid disease, especially during the first cycle of IFNA. Genotype 1 virus was associated with a twofold risk of developing the illness. However, approximately 34% of thyroid disease was transient.
In a retrospective study of 288 patients who received IFNA-ribavirin for HCV over a 2-year period, thyroid function was assessed during a 24- or 48-week course of therapy.21 The authors concluded that although thyroid disease was common in this cohort, just 2.3% patients required ongoing therapy. Interestingly, pre-IFNA-ribavirin serum thyroid-stimulating hormone and thyroid peroxidase antibody titers were found to predict development of thyroid disease in this group of patients. This finding highlights the fact that IFNA-associated thyroiditis during treatment of HCV infection is critically influenced by individual factors. In another report, a case of fluctuating and wavering thyrotropin autoantibodies of both a stimulating and blocking nature was described.22 The autoantibody profile was clearly modified during IFNA therapy and settled into a new equilibrium on completion of treatment. This case highlights the possible existence of a dual thyroid autoantibody population associated with HCV, and its possible modulation by IFNA therapy. There has also been a report of a 69-year-old Japanese man who developed Graves’ ophthalmopathy while receiving IFNA-ribavirin.23
However, several other studies have shown that the prevalence of thyroid disease is increased regardless in patients infected with HCV compared with normal subjects, after exclusion of patients being treated with IFNA.24–26 In this regard, genetic and environmental factors seem to play an important role,27–29 and subclinical hypothyroidism is observed in a significant proportion of patients with chronic HCV infection.30,31 To explain the relationship between HCV infection and thyroid disease, it has been hypothesized recently that HCV envelope proteins can induce thyroidal inflammation directly, thereby triggering thyroiditis via a so-called “bystander activation” mechanism. In a recent study,32 significant levels of CD81 mRNA as well as CD81 protein (one of the putative HCV cell receptors33) were found on thyroid cells. Incubation of thyroid cells with HCV envelope glycoprotein E2 resulted in binding of E2 to thyroid cells, with activation of interleukin-8, an important proinflammatory cytokine. Intriguingly, thyroid cells incubated with E2 continued to proliferate normally and did not undergo apoptosis, as was reported in hepatocytes. The authors of this study concluded that HCV envelope glycoprotein E2 can bind to CD81 receptors expressed on thyroid cells and induce a cascade of signals, with possible onset of thyroiditis in genetically susceptible individuals. In conclusion, it is advisable for the clinician to monitor thyroid function regularly in the course of chronic HCV, and in particular during treatment with IFNA-based regimens.
Rheumatoid arthritis
Polyarthritis can be observed in the course of HCV infection, and in some cases is associated with mixed cryoglobulinemia.34,35 A recent study investigated 45 HCV-infected patients and 30 patients with rheumatoid arthritis fulfilling American College of Rheumatology classification criteria for rheumatoid arthritis but negative for HCV.36 The study focused on the significance of anti-mutated citrullinated vimentin (MCV) antibodies, which were recently suggested for inclusion in the diagnostic workup for rheumatoid arthritis. Anti-MCV antibodies, anti-cyclic citrullinated peptide (CCP) antibodies, rheumatoid factor, and cryoglobulins were measured. The most frequent pattern was symmetric polyarthralgia, and the joints most frequently involved were the wrists, metacarpophalangeal joints, shoulders, and knees. In HCV arthropathy, anti-MCV was positive in 30% of cases, anti-CCP in 0%, and rheumatoid factor in 73.3%, whereas in rheumatoid arthritis, anti-MCV was positive in 93.3%, anti-CCP in 96.7%, and rheumatoid factor in 86.7% of cases. Therefore, the authors concluded that anti-CCP still plays a major role in differentiating between rheumatoid arthritis and HCV arthropathy.
Another recent study assessed the diagnostic utility of anti-CCP antibodies in comparison with rheumatoid factor in distinguishing between rheumatoid arthritis and HCV-related polyarthropathy.37 Serum anti-CCP antibodies and rheumatoid factor were measured in 30 patients with rheumatoid arthritis and 22 patients with HCV-related polyarthropathy. Anti-CCP antibodies were positive in 83.3% of patients with rheumatoid arthritis and in 4.5% in patients with HCV and polyarthropathy. Rheumatoid factor was positive in 90% of patients with rheumatoid arthritis and in 81.1% of HCV patients with polyarthropathy. Anti-CCP antibodies showed higher specificity than rheumatoid factor for rheumatoid arthritis (95.4% versus 18.2%). However, the sensitivity of anti-CCP was comparable with that of rheumatoid factor (83.3% versus 90%). The conclusion of this study is again that anti-CCP antibodies are the most reliable laboratory markers for differentiating between rheumatoid arthritis and HCV-related polyarthropathy. Therefore, there is consolidated evidence indicating that “true” rheumatoid arthritis is uncommon in HCV patients, and that the arthritis observed is, in most cases, a nonerosive intermittent oligoarticular arthritis.
Sjögren’s syndrome
Sjögren’s syndrome is an autoimmune disease characterized by involvement of exocrine glands showing significant T cell infiltration. The inflammatory process involves mainly the salivary and lacrimal glands, leading to both xerostomia and xerophthalmia. Sjögren’s syndrome is also characterized by nonerosive arthritis, which may be accompanied by systemic symptoms, including asthenia. Laboratory investigation shows positivity for SSA and/or SSB autoantibodies, often associated with the presence of rheumatoid factor. Sjögren’s syndrome can present as a primary disease or secondary to rheumatoid arthritis or systemic lupus erythematosus.38 Definitive evidence that HCV infection may trigger Sjögren’s syndrome is still lacking. A correlation between Sjögren’s syndrome and mixed cryoglobulinemia has been repeatedly reported.39,40 However, different studies have been carried out subsequently to ascertain the possible relationship between HCV infection and Sjögren’s syndrome.41,42 In an interesting study, the clinical and immunologic characteristics of 35 patients with chronic HCV infection and a well documented diagnosis of Sjögren’s syndrome were reported.43 Compared with 60 patients with primary Sjögren’s syndrome who tested negative for HCV antibodies, patients with Sjögren’s syndrome and HCV showed a higher mean age, a lower prevalence of parotidomegaly, and a higher prevalence of liver involvement. Moreover, the patients with HCV-related Sjögren’s syndrome showed a higher prevalence of anti-gastric parietal cell antibodies, anti-mitochondrial antibodies, cryoglobulinemia, hypocomplementemia, and a lower prevalence of SSA autoantibodies.44 Therefore, the immunologic characteristics of HCV infection and Sjögren’s syndrome appear to be different, although common mechanisms linking HCV-associated Sjögren’s syndrome and lymphoproliferative disorders have been hypothesized.45 Further studies are needed to clarify the role of HCV infection in Sjögren’s syndrome.
Anecdotal observations
Many other autoimmune conditions have been related to HCV infection. Glomerulonephritis has been associated with HCV, especially in children,46,47 and immune- mediated skin diseases, especially oral lichen planus, have been linked with HCV.48–50 Neurologic autoimmune diseases, including myelitis51 and encephalomyelitis,52 as well as several neuromuscular diseases, have also been reported in the course of HCV infection.53 A case of relapsing polychondritis associated with HCV infection has been recently observed.54 Finally, autoimmune mechanisms have been implicated in thrombocytopenia associated with chronic HCV.55–57
Conclusion
HCV infection not only affects the liver, but is also associated with extrahepatic disorders, including autoimmune disorders. There is experimental evidence showing that HCV can subvert the immune system, possibly leading to the onset of autoimmunity. However, IFNA, used in the treatment of HCV infection, is a powerful inducer of autoimmunity, and it is sometimes difficult to discriminate between the effect of HCV and IFNA in inducing an autoimmune reaction. On the other hand, IFNA has been found to improve some HCV-related autoimmune diseases, suggesting that these might be mediated by inhibition of HCV replication or viral clearance. Therefore, IFNA can be considered on the one hand as a trigger for autoimmunity in patients with HCV but, on the other hand, as a therapeutic tool in some cases of autoimmune disease. In light of all these considerations, further study is needed to clarify the actual role of HCV infection in the induction of clinically significant autoimmunity, and to identify additional autoimmune disorders in which concomitant presence of HCV might possibly be involved in the pathogenesis.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB. Extrahepatic manifestations of hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39(1):2–17. doi: 10.1016/j.dld.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Zignego AL, Brechot C. Extrahepatic manifestations of HCV infection: facts and controversies. J Hepatol. 1999;31(2):369–376. doi: 10.1016/s0168-8278(99)80239-6. [DOI] [PubMed] [Google Scholar]
- 4.Cacoub P, Poynard T, Ghillani P, et al. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42(10):2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Misiani R, Bellavita P, Fenili D, et al. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117(7):573–577. doi: 10.7326/0003-4819-117-7-573. [DOI] [PubMed] [Google Scholar]
- 6.Ferri C, Monti M, La Civita L, et al. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993;82(12):3701–3704. [PubMed] [Google Scholar]
- 7.Zignego AL, Ferri C, Giannini C, et al. Hepatitis C virus infection in mixed cryoglobulinemia and B-cell non-Hodgkin’s lymphoma: evidence for a pathogenetic role. Arch Virol. 1997;142(3):545–555. doi: 10.1007/s007050050100. [DOI] [PubMed] [Google Scholar]
- 8.Pozzato G, Mazzaro C, Crovatto M, et al. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994;84(9):3047–3053. [PubMed] [Google Scholar]
- 9.Ferri C, Monti M, La Civita L, et al. Hepatitis C virus infection in non-Hodgkin’s B-cell lymphoma complicating mixed cryoglobulinaemia. Eur J Clin Invest. 1994;24(11):781–784. doi: 10.1111/j.1365-2362.1994.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 10.Luppi M, Grazia Ferrari M, Bonaccorsi G, et al. Hepatitis C virus infection in subsets of neoplastic lymphoproliferations not associated with cryoglobulinemia. Leukemia. 1996;10(2):351–355. [PubMed] [Google Scholar]
- 11.Zuckerman E, Zuckerman T, Levine AM, et al. Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med. 1997;127(6):423–428. doi: 10.7326/0003-4819-127-6-199709150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Monti G, Pioltelli P, Saccardo F, et al. Incidence and characteristics of non-Hodgkin lymphomas in a multicenter case file of patients with hepatitis C virus-related symptomatic mixed cryoglobulinemias. Arch Intern Med. 2005;165(1):101–105. doi: 10.1001/archinte.165.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Andreone P, Zignego AL, Cursaro C, et al. Prevalence of monoclonal gammopathies in patients with hepatitis C virus infection. Ann Intern Med. 1998;129(4):294–298. doi: 10.7326/0003-4819-129-4-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Terrier B, Joly F, Vazquez T, et al. Expansion of functionally anergic CD21−/low marginal zone-like B cell clones in hepatitis C virus infection-related autoimmunity. J Immunol. 2011;187(12):6550–6563. doi: 10.4049/jimmunol.1102022. [DOI] [PubMed] [Google Scholar]
- 15.Franceschini D, Del Porto P, Piconese S, et al. Polyfunctional type-1, -2, and -17 CD8(+) T cell responses to apoptotic self-antigens correlate with the chronic evolution of hepatitis C virus infection. PLoS Pathog. 2012;8(6):e1002759. doi: 10.1371/journal.ppat.1002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roughan JE, Reardon KM, Cogburn KE, Quendler H, Pockros PJ, Law M. Chronic hepatitis C virus infection breaks tolerance and drives polyclonal expansion of autoreactive B cells. Clin Vaccine Immunol. 2012;19(7):1027–1037. doi: 10.1128/CVI.00194-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu Z, Hamalainen-Laanaya HK, Nishitani C, Kuroki Y, Crispe IN, Orloff MS. HCV core and NS3 proteins manipulate human blood-derived dendritic cell development and promote Th 17 differentiation. Int Immunol. 2012;24(2):97–106. doi: 10.1093/intimm/dxr104. [DOI] [PubMed] [Google Scholar]
- 18.Sutti S, Vidali M, Mombello C, Sartori M, Ingelman-Sundberg M, Albano E. Breaking self-tolerance toward cytochrome P4502E1 (CYP2E1) in chronic hepatitis C: possible role for molecular mimicry. J Hepatol. 2010;53(3):431–438. doi: 10.1016/j.jhep.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Choubey D, Moudgil KD. Interferons in autoimmune and inflammatory diseases: regulation and roles. J Interferon Cytokine Res. 2011;31(12):857–865. doi: 10.1089/jir.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavan MH, Pavin EJ, Goncales FL, Jr, Wittmann DE. Virus C genotype predisposes to primary hypothyroidism during interferon-alpha treatment for chronic hepatitis C. Braz J Infect Dis. 2011;15(5):449–456. doi: 10.1016/s1413-8670(11)70226-4. [DOI] [PubMed] [Google Scholar]
- 21.Costelloe SJ, Wassef N, Schulz J, et al. Thyroid dysfunction in a UK hepatitis C population treated with interferon-alpha and ribavirin combination therapy. Clin Endocrinol (Oxf) 2010;73(2):249–256. doi: 10.1111/j.1365-2265.2010.03785.x. [DOI] [PubMed] [Google Scholar]
- 22.Tran HA, Reeves GE. The influence of hepatitis C infection and interferon-alpha therapy on thyrotropin blocking and stimulating autoantibodies in Graves’ ophthalmopathy: a case report. 2009;2(1):12. doi: 10.1186/1756-6614-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagao Y, Hiromatsu Y, Nakashima T, Sata M. Graves’ ophthalmopathy and tongue cancer complicated by PEG-interferon alpha-2b and ribavirin therapy for chronic hepatitis C: a case report and review of the literature. Mol Med Report. 2008;1(5):625–631. doi: 10.3892/mmr_00000003. [DOI] [PubMed] [Google Scholar]
- 24.Huang MJ, Tsai SL, Huang BY, Sheen IS, Yeh CT, Liaw YF. Prevalence and significance of thyroid autoantibodies in patients with chronic hepatitis C virus infection: a prospective controlled study. Clin Endocrinol (Oxf) 1999;50(4):503–509. doi: 10.1046/j.1365-2265.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 25.Ganne-Carrie N, Medini A, Coderc E, et al. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. J Autoimmun. 2000;14(2):189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 26.Salazar LA, Garcia-Samper X, Suarez-Carpio R, et al. Hypothyroidism in noninterferon treated-HCV infected individuals is associated with abnormalities in the regulation of Th17 Cells. Hepat Res Treat. 2010;2010:971095. doi: 10.1155/2010/971095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenzi M, Johnson PJ, McFarlane IG, et al. Antibodies to hepatitis C virus in autoimmune liver disease: evidence for geographical heterogeneity. Lancet. 1991;338(8762):277–280. doi: 10.1016/0140-6736(91)90418-o. [DOI] [PubMed] [Google Scholar]
- 28.Prentice LM, Phillips DI, Sarsero D, Beever K, McLachlan SM, Smith BR. Geographical distribution of subclinical autoimmune thyroid disease in Britain: a study using highly sensitive direct assays for autoantibodies to thyroglobulin and thyroid peroxidase. Acta Endocrinol (Copenh) 1990;123(5):493–498. doi: 10.1530/acta.0.1230493. [DOI] [PubMed] [Google Scholar]
- 29.Minelli R, Braverman LE, Giuberti T, et al. Effects of excess iodine administration on thyroid function in euthyroid patients with a previous episode of thyroid dysfunction induced by interferon-alpha treatment. Clin Endocrinol (Oxf) 1997;47(3):357–361. doi: 10.1046/j.1365-2265.1997.2721081.x. [DOI] [PubMed] [Google Scholar]
- 30.Preziati D, La Rosa L, Covini G, et al. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol. 1995;132(5):587–593. doi: 10.1530/eje.0.1320587. [DOI] [PubMed] [Google Scholar]
- 31.Marazuela M, Garcia-Buey L, Gonzalez-Fernandez B, et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44(6):635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 32.Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. J Autoimmun. 2008;31(4):339–344. doi: 10.1016/j.jaut.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pileri P, Uematsu Y, Campagnoli S, et al. Binding of hepatitis C virus to CD81. Science. 1998;282(5390):938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 34.Ferri C, Sebastiani M, Giuggioli D, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33(6):355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Rosner I, Rozenbaum M, Toubi E, Kessel A, Naschitz JE, Zuckerman E. The case for hepatitis C arthritis. Semin Arthritis Rheum. 2004;33(6):375–387. doi: 10.1016/j.semarthrit.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Zehairy M, Soliman E, Daghaidy A. Antibodies to mutated citrullinated vimentin in patients with chronic hepatitis C virus genotype IV infection-related arthropathy. Rheumatol Int. 2011 Nov 9; doi: 10.1007/s00296-011-2193-3. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 37.Ezzat WM, Raslan HM, Aly AA, Emara NA, El Menyawi MM, Edrees A. Anti-cyclic citrullinated peptide antibodies as a discriminating marker between rheumatoid arthritis and chronic hepatitis C-related polyarthropathy. Rheumatol Int. 2011;31(1):65–69. doi: 10.1007/s00296-009-1225-8. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson R, Vogelsang P, Volchenkov R, Espinosa A, Wahren-Herlenius M, Appel S. The complexity of Sjogren’s syndrome: novel aspects on pathogenesis. Immunol Lett. 2011;141(1):1–9. doi: 10.1016/j.imlet.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Meltzer M, Franklin EC. Cryoglobulinemia – a study of twenty- nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966;40(6):828–836. doi: 10.1016/0002-9343(66)90199-9. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer M, Franklin EC, Elias K, McCluskey RT, Cooper N. Cryoglobulinemia – a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40(6):837–856. doi: 10.1016/0002-9343(66)90200-2. [DOI] [PubMed] [Google Scholar]
- 41.Koike K, Moriya K, Ishibashi K, et al. Sialadenitis histologicly resembling Sjogren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc Natl Acad Sci U S A. 1997;94(1):233–236. doi: 10.1073/pnas.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott CA, Avellini C, Desinan L, et al. Chronic lymphocytic sialoadenitis in HCV-related chronic liver disease: comparison of Sjogren’s syndrome. Histopathology. 1997;30(1):41–48. doi: 10.1046/j.1365-2559.1997.d01-561.x. [DOI] [PubMed] [Google Scholar]
- 43.Vitali C. Immunopathologic differences of Sjogren’s syndrome versus sicca syndrome in HCV and HIV infection. Arthritis Res Ther. 2011;13(4):233. doi: 10.1186/ar3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramos-Casals M, Garcia-Carrasco M, Cervera R, et al. Hepatitis C virus infection mimicking primary Sjogren syndrome. A clinical and immunologic description of 35 cases. Medicine (Baltimore) 2001;80(1):1–8. doi: 10.1097/00005792-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Ramos-Casals M, De Vita S, Tzioufas AG. Hepatitis C virus, Sjogren’s syndrome and B-cell lymphoma: linking infection, autoimmunity and cancer. Autoimmun Rev. 2005;4(1):8–15. doi: 10.1016/j.autrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto S, Nakajima S, Nakamura K, et al. Interferon treatment on glomerulonephritis associated with hepatitis C virus. Pediatr Nephrol. 2000;15(3/4):271–273. doi: 10.1007/s004670000467. [DOI] [PubMed] [Google Scholar]
- 47.Romas E, Power DA, Machet D, Powell H, d’Apice AJ. Membranous glomerulonephritis associated with hepatitis C virus infection in an adolescent. Pathology. 1994;26(4):399–402. doi: 10.1080/00313029400169052. [DOI] [PubMed] [Google Scholar]
- 48.Nagao Y, Sata M, Tanikawa K, Itoh K, Kameyama T. Lichen planus and hepatitis C virus in the northern Kyushu region of Japan. Eur J Clin Invest. 1995;25(12):910–914. doi: 10.1111/j.1365-2362.1995.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 49.Bagan JV, Ramon C, Gonzalez L, et al. Preliminary investigation of the association of oral lichen planus and hepatitis C. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(5):532–536. doi: 10.1016/s1079-2104(98)90286-4. [DOI] [PubMed] [Google Scholar]
- 50.Mignogna MD, Lo Muzio L, Favia G, Mignogna RE, Carbone R, Bucci E. Oral lichen planus and HCV infection: a clinical evaluation of 263 cases. Int J Dermatol. 1998;37(8):575–578. doi: 10.1046/j.1365-4362.1998.00510.x. [DOI] [PubMed] [Google Scholar]
- 51.Stubgen JP. Immune-mediated myelitis associated with hepatitis virus infections. J Neuroimmunol. 2011;239(1/2):21–27. doi: 10.1016/j.jneuroim.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Sim JE, Lee JB, Cho YN, Suh SH, Kim JK, Lee KY. A case of acute disseminated encephalomyelitis associated with hepatitis C virus infection. Yonsei Med J. 2012;53(4):856–858. doi: 10.3349/ymj.2012.53.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stubgen JP. Neuromuscular diseases associated with chronic hepatitis C virus infection. J Clin Neuromuscul Dis. 2011;13(1):14–25. doi: 10.1097/CND.0b013e3181d4a527. [DOI] [PubMed] [Google Scholar]
- 54.Hemmati I, Yoshida E, Shojania K. Relapsing polychondritis associated with hepatitis C virus infection. Clin Rheumatol. 2012;31(2):391–394. doi: 10.1007/s10067-011-1881-4. [DOI] [PubMed] [Google Scholar]
- 55.Dimitroulis D, Valsami S, Stamopoulos P, Kouraklis G. Immunologic HCV-associated thrombocytopenia: short review. Clin Dev Immunol. 2012;2012:378653. doi: 10.1155/2012/378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aref S, Ibrahem L, El Menshawy N, Bassam A. Effect of splenectomy on platelets associated antibodies in hepatitis C patients with thrombocytopenia. Hematology. 2012;17(2):118–121. doi: 10.1179/102453312X13221316477705. [DOI] [PubMed] [Google Scholar]
- 57.Olariu M, Olariu C, Olteanu D. Thrombocytopenia in chronic hepatitis C. J Gastrointestin Liver Dis. 2010;19(4):381–385. [PubMed] [Google Scholar]