Abstract
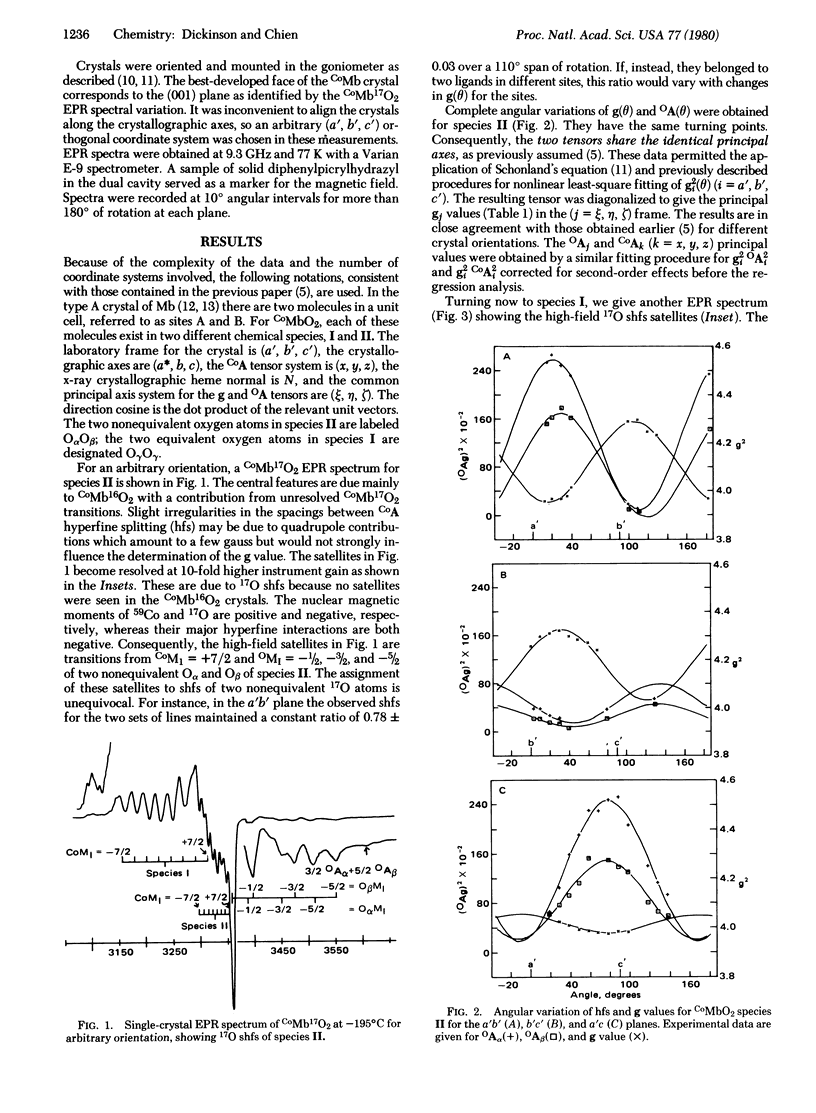
An electron paramagnetic resonance crystallographic study was made on oxycobaltomyoglobin with the dioxygen ligand enriched to 19.1% in 17O. There are two spectroscopically distinct cobalt dioxygen species. The less abundant species, II (40%), has nonequivalent oxygen atoms with superhyperfine tensors OAα = (5, -67.5, 22.4)G and OAβ = (5.4, -83.3, 30.3)G. Together with the previously reported 59Co hyperfine tensor [Chien, J. C. W. & Dickinson, L. C. (1972) Proc. Natl. Acad. Sci. USA 69, 2783-2787], the orbital spin densities are found to be Oα(pη) = 0.48, Oα(pζ) = -0.11, Oβ(pη) = 0.74, Oβ(pζ) = -0.16, Co(dxz) = -0.01, Co(dyz) = 0.06 for a total electron density of 1.01. The O—O axis is directed toward His-E7, suggesting a possible hydrogen bonding interaction which may contribute to the nonequivalency of the oxygen atoms; its projection approximately bisects N1—Fe—N2. The z axis of the CoA tensor is tilted at an angle of 28° from the heme normal, resulting in a Co—O—O angle of 120°. The more abundant species, I (60%), has equivalent oxygen atoms with OAγ = (12, -72.5, 20)G and orbital spin densities of Oγ(pη) = 0.54, Oγ(pζ) = -0.05, Co(dxz) = -0.02, Co(dyz) = 0.09 for a total spin density of 1.10. Although the direction cosines for this molecule cannot be precisely determined, the projection of its O—O axis approximately bisects N2—Fe—N3 and is parallel to the imidazole ring of His-F8. Increase of temperature changes g, CoA, and OA values, with the largest effect seen with OA. This temperature dependence indicates averaging of the two bond structures which are stabilized at 77 K.
Keywords: sperm whale myoglobin, metal-replaced enzymes, 17O and 59Co hyperfine tensors
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chien J. C., Dickinson L. C. Electron paramagnetic resonance of single crystal oxycobaltmyoglobin and deoxycobaltmyoglobin. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2783–2787. doi: 10.1073/pnas.69.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien J. C. Electron paramagnetic resonance crystallography of spin-labeled hemoglobin-protein fine structures. J Mol Biol. 1979 Sep 25;133(3):385–398. doi: 10.1016/0022-2836(79)90399-1. [DOI] [PubMed] [Google Scholar]
- Chien J. C. Electron paramagnetic resonance study of the stereochemistry of nitrosylhemoglobin. J Chem Phys. 1969 Nov 15;51(10):4220–4227. doi: 10.1063/1.1671782. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Gagne R. R., Reed C. A., Robinson W. T., Rodley G. A. Structure of an iron(II) dioxygen complex; a model for oxygen carrying hemeproteins. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1326–1329. doi: 10.1073/pnas.71.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson L. C., Chien C. W. Comparative biological chemistry of cobalt hemoglobin. J Biol Chem. 1973 Jul 25;248(14):5005–5011. [PubMed] [Google Scholar]
- Dickinson L. C., Chien J. C. An electron paramagnetic resonance study of nitrosylmyoglobin. J Am Chem Soc. 1971 Oct 6;93(20):5036–5040. doi: 10.1021/ja00749a011. [DOI] [PubMed] [Google Scholar]
- Dickinson L. C., Chien J. C. Crystallization of reconstituted sperm whale myoglobins. Nat New Biol. 1971 Nov 24;234(47):107–107. doi: 10.1038/newbio234107a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Mildvan A. S., Yonetani T., Srivastava T. S. EPR study of 17O nuclear hyperfine interaction in cobalt-oxyhemoglobin: conformation of bound oxygen. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1005–1012. doi: 10.1016/0006-291x(75)90774-3. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Petering D. H. Coboglobins: oxygen-carrying cobalt-reconstituted hemoglobin and myoglobin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):637–643. doi: 10.1073/pnas.67.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt W. R. Stereochemistry of low-spin cobalt porphyrins. 3. The crystal structure and molecular stereochemistry of bis(piperidine)-alpha, beta, gamma, delta-tetraphenylporphinatocobalt(II). J Am Chem Soc. 1974 Jan 9;96(1):84–89. doi: 10.1021/ja00808a013. [DOI] [PubMed] [Google Scholar]
- Snyder F. W., Jr, Chien C. W. Cobalt hemoglobin: pH dependence of Adair constants and the Bohr effects. Eur J Biochem. 1978 Nov 2;91(1):83–88. doi: 10.1111/j.1432-1033.1978.tb20939.x. [DOI] [PubMed] [Google Scholar]
- Symons M. C., Petersen R. L. Electron capture by oxyhaemoglobin: an e.s.r. study. Proc R Soc Lond B Biol Sci. 1978 May 16;201(1144):285–300. doi: 10.1098/rspb.1978.0046. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Iizuka T. Studies on cobalt myoglobins and hemoglobins. 3. Electron paramagnetic resonance studies of reversible oxygenation of cobalt myoglobins and hemoglobins. J Biol Chem. 1974 Apr 10;249(7):2168–2174. [PubMed] [Google Scholar]


