Abstract
In an effort to isolate novel meiotic mutants that are severely defective in chromosome segregation and/or exchange, we employed a germline clone screen of the X chromosome of Drosophila melanogaster. We screened over 120,000 EMS-mutagenized chromosomes and isolated 19 mutants, which comprised nine complementation groups. Four of these complementation groups mapped to known meiotic genes, including mei-217, mei-218, mei-9, and nod. Importantly, we have identified two novel complementation groups with strong meiotic phenotypes, as assayed by X chromosome nondisjunction. One complementation group is defined by three alleles, and the second novel complementation group is defined by a single allele. All 19 mutants are homozygous viable, fertile, and fully recessive. Of the 9 mutants that have been molecularly characterized, 5 are canonical EMS-induced transitions, and the remaining 4 are transversions. In sum, we have identified two new genes that are defined by novel meiotic mutants, in addition to isolating new alleles of mei-217, mei-218, mei-9, and nod.
Keywords: meiosis, mutant screens, chromosome segregation
A cornerstone of investigating Drosophila female meiosis has been the power and success of genetic screens. In the first EMS mutagenesis screen for meiotic mutants in flies, Baker and Carpenter (1972) screened 209 mutagenized X chromosomes and isolated six strong meiotic mutants, whose further investigation proved to be a critical foundation for studies of the mechanisms of meiotic chromosome segregation and recombination (Hawley 1993). For example, the Baker and Carpenter (1972) screen identified nod, mei-218, mei-9, mei-38, mei-41, and Klp3Amei-352, among other mutants. Since the landmark Baker and Carpenter screen, two additional screens for X-linked meiotic mutants have been performed. Sekelsky et al. (1999) screened 2311 mutagenized X chromosomes using P element mutagenesis and identified an essential meiotic regulator, mei-P26. Most recently, Liu et al. (2000) screened 2106 EMS-mutagenized X chromosomes and identified mei-217 and hdm.
Similarly, traditional screening of either wild populations and/or EMS-mutagenized chromosomes have proved fruitful in their identification of genes such as ord, mei-S332, mei-S282, mei-P22, CycE, mei-W68, mei-S51, and sub (Giunta et al. 2002; Mason 1976; Sandler 1971; Sandler et al. 1968; Sekelsky et al. 1999). Taken together, the characterization of the mutants resulting from these screens has greatly contributed to our current understanding of fundamental processes in Drosophila female meiosis, including recombination, cohesion, achiasmate chromosome segregation, and meiotic spindle organization.
Most recently, Page et al. (2007) advanced the art of genetic screens in Drosophila female meiosis when they performed a germline clone screen to identify autosomal meiotic mutants. This screen created germline clones in females heterozygous for the mutagenized autosome. In this screen, the use of dominant female sterile mutation ovoD1-18 on the un-mutagenized homolog ensured that the only fertile progeny resulted from germline clones homozygous for the EMS-mutagenized chromosome. Meiotic mutants were selected by crossing these females to a compound autosome, thus demanding that the female nondisjoin an autosome in order to have viable progeny.
This strategy has three salient advantages: it is in essence an F1 screen; it is a selection and not a screen for mutants; and it has the ability to isolate lethal mutants. Using this technique, Page et al. (2007) isolated 11 new meiotic mutants on 2L and 3R in a screen of 46,388 EMS-mutagenized chromosomes. Importantly, this screen identified three novel meiotic mutants: cona, mcm5, and trem (Page et al. 2007), whose subsequent characterization has illuminated meiotic chromosome synapsis (cona), a requirement for mcm5 in the resolution of crossovers and the mechanism by which double-strand breaks are initiated in meiosis (trem) (Lake and Hawley 2012; Lake et al. 2007, 2011; Page et al. 2008).
In a continuation of the germline clone approach for meiotic mutant isolation, we sought to identify novel fertile meiotic mutants on the X chromosome with strong effects on either chromosome segregation or recombination. A limitation of this strategy is that the germline clone-bearing female must undergo high levels of nondisjunction and be reasonably fertile. Prior to our screen, the notable meiotic mutations on the X included mei-217, mei-218, mei-9, hdm, mei-P26, mei-41, mei-38, east, Cap, Klp3Amei-352, and nod. As alleles of mei-217, mei-218, mei-9, and hdm are fertile, we anticipated isolating new alleles of these genes. As strong hypomorphic and null alleles of mei-P26, mei-41, Klp3Amei-352, and Cap have greatly reduced fertility or are sterile, we did not anticipate isolating strongly hypomorphic alleles of these genes. Finally, as nod primarily affects the achiasmate chromosome segregation pathway, we did not expect to isolate any alleles of this gene (Carpenter 1973).
Here we describe the isolation of 19 novel meiotic mutants on the X chromosome using the approach pioneered by Page et al. (2007). Among the 19 mutants, we isolated nine complementation groups, of which four correspond to mei-217, mei-218, mei-9, and nod. Three of the unidentified complementation groups isolated demonstrate only weak to moderate levels of meiotic nondisjunction and will not be pursued further. The final two complementation groups represent novel genes. Mutants in both of these complementation groups display strong chromosome nondisjunction phenotypes, and the characterization of these mutants will be described elsewhere.
We molecularly characterized the lesions in mei-217, mei-218, mei-9, and nod and were able to identify mutations in 9 of the 10 mutants within the coding regions. Of these lesions, six were nonsense mutations, and three were missense mutations. Only five of the mutations were traditional EMS-induced transitions. In sum, we have identified two novel meiotic mutants in addition to isolating new alleles of mei-217, mei-218, mei-9, and nod.
Materials and Methods
Drosophila stocks
All stocks were maintained on standard medium containing yeast, cornmeal, corn syrup, malt extract, and agar at 25° with the exception of the stocks containing P{hs-hid}Y, which were maintained at room temperature.
Stocks used in the screen
y1 w1118 FRT19A/P{hs-hid}Y derived from Bloomington 1744 y1 w1118 P{ry[+t7.2]=neoFRT}19A
P{ovoD1-18}P4.1, P{hsp70-flp}1, y1 w1118 sn3 P{neoFRT}19A/ P{hs-hid}Y derived from Bloomington 23880 P{ovoD1-18}P4.1, P{hsp70-flp}1, y1 w1118 sn3 P{neoFRT}19A/C(1)DX, y1 w1 f1
C(2)EN, b1pr1 (Bloomington 1112)
C(1)DX, y1 f1/FM7i /Y (Bloomington 5263)
FM7a/y+Y
X^Y, In(1)EN,v f B; C(4)RM,ci eyR x C(1)RM,y v; C(4)RM, ci eyR
w1118 PBac{WH}mei-217f04441 mei-218f04441 (Bloomington 18770)
y mei-2181 / C(1)DX, y f / y+Y; spapol
Dp(1;1)scV1, y1 mei-2181 car1, y+/C(1)DX, y1 f1 (Bloomington 4914)
y hdmg7/C(1)DX, y f/y+Y; spapol (K. S. McKim)
w1 mei-9A2/C(1)DX, y1 f1 (Bloomington 4280)
y noda/C(1)DX, y1 f1 /y+Y ; spapol
Df(1)BSC719, P+PBac{XP3.WH3}BSC719 w1118/FM7h/Dp(2;Y)G, P{hs-hid}Y(Bloomington 26571; Df(mei-38))
Df(1)BSC537, w1118/FM7h/Dp(2;Y)G, P{hs-hid}Y (Bloomington 25065; Df(mei-P26))
Df(1)BSC760, w1118 P+PBac{XP3.WH3}BSC760/Binsinscy (Bloomington 26857; Df(Cap))
Df(1)ED7364, w1118 P{3′.RS5+3.3′}ED7364/FM7h (Bloomington 9905; Df(mei-41))
Df(1)ED6565, P{3′.RS5+3.3′}ED6565 w1118/FM7h (Bloomington 9299; Df(east))
Df(1)ED411, P{3′.RS5+3.3′}ED411 w1118/FM7j, B1 (Bloomington 8031; Df(Klp3Amei-352))
Determination of lethal hit rate
To determine the lethal hit rate induced by 35 mM EMS mutagenesis in females, 200 vials of the following cross were analyzed for the presence of Bar and non-Bar male progeny: y1 w1118P{ry[+t7.2]=neoFRT}19A**/FM7a; spapol/+ crossed to y1 w1118P{ry[+t7.2]=neoFRT}19A**/y+Y; spapol /+, where asterisks (**) indicate the mutagenized chromosome. Ten of 195 vials had exclusively Bar male progeny and therefore represent a lethal hit on the X chromosome. As 35 mM EMS was used for all rounds of mutagenesis, this represents a 5.1% lethal hit rate in the screen. The 35 mM concentration of EMS was chosen because higher doses of EMS did not result in an increased rate of male lethality in the assay described above.
Germline clone screen genetics
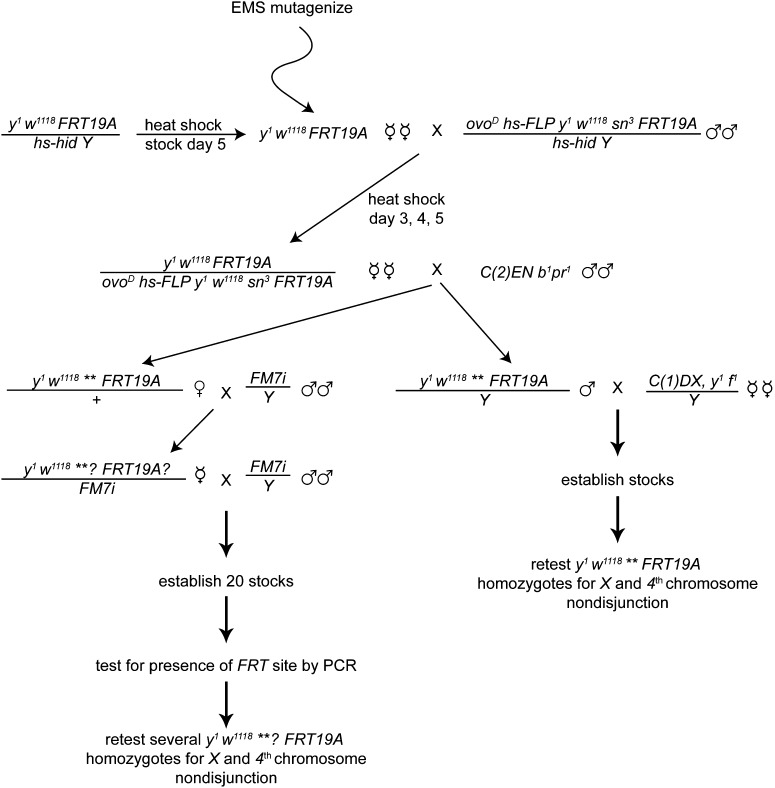
y1 w1118 P{ry[+t7.2]=neoFRT}19A / P{hs-hid}Y stock bottles were heat shocked for one hour at 38° on day 5 after egg laying, and the resulting virgin females were collected for EMS mutagenesis. Mutagenized virgins were mated to P{ovoD1-18}P4.1, P{hsp70-flp}1, y1 w1118 sn3 P{neoFRT}19A/ P{hs-hid}Y males in bottles. The parents were brooded into new bottles at day 3 and allowed to lay for three additional days before bottles were cleared. For both broods, the larvae were heat shocked at days 3, 4, and 5 for one hour at 38° to induce germline clone formation via mitotic recombination and to induce expression of hid. The resulting virgin females containing germline clones of the genotype y1 w1118 P{ry[+t7.2]=neoFRT}19A / P{ovoD1-18}P4.1, P{hsp70-flp}1, y1 w1118 sn3 P{neoFRT}19A were crossed to C(2)EN, b1 pr1 males. The C(2)EN, b1 pr1 crosses were tested with 1, 3, or 10 virgins in a vial, as well as 25 or 30 females in a bottle. In all, six rounds of EMS mutagenesis were performed, and the ideal culture conditions were determined to be 10 virgins in a vial. Vials or bottles were screened initially on day 10 for the presence of pupae. All vials or bottles with zero or one pupa were discarded at day 10. Progeny from the remaining vials were collected until day 18. Males or females that were not bearing C(2)EN b1 pr1 were isolated for stock establishment. Male progeny were genotypically y1 w1118 ** P{ry[+t7.2]=neoFRT}19A / Y and were crossed as single males to C(1)DX, y1 f1 /Y virgin females to establish stocks. The mutant is indicated by (**). Female progeny were y1w1118 ** P{ry[+t7.2]=neoFRT}19A / + and were mated singly with FM7i / Y males. From this cross, y1 w1118 **? P{ry[+t7.2]=neoFRT}19A? / FM7i virgin females were again crossed to FM7i / Y males for stock establishment. The “?” indicates unknown presence of the mutant (**) and the FRT site in the line. Once stocks were established, females were tested for the presence of the FRT site by PCR. Homozygous y1 w1118 **? P{ry[+t7.2]=neoFRT}19A mutant chromosomes were then retested for X and 4th chromosome nondisjunction. Similarly, male stocks were made homozygous (y1 w1118 ** P{ry[+t7.2]=neoFRT}19A) and were then retested for X and 4th chromosome nondisjunction. Of the stocks that were initially isolated from females, all were able to be maintained in the male as y1 w1118 mutant P{ry[+t7.2]=neoFRT}19A / C(1)DX, y1 f1 /Y stocks.
EMS mutagenesis
Virgin females (75 females per bottle, 20 bottles) were starved for six hours in bottles lacking medium. While females were starving, empty bottles with four Whatman #3 circular filter papers were securely taped to the bottom of empty 8 oz round-bottom fly bottles. Three milliliters of 35 mM EMS in 3% sucrose was pipetted into each bottle containing the Whatman filter paper. EMS was allowed to absorb fully before adding starved virgins at a density of 75 virgins/bottle. Flies were allowed to ingest EMS for 24 hr, and then the flies were transferred to bottles containing normal fly food medium for 24 hr. Next, 100 males were transferred into fresh food bottles, and the mutagenized females were added to these bottles. Progeny were reared at 25°.
Heat-shock procedure
Heat-shock treatment of bottles was performed as previously published (Page et al. 2007) for round 1 of the screen, but for rounds 2–6, the heat shock was done on days three, four, and five, as it was experimentally determined that heat shocking larvae on these three consecutive days yielded the maximal number of germline clone-containing progeny, as assayed by sn3 mosacism in the y1 w1118 P{ry[+t7.2]=neoFRT}19A / P{ovoD1-18}P4.1, P{hsp70-flp}1, y1 w1118 sn3 P{neoFRT}19A females.
Screening for FRT19A in mutants recovered from females
Single fly squashes were performed on aged females according to Gloor et al. (1993), and the resulting DNA was assayed for the presence of the FRT site using primers 5′cgcagatataggtgcgacgtg3′ and 5′gccgtatgggtctacttgacag3′, which yielded a PCR product of 403 bp when the FRT site was present.
Complementation testing and assays for chromosome nondisjunction
Complementation was assayed within the 19 mutants by crossing transheterozygote virgins to X^Y, In(1)EN,v f B; C(4)RM,ci eyR males and assaying X and 4th chromosome nondisjunction by methods reported previously (Hawley et al. 1993; Zitron and Hawley 1989). All of the mutations isolated are fully recessive.
For complementation testing of the mutants against known meiotic mutants on the X chromosome, either deficiency stocks or mutant alleles for mei-217, mei-218, mei-38, east, Klp3Amei-352, mei-P26, mei-41, Cap, mei-9, and nod were used. Due to the inconsistent levels of nondisjunction observed in homozygotes of the three weakest complementation groups, we were unable to determine whether any of these three groups represent new alleles of hdm. Transheterozygotes were tested for complementation by crossing to y sc cv v f car / B[S]Y males; spapol males and assaying X chromosome nondisjunction (Matsubayashi and Yamamoto 2003; Zimmering 1976).
Sequencing
DNA was isolated from a single aged male according to Gloor et al. (1993). Sequencing primers for mei-217, mei-218, mei-9, and nod are available upon request.
Metaphase I oocytes preparations and microscopy
DAPI-only preparations of metaphase I oocytes and microscopy was performed as previously described (Gilliland et al. 2009).
Saturation calculations
The number of alleles per mutable locus (m) is calculated as the number of alleles divided by the number of loci. Percentage saturation is calculated as 100 (1 − e−m) (Laurencon et al. 2004).
Results and Discussion
To identify novel fertile meiotic mutants on the X chromosome of Drosophila melanogaster, we undertook a large-scale screen employing a FLP-FRT–mediated germline clone strategy that is analogous to the strategy used in screens for meiotic mutants on 2L and 3R by the Hawley Laboratory (Page et al. 2007). While at least three screens for meiotic mutants on the X chromosome have been performed, we suspected that additional fertile meiotic mutants were yet to be discovered, as only 4626 mutagenized X chromosomes had been screened (Baker and Carpenter 1972; Liu et al. 2000; Sekelsky et al. 1999). Similar to the screens performed by Page and colleagues, we utilized a dominant female sterile mutation ovoD (P{ovoD1-18}P4.1) in combination with the creation of germline clones such that the only fertile offspring following germline clone induction are due to FLP-induced recombinants that lack ovoD and are therefore homozygous for the y1 w1118 P{ry[+t7.2]=neoFRT}19A chromosome.
We performed EMS mutagenesis on y1 w1118 P{ry[+t7.2]=neoFRT}19A females and then crossed them to P{ovoD1-18}P4.1, P{hsp70-flp}1, y1 w1118 sn3 P{neoFRT}19A/P{hs-hid}Y males (Figure 1). We then crossed the resulting germline clone containing female progeny of the genotype y1 w1118 P{ry[+t7.2]=neoFRT}19A/ P{ovoD1-18}P4.1, P{hsp70-flp}1, y1 w1118 sn3 P{neoFRT}19A to males bearing a compound second chromosome, C(2)EN b1 pr1 (Figure 1).
Figure 1 .
Schematic representing the cross schemes used in the screen to isolate new meiotic mutants on the X chromosome. Specifics of the screen are detailed in Materials and Methods.
This cross selects for meiotic mutants because progeny only arise following nondisjunction of the second chromosome in the female [see Page et al. (2007)] (Figure 1A). Nondisjunctional progeny arise from the combination of an X; diplo 2 egg and a Y; nullo 2 sperm or from an X; diplo 2 egg and an X; nullo 2 sperm. Although C(2)EN b1 pr1 progeny will also arise, these were discarded because the presence of the compound autosome prohibited further analysis of the mutant. Mutant stocks were preferentially established from males by crossing a single male to C(1)DX, y1 f1/Y females (Figure 1). When available, males were chosen for the stock establishment because establishing stocks from a female required an extra generation and a PCR screening step to identify stocks with the FRT site (Figure 1).
In six rounds of EMS mutagenesis, 121,048 X chromosomes were screened. Any vial or bottle with more than one pupa was kept for the chromosome recovery step (see Materials and Methods). In all, 77 putative mutants were isolated, with the majority (63.6%) being from vials with two or three progeny. All 77 stocks were retested for X and 4th chromosome nondisjunction by crossing homozygous mutant females to X^Y, In(1)EN,v f B; C(4)RM,ci eyR males. Of the 77 stocks, 19 mutants (24.7%) showed elevated levels of X and/or 4th chromosome nondisjunction, and all are homozygous viable (Table 1 and data not shown). Surprisingly, the number of exceptions per vial was not a strong indicator of whether the vial contained a meiotic mutant (data not shown).
Table 1. Nondisjunction frequencies in 19 novel meiotic mutants.
| Gamete Type |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal | Paternal | y w | mei-391 | mei-39166 | mei-39129 | mei-826 | mei-2171330 | mei-218125 | mei-218621 | mei-2181940 | mei-218136 |
| X; 4 | XY; 44 | 366 | 62 | 95 | 121 | 88 | 141 | 52 | 44 | 80 | 78 |
| X; 4 | 0; 44 | 442 | 99 | 135 | 140 | 137 | 222 | 39 | 75 | 110 | 108 |
| X ND | |||||||||||
| 0; 4 | XY; 44 | 1 | 20 | 33 | 22 | 30 | 25 | 14 | 10 | 15 | 17 |
| XX; 4 | 0; 44 | 2 | 32 | 33 | 37 | 45 | 48 | 13 | 15 | 26 | 31 |
| 4 ND | |||||||||||
| X; 0 | XY; 44 | 1 | 21 | 22 | 12 | 22 | 18 | 7 | 8 | 9 | 11 |
| X; 0 | 0; 44 | 3 | 27 | 22 | 28 | 37 | 30 | 1 | 6 | 26 | 8 |
| X; 44 | XY; 0 | 2 | 8 | 11 | 24 | 18 | 18 | 11 | 14 | 11 | 6 |
| X; 44 | 0; 0 | 0 | 13 | 16 | 18 | 22 | 20 | 2 | 8 | 12 | 10 |
| X; 4 ND | |||||||||||
| 0; 0 | XY; 44 | 0 | 17 | 45 | 30 | 14 | 15 | 6 | 2 | 22 | 17 |
| XX; 44 | 0; 0 | 0 | 8 | 18 | 11 | 16 | 11 | 2 | 2 | 5 | 14 |
| 0; 44 | XY; 0 | 0 | 3 | 0 | 1 | 4 | 2 | 5 | 0 | 0 | 3 |
| XX; 0 | 0; 44 | 0 | 4 | 8 | 6 | 4 | 6 | 0 | 1 | 7 | 4 |
| Total progeny | 808 | 314 | 438 | 450 | 437 | 556 | 152 | 185 | 323 | 307 | |
| Adjusted total | 820 | 398 | 575 | 557 | 550 | 663 | 192 | 215 | 398 | 393 | |
| % X nondisjunction | 0.7 | 42.2 | 47.7 | 38.4 | 41.1 | 32.3 | 41.7 | 27.9 | 37.7 | 43.8 | |
| % 4 nondisjunction | 0.7 | 33.4 | 37 | 32 | 31.8 | 23.2 | 24.5 | 21.4 | 31.7 | 28.2 | |
| % nullo-X | 0.24 | 20.1 | 27.1 | 19.0 | 17.5 | 12.7 | 26.0 | 11.2 | 18.6 | 18.8 | |
| % diplo-X | 0.49 | 22.1 | 20.5 | 19.4 | 23.6 | 19.6 | 15.6 | 16.7 | 19.1 | 24.9 | |
| % nullo-4 | 0.49 | 22.6 | 26.1 | 20.1 | 17.3 | 13.6 | 10.4 | 9.3 | 23.4 | 15.5 | |
| % diplo-4 | 0.24 | 10.8 | 11.0 | 11.8 | 14.5 | 9.7 | 14.1 | 12.1 | 8.3 | 12.7 | |
| Gamete Type |
|||||||||||
| Maternal | Paternal | mei-218646 | mei-2181057 | mei-9140 | mei-9357 | nod143 | mei-105 | mei-175 | mei-86 | mei-114 | mei-889 |
| X; 4 | XY; 44 | 129 | 145 | 101 | 175 | 89 | 170 | 357 | 476 | 32 | 255 |
| X; 4 | 0; 44 | 173 | 161 | 106 | 229 | 104 | 257 | 558 | 645 | 90 | 324 |
| X ND | |||||||||||
| 0; 4 | XY; 44 | 26 | 31 | 10 | 12 | 6 | 16 | 7 | 28 | 4 | 5 |
| XX; 4 | 0; 44 | 42 | 40 | 14 | 36 | 3 | 24 | 3 | 6 | 3 | 4 |
| 4 ND | |||||||||||
| X; 0 | XY; 44 | 11 | 18 | 9 | 13 | 355 | 14 | 17 | 14 | 2 | 20 |
| X; 0 | 0; 44 | 19 | 23 | 13 | 17 | 555 | 11 | 20 | 24 | 8 | 30 |
| X; 44 | XY; 0 | 23 | 12 | 13 | 23 | 15 | 11 | 11 | 24 | 7 | 28 |
| X; 44 | 0; 0 | 18 | 13 | 9 | 28 | 18 | 15 | 13 | 21 | 13 | 17 |
| X; 4 ND | |||||||||||
| 0; 0 | XY; 44 | 19 | 21 | 5 | 3 | 12 | 10 | 2 | 0 | 0 | 0 |
| XX; 44 | 0; 0 | 12 | 10 | 5 | 10 | 0 | 5 | 0 | 0 | 0 | 0 |
| 0; 44 | XY; 0 | 3 | 5 | 3 | 5 | 0 | 1 | 0 | 2 | 0 | 2 |
| XX; 0 | 0; 44 | 10 | 9 | 3 | 2 | 7 | 3 | 0 | 6 | 1 | 0 |
| Total progeny | 485 | 488 | 291 | 553 | 1164 | 537 | 988 | 1246 | 160 | 685 | |
| Adjusted total | 597 | 604 | 331 | 621 | 1192 | 596 | 1000 | 1288 | 168 | 696 | |
| % X nondisjunction | 37.5 | 38.4 | 24.2 | 21.9 | 4.7 | 19.8 | 2.4 | 6.5 | 9.5 | 3.2 | |
| % 4 nondisjunction | 26.6 | 25.8 | 23 | 19.5 | 82.3 | 14.9 | 6.5 | 7.7 | 19 | 14.2 | |
| % nullo-X | 16.1 | 18.9 | 10.9 | 6.4 | 3.0 | 9.1 | 1.8 | 4.7 | 4.8 | 2.0 | |
| % diplo-X | 21.4 | 19.5 | 13.3 | 15.5 | 1.7 | 10.7 | 0.6 | 1.9 | 4.8 | 1.1 | |
| % nullo-4 | 14.7 | 16.7 | 11.5 | 6.4 | 79.5 | 8.6 | 4.1 | 3.9 | 7.1 | 7.2 | |
| % diplo-4 | 11.9 | 9.1 | 11.5 | 13.0 | 2.8 | 6.4 | 2.4 | 3.8 | 11.9 | 7.0 | |
All mutants are homozygous viable and were tested as y1 w1118** P{ry[+t7.2]=neoFRT}19A ; spapol homozygote females crossed to X^Y, In(1)EN,v f B; C(4)RM,ci eyR males to assay X and 4th chromosome nondisjunction (where ** indicates the mutation). In all cases, 25 females were analyzed.
We placed the mutants into complementation groups by testing transheterozygotes for X chromosome nondisjunction. We found nine complementation groups (data not shown), of which four had multiple alleles. Next, we tested representative members of each complementation group against known meiotic mutants on the X chromosome using deficiency stocks and/or mutant alleles of mei-217, mei-218, east, mei-P26, mei-41, Cap, mei-9, mei-38, Klp3Amei-352, and nod (data not shown). We found that the largest complementation group represents six new alleles of mei-218 (which we name mei-218125, mei-218136, mei-218621, mei-2181057, mei-218646, and mei-2181940). In addition, another complementation group identified two novel alleles of mei-9 (mei-9357 and mei-9140). This analysis also identified mutant line 143 as an allele of nod (nod143) and mutant line 1330 as an allele of mei-217 (mei-2171330).
Strikingly, the two complementation groups with the strongest nondisjunction phenotype complemented all meiotic mutants tested and therefore represent two novel complementation groups. The first group is composed of three alleles, and we have preliminarily named mutant line 39 (mei-391), mutant line 129 (mei-39129), and mutant line 166 (mei-39166). The second novel complementation group is composed of a single allele preliminarily named mei-826.
Complementation analysis of one of the groups (mei-114, mei-86, and mei-175) was not completed, as these homozygotes did not have a reproducible nondisjunction phenotype in this assay. Therefore, we were unable to ascertain whether mei-114, mei-889, and mei-105 are alleles of known meiotic genes. However, it was clear that mutant lines mei-114, mei-889, and mei-105 failed to complement one another in all combinations.
One meiotic mutant that was not included in the initial complementation testing is hdm, as all strong complementation groups were accounted for by mei-217, mei-218, mei-9, and nod, and the two novel complementation groups that show the highest level of nondisjunction do not map to hdm (data not shown). It is possible that one of the weak complementation groups (represented by mei-114, mei-889, and mei-105) are allelic to hdm; however, results were inconclusive due to low levels of nondisjunction in hdm homozygotes and due to variable nondisjunction frequencies in the mei-114, mei-889, and mei-105 homozygotes. Characterization of the two novel complementation groups (represented by mei-391 and mei-826) will be described in subsequent articles.
To accurately assay 4th chromosome nondisjunction in our mutant stocks, we crossed y1 w1118 ** P{ry[+t7.2]=neoFRT}19A; spapol females to X^Y, In(1)EN,v f B; C(4)RM,ci eyR males and scored all resulting progeny (Table 1). Mutants within a complementation group exhibited similar frequencies of nondisjunction.
Eleven mutants demonstrated X chromosome nondisjunction at 25% or above. Among these 11 mutants, 5 showed 40% or greater X chromosome nondisjunction (mei-391, mei-39166, mei-826, mei-218125, and mei-218136) and 6 showed X chromosome nondisjunction between 25 and 40% (mei-2171330, mei-218621, mei-2181940, mei-218646, mei-2181057, and mei-39129). The remaining 8 mutant stocks exhibited weaker X chromosome nondisjunction phenotypes, ranging between 2.4 and 24.2% (mei-9140, mei-9357, nod143, and mei-105, mei-175, mei-86, mei-114, and mei-889). All mutants displayed an approximately equal ratio of nullo-X to diplo-X eggs, although mutant lines mei-391, mei-39166, mei-39129, and mei-2181940 yielded about twice as many nullo-4 eggs to diplo-4 eggs, potentially indicating a 4th chromosome loss phenotype (Table 1).
To molecularly characterize the lesions in mei-217, mei-218, mei-9, and nod, we sequenced the exons of all alleles in the respective complementation groups and compared them with the parental y1 w1118 P{ry[+t7.2]=neoFRT}19A sequence. In 9 of 10 mutants sequenced, we identified lesions in the coding sequence (Table 2). In 6 of 10 cases, the mutation encoded a nonsense mutation, whereas three cases were missense mutations. We were unable to identify any mutations in the exons of mei-2181057. This may be due to the fact that we did not obtain high-quality sequence reads for a small region in exons 6 and 7. Alternatively, the mutation in mei-2181057 may lie in the regulatory regions.
Table 2. Mutations identified in novel meiotic mutants.
| Allele | Mutation | Canonical | Amino Acid Change |
|---|---|---|---|
| mei-2171330 | A to T | No | K111 ter |
| mei-218125 | C to T | Yes | Q339 ter |
| mei-218621 | A to T | No | K320 ter |
| mei-218136 | G to A | Yes | W365 ter |
| mei-218646 | C to T | Yes | Q1014 ter |
| mei-2181940 | T to A | No | S845R |
| mei-2181057 | Unknown | Unknown | Unknown |
| mei-9140 | G to A | Yes | G930I |
| mei-9357 | A to T | No | K408 ter |
| nod143 | T to C | Yes | I620T |
Exons of mei-217, mei-218, mei-9, and nod were sequenced for the mutants that failed to complement mei-217, mei-218, mei-9, or nod, respectively. All mutations were identified within coding regions with the exception of mei-2181057, for which no mutation was identified. The mei-2181057 lesion may be in noncoding or regulatory regions. Alternatively, the mutation could be within one of two gaps (176 bp in total) of exon 6 and exon 7 for which we were unable to obtain high quality sequence in mei-2181057.
Of the nine mutants that we molecularly characterized, five were canonical EMS-induced transitions, and four were non-traditional transversions. Unlike the previous germline clone screen for meiotic mutants (Page et al. 2007), we did not obtain mutants due to mobilization of Doc elements. Note that we will make all recovered alleles fully available for at least one year, but we may not maintain the weaker mutants past one year.
Isolation of novel meiotic mutants in a germline clone screen
In our screen of 121,048 mutagenized X chromosomes, all 19 novel meiotic mutants that we recovered are homozygous viable. Because our screen could have identified homozygous lethal mutants, it is surprising that we did not create a null mutation in an essential gene that yielded a meiotic phenotype. When we also consider that the Page et al. (2007) germline clone screen found only 2 of 11 mutants to be homozygous lethal, together, both screens isolated only 2 of 30 (6.7%) homozygous lethal mutants. Therefore, neither screen yielded a substantial number of meiotic mutants that were recessive lethal. Perhaps our dearth of homozygous-lethal mutants is related to a serious limitation of the screen: an inability to obtain maternal-effect lethals.
Among the nine complementation groups we identified, four represented previously characterized genes (mei-217, mei-218, mei-9, and nod). It is our hope that the new alleles may help shed light on the understanding of their respective gene functions. For example, Nod is a chromokinesin-like protein that functions in achiasmate (or nonexchange) chromosome segregation. In vitro, Nod can stimulate microtubule assembly and is responsible for the polar ejection force that maintains achiasmate chromosomes on the meiotic spindle (Cui et al. 2005; Theurkauf and Hawley 1992). Indeed, live imaging of nod null oocytes reveals that achiasmate X and 4th chromosomes are rapidly ejected from the spindle shortly following spindle assembly (Hughes et al. 2009; Theurkauf and Hawley 1992). Our novel allele, nod143, has a nondisjunction phenotype that is nearly identical to the null allele noda (Carpenter 1973). Interestingly, the mutation occurs at amino acid 620, which is the same amino acid in which the complex rearrangement in nodb17 begins (Rasooly et al. 1994) (Table 2). Nod143 represents the only allele of nod isolated to date that is a genetic null and a single missense mutation, as noda truncates the last 12 amino acids of the protein, and all other genetically null alleles are the result of complex rearrangements or contain deletions (Rasooly et al. 1994). Because Nod functions primarily to ensure that achiasmate X and 4th chromosomes properly disjoin at meiosis I, we did not anticipate the isolation of an allele of nod in a screen for autosomal nondisjunction mutants. Retrospectively, we note that Carpenter (1973) showed that noda homozygotes exhibit autosomal nondisjunction when crossed to C(2)RM or C(3)RM males. Considering that noda exhibits some autosomal nondisjunction and that nod143 phenocopies noda with respect to X and 4th nondisjunction; this provides an explanation for the unanticipated isolation of an allele of nod in our screen.
Mei-218 is enigmatic in that its protein localization is predominantly cytoplasmic, yet it functions in the resolution of crossovers (Joyce et al. 2012; Manheim et al. 2002). In mei-218 mutants, recombination is reduced to about 10% of wild type, and the crossovers that remain do not show a normal distribution (Carpenter and Sandler 1974; Manheim et al. 2002; McKim et al. 1996). The six alleles of mei-218 isolated here exhibit a slightly stronger meiotic phenotype than do previously reported alleles of mei-218. The strongest mei-218 alleles (McKim et al. 1996) are thought to be genetically null, as the alleles over deficiencies phenocopied the homozygotes.
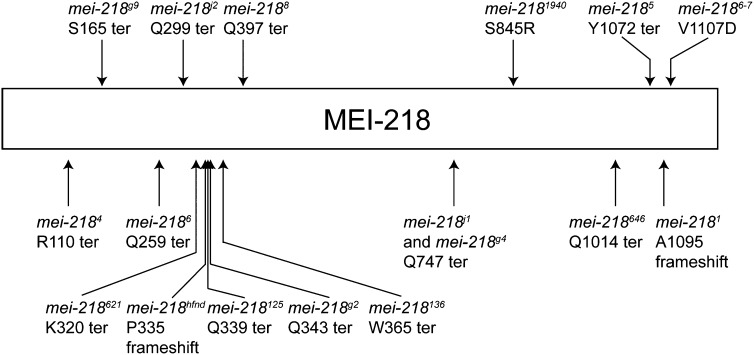
The molecular lesions of the mei-218 alleles that we isolated in addition to other known mei-218 alleles are shown in Figure 2 and Table 2. Notably, the mutations in mei-218621, mei-218hfnd, and mei-218125 are all early terminations or frameshift mutations that occur within 17 amino acids of an 1186 amino acid protein. As mei-218hfnd is thought to be a null, this strongly suggests that mei-218621 and mei-218125 are also nulls that have additional mutations contributing to their stronger meiotic nondisjunction phenotype.
Figure 2 .
Schematic representing mutations of mei-218 alleles. Mutations are shown for the alleles identified in this screen (with the exception of mei-2181057) as well as for the following previously identified alleles: mei-2184, mei-218g9, mei-2186, mei-218j2, mei-218hfnd, mei-218g2, mei-2188, mei-218j1, mei-218g4, mei-2185, mei-2181, and mei-2186-7. The mutation in mei-2187 is not shown because it encodes for a splice acceptor mutation prior to the 4th exon.
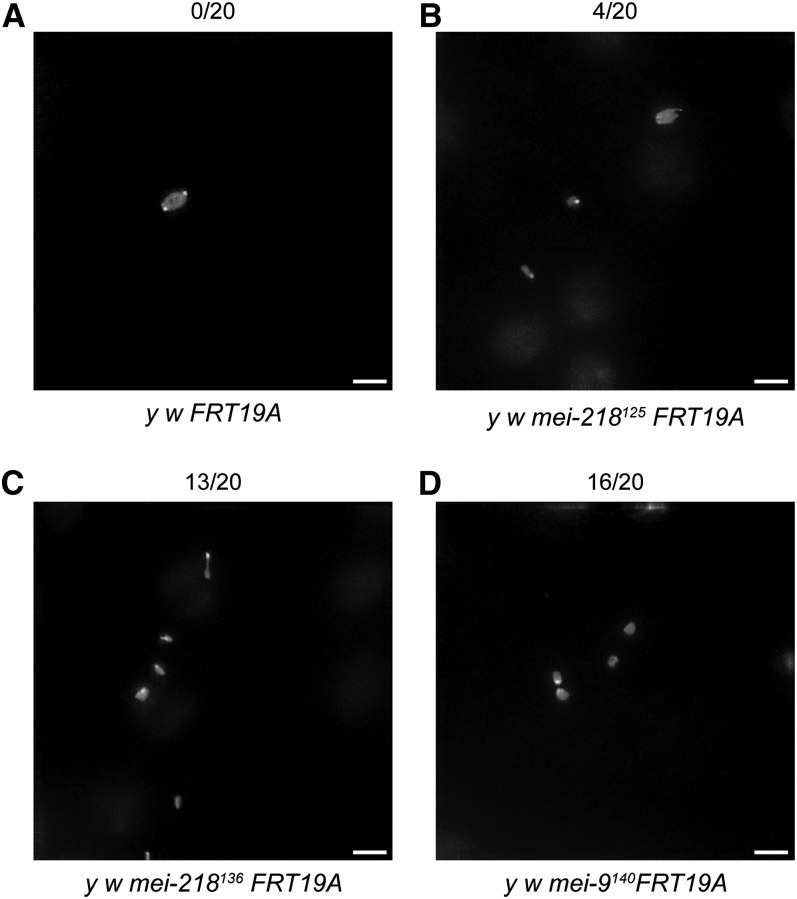
Consistent with a defect in recombination, all of the new mei-218 alleles that we analyzed exhibited multiple chromosome masses at metaphase I (Figure 3 and data not shown). In wild-type oocytes (y1 w1118 P{ry[+t7.2]=neoFRT}19A), none of the 20 had multiple chromosome masses at metaphase I, indicating that chromosome congression was normal (Gilliland et al. 2009). In contrast, multiple chromosome masses were seen in our mei-218 mutants. Multiple chromosome masses at metaphase I were seen in 10 out of 20 mei-218621 oocytes, 11 out of 20 mei-218646 oocytes, 14 out of 20 mei-2181057 oocytes, 13 out of 20 mei-218136 oocytes, and 4 out of 20 mei-218125 oocytes (Figure 3 and data not shown). The control, mei-2181, had 3 out of 20 oocytes with multiple chromosome masses at metaphase I (data not shown). Mei-2181940 was not analyzed.
Figure 3 .
Metaphase I preparations reveal multiple chromosome masses in novel mei-218 and mei-9 mutants. The number of oocytes with multiple chromosome masses is indicated above each representative figure. (A) Metaphase I preparations of y1 w1118 FRT19A oocytes show one chromosome mass, indicating that chromosome congression is complete. (B–D) Metaphase I preparation of y1 w1118 mei-218125 FRT19A (B), y1 w1118 mei-218136 FRT19A (C), and y1 w1118 mei-9140 FRT19A (D) oocytes show multiple chromosome masses, suggestive of a defect in recombination. Scale bar: 5 μ.
Mei-217 is expressed from the same message as mei-218, although they share only one part of one exon in the coding sequence. Intriguingly, mei-217 mutants exhibit a similar recombination-defective phenotype to mei-218 mutants. It will be interesting to localize Mei-217 to determine whether it, too, shows predominantly cytoplasmic localization. Our new allele, mei-2171330, is phenotypically similar to the two previously identified alleles of mei-217, called mei-217r1 and mei-217g10. Mei-217r1 and mei-217g10 have X chromosome nondisjunction rates of 34.4% and 34.5%, respectively (Liu et al. 2000). The mei-217g10 allele is thought to be a null, as mei-217g10/Df (1)815-6 phenocopies mei-217g10 homozygotes. As mei-2171330 has an X nondisjunction frequency of 32.3%, it is likely that mei-2171330 represents another mei-217 null.
Mei-9 is a well-characterized gene that functions in meiotic recombination and nucleotide excision repair (Sekelsky et al. 1995). Our two alleles of mei-9, (mei-9357 and mei-9140) are likely hypomorphic, as they have X chromosome nondisjunction frequencies (21.9 and 24.2%, respectively) that are lower than the most severe mei-9 alleles reported to date. The first allele of mei-9 reported by Baker and Carpenter (1972) had an X chromosome nondisjunction frequency of 27.6%, whereas mei-911/mei-9A2 females nondisjoin X chromosomes 39% of the time (Yildiz et al. 2004). However, we have not yet assayed the mei-9 alleles reported here over a deficiency for mei-9 to distinguish whether our new mei-9 alleles are hypomorphic or null. Similar to other alleles, our novel mei-9 alleles also appear to be defective in recombination, as assayed by metaphase I chromosome preparations (Figure 3D and data not shown). Wild-type oocytes showed none out of 20 with multiple chromosome masses, whereas mei-9357 and mei-9140 oocytes had 13 out of 20 and 16 out of 20 with multiple chromosome masses, respectively. It will be interesting to determine whether mei-9357 and mei-9140 exhibit DNA damage sensitivity, a hallmark of mei-9 mutants.
Have we reached saturation in screening the X chromosome for meiotics?
To determine whether we have now reached saturation in screening the X chromosome for fertile meiotic mutants, we used the traditional Poisson method in which the percentage saturation of a screen is determined by 100 (1 – e−m), where m is defined by the average number of alleles of a given locus. Unfortunately, the Poisson distribution assumes that all genes are equally mutable (Smith et al. 1980). A problem inherent to this analysis is that all genes are not equally mutable: in fact, it is not uncommon for a screen to isolate many alleles of a given complementation group (Laurencon et al. 2004). When m is calculated as the average number of alleles per locus, these “hypermutable” loci lead to an increase in m, distorting the estimate of saturation. However, excluding what appear to be hypermutable loci at the researchers’ discretion can also be problematic.
To determine whether saturation has been reached with respect to screening for meiotic mutants on the X chromosome, we compiled a list of all known meiotic mutants on the X that were isolated from unbiased genetic screens that were fertile enough to survive a nondisjunction assay. Screens for additional alleles of a given gene were excluded from this analysis. To our knowledge, four such screens have been performed: Baker and Carpenter (1972), Liu et al. (2000), Sekelsky et al. (1999), and this article. Table 3 represents a list of all known mutations on the X isolated from these four screens, presented in cytological order on the X.
Table 3. Meiotic mutants on the X chromosome identified by genetic screens.
| Gene | Allele | Publication |
|---|---|---|
| mei-38 | mei-381 | Baker and Carpenter (1972) |
| Klp3A | Klp3A352 | Baker and Carpenter (1972) |
| mei-9 | mei-9a | Baker and Carpenter (1972) |
| mei-9 | mei-9j3 | Liu et al. (2000) |
| mei-9 | mei-9357 | This article |
| mei-9 | mei-9140 | This article |
| hdm | hdmg6 | Liu et al. (2000) |
| hdm | hdmg7 | Liu et al. (2000) |
| hdm | hdmg8 | Liu et al. (2000) |
| mei-P26 | mei-P261 | Sekelsky et al. (1999) |
| nod | noda | Baker and Carpenter (1972) |
| nod | nod143 | This article |
| mei-41 | mei-411 | Baker and Carpenter (1972) |
| mei-217 | mei-217g10 | Liu et al. (2000) |
| mei-217 | mei-217r1 | Liu et al. (2000) |
| mei-217 | mei-2171330 | This article |
| mei-218 | mei-2181 | Baker and Carpenter (1972) |
| mei-218 | mei-2186-7 | Baker and Carpenter (1972) |
| mei-218 | mei-218j1 | Liu et al. (2000) |
| mei-218 | mei-218j2 | Liu et al. (2000) |
| mei-218 | mei-218g1 | Liu et al. (2000) |
| mei-218 | mei-218g4 | Liu et al. (2000) |
| mei-218 | mei-218g9 | Liu et al. (2000) |
| mei-218 | mei-2181057 | This article |
| mei-218 | mei-218646 | This article |
| mei-218 | mei-218136 | This article |
| mei-218 | mei-2181940 | This article |
| mei-218 | mei-218621 | This article |
| mei-218 | mei-218125 | This article |
| mei-39 | mei-391 | This article |
| mei-39 | mei-39129 | This article |
| mei-39 | mei-39166 | This article |
| mei-826 | mei-826 | This article |
Summary of meiotic mutants on the X identified through unbiased genetic screens. Alleles of these genes that were identified through a targeted screen are not included in this table. Mei-2186-7 is an unpublished allele from Baker and Carpenter (1972). Complementation groups are indicated by boxes.
Prior to the screen reported here, we estimated the saturation frequency of the X chromosome for fertile meiotics to be 87.9%; with all four screens taken together, we now estimate the saturation frequency to be 95%, resulting in a net increase of 7.1%. In all cases, m is calculated as the number of alleles divided by the number of loci, and percentage saturation is calculated as 100 (1 – e−m). If mei-218 is excluded from this classical Poisson analysis (as a possible hypermutable locus), the saturation frequencies are 77.7% prior to this screen and 86.5% after this screen, resulting in a net increase of 8.8%. Regardless of whether mei-218 is included, the X chromosome is reaching saturation for fertile meiotic mutants.
An alternative method relies on using a variant of the Poisson distribution in which m is defined based on the proportion of loci with one and two alleles, respectively. This method is ideal in the case where the proportion of loci with one and two alleles is not small, as it does not require the exclusion of data (Laurencon et al. 2004). However, this method is not suited to the screens of the X chromosome under discussion, as the number of genes defined by two alleles is equal to one when the data from all four screens are considered.
The number of fertile meiotics isolated by the Baker and Carpenter (1972) screen of 209 mutagenized chromosomes has never been matched. As shown in Table 4, six essential meiotic genes were identified in the Baker and Carpenter screen, whereas the subsequent Liu et al. (2000) screen isolated two meiotic genes. The Sekelsky et al. (1999) screen identified one critical meiotic regulator on the X chromosome, and the screen reported here identified two novel meiotic genes (represented by mei-39 and mei-826). In total, 125,674 X chromosomes have been screened by these four screens alone (Table 4).
Table 4. X chromosome screens for meiotic mutants.
| Publication | Number of X Chromosomes Screened | Mutagen | Novel Genes |
|---|---|---|---|
| Baker and Carpenter (1972) | 209 | EMS | nod, mei-218, mei-9, mei-38, mei-41, Klp3Amei-352 |
| Liu et al. (2000) | 2,106 | EMS | mei-217, hdm |
| Sekelsky et al. (1999) | 2,311 | P element | mei-P26 |
| This article | 121,048 | EMS | mei-39, mei-826 |
| Total chromosomes | 125,674 |
What mutants did we fail to isolate from the screen?
As the screen required that the germline clone containing females be fertile, one limitation of the screen is that we will not recover mutants that result in a severely reduced fertility phenotype or are sterile. However, we could have recovered weak hypomorphic alleles of genes that have reduced fertility, such as Klp3Amei-352, mei-P26, Cap, and mei-41. One possibility for why we did not isolate such weak hypomorphic alleles is that such mutations did not cause enough autosomal nondisjunction to be picked up in the screen. Additional limitations of our screen are that maternal-effect lethals will not be identified and mutations in genes required for pre-meiotic mitoses may not be identified.
One notable gene for which we did not isolate mutants is mei-38. As mei-38 mutants are very fertile (Baker and Carpenter 1972), we should have been able to isolate mutants in this gene if we were truly screening to saturation. It is possible that one of the complementation groups represented by mutant lines mei-889, mei-114, and mei-105 is allelic to mei-38. However, we were unable to complete these complementation assays due to inconsistencies in the mutant homozygote phenotype.
In conclusion, the germline clone screen described here led to the identification of two novel meiotic mutants, whose characterization will be described shortly in subsequent publications. In addition to the novel complementation groups, new alleles of mei-217, mei-218, mei-9, and nod were isolated. Due to the success of this screen and the previous germline clone screens on 2L and 3R in identifying new meiotic genes (Page et al. 2007), it would be prudent to continue to mine chromosome arms 2R and 3L for meiotic mutants. Indeed, these screens are currently under way.
Acknowledgments
We thank Stacie Hughes, Satomi Takeo, and Rachel Nielsen for valuable input on the screen design. We thank Kim McKim for the hdm stocks. We thank the Bioinformatics Core Facility for discussions on saturation. Finally, we thank all Hawley Laboratory members for their contributions in setting up C(2)EN, b1 pr1 crosses during the screen and comments on the manuscript. K.P.K. acknowledges National Institutes of Health grant GM-061252.
Footnotes
Communicating editor: K. S. McKim
Literature Cited
- Baker B. S., Carpenter A. T. C., 1972. Genetic analysis of sex chromosomal meiotic mutants in Drosophila melanogaster. Genetics 71: 255–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T. C., 1973. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73: 393–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T. C., Sandler L., 1974. On recombination-defective meiotic mutants in Drosophila melanogaster. Genetics 76: 453–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Sproul L. R., Gustafson S. M., Matthies H. J., Gilbert S. P., et al. , 2005. Drosophila Nod protein binds preferentially to the plus ends of microtubules and promotes microtubule polymerization in vitro. Mol. Biol. Cell 16: 5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland W. D., Hughes S. F., Vietti D. R., Hawley R. S., 2009. Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes. Dev. Biol. 325: 122–128 [DOI] [PubMed] [Google Scholar]
- Giunta K. L., Jang J. K., Manheim E. A., Subramanian G., McKim K. S., 2002. subito encodes a kinesin-like protein required for meiotic spindle pole formation in Drosophila melanogaster. Genetics 160: 1489–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor G. B., Preston C. R., Johnson-Schlitz D. M., Nassif N. A., Phillis R. W., et al. , 1993. Type I repressors of P element mobility. Genetics 135: 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. S., 1993. Meiosis as an “M” thing: twenty-five years of meiotic mutants in Drosophila. Genetics 135: 613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. S., Irick H. A., Zitron A. E., Haddox D. A., Lohe A. R., et al. , 1993. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467 [DOI] [PubMed] [Google Scholar]
- Hughes S. E., Gilliland W. D., Cotitta J. L., Takeo S., Collins K. A., et al. , 2009. Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes. PLoS Genet. 5: e1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce E. F., Paul A., Chen K. E., Tanneti N., McKim K. S., 2012. Multiple barriers to non-homologous DNA end joining during meiosis in drosophila. Genetics. 191: 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake C. M., Hawley R. S., 2012. The molecular control of meiotic chromosomal behavior: events in early meiotic prophase in Drosophila oocytes. Annu. Rev. Physiol. 74: 425–451 [DOI] [PubMed] [Google Scholar]
- Lake C. M., Teeter K., Page S. L., Nielsen R., Hawley R. S., 2007. A genetic analysis of the Drosophila mcm5 gene defines a domain specifically required for meiotic recombination. Genetics 176: 2151–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake C. M., Nielsen R. J., Hawley R. S., 2011. The Drosophila zinc finger protein trade embargo is required for double strand break formation in meiosis. PLoS Genet. 7: e1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurencon A., Orme C. M., Peters H. K., Boulton C. L., Vladar E. K., et al. , 2004. A large-scale screen for mutagen-sensitive loci in Drosophila. Genetics 167: 217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Jang J. K., Graham J., Nycz K., McKim K. S., 2000. Two genes required for meiotic recombination in Drosophila are expressed from a dicistronic message. Genetics 154: 1735–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manheim E. A., Jang J. K., Dominic D., McKim K. S., 2002. Cytoplasmic localization and evolutionary conservation of MEI-218, a protein required for meiotic crossing-over in Drosophila. Mol. Biol. Cell 13: 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., 1976. Orientation disruptor (ord): a recombination-defective and disjunction-defective meiotic mutant in Drosophila melanogaster. Genetics 84: 545–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H., Yamamoto M. T., 2003. REC, a new member of the MCM-related protein family, is required for meiotic recombination in Drosophila. Genes Genet. Syst. 78: 363–371 [DOI] [PubMed] [Google Scholar]
- McKim K. S., Dahmus J. B., Hawley R. S., 1996. Cloning of the Drosophila melanogaster meiotic recombination gene mei-218: a genetic and molecular analysis of interval 15E. Genetics 144: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. L., Nielsen R. J., Teeter K., Lake C. M., Ong S., et al. , 2007. A germline clone screen for meiotic mutants in Drosophila melanogaster. Fly (Austin) 1: 172–181 [DOI] [PubMed] [Google Scholar]
- Page S. L., Khetani R. S., Lake C. M., Nielsen R. J., Jeffress J. K., et al. , 2008. Corona is required for higher-order assembly of transverse filaments into full-length synaptonemal complex in Drosophila oocytes. PLoS Genet. 4: e1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasooly R. S., Zhang P., Tibolla A. K., Hawley R. S., 1994. A structure-function analysis of NOD, a kinesin-like protein from Drosophila melanogaster. Mol. Gen. Genet. 242: 145–151 [DOI] [PubMed] [Google Scholar]
- Sandler L., 1971. Induction of autosomal meiotic mutants by EMS in D. melanogaster. Drosoph. Inf. Serv. 47: 68 [Google Scholar]
- Sandler L., Lindsley D. L., Nicoletti B., Trippa G., 1968. Mutants affecting meiosis in natural populations of Drosophila melanogaster. Genetics 60: 525–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J. J., McKim K. S., Chin G. M., Hawley R. S., 1995. The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics 141: 619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J. J., McKim K. S., Messina L., French R. L., Hurley W. D., et al. , 1999. Identification of novel Drosophila meiotic genes recovered in a P-element screen. Genetics 152: 529–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Snyder R. D., Dusenbery R. L., 1980. Isolation and characterization of repair-deficient mutants of Drosophila melanogaster. Basic Life Sci. 15: 175–188 [DOI] [PubMed] [Google Scholar]
- Theurkauf W. E., Hawley R. S., 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116: 1167–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz O., Kearney H., Kramer B. C., Sekelsky J. J., 2004. Mutational analysis of the Drosophila DNA repair and recombination gene mei-9. Genetics 167: 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmering S., 1976 Genetic and cytogenetic aspects of altered segregation phenomena in Drosophila, pp. 569–613 in The Genetics and Biology of Drosophila, Vol. 1a, edited by M. Ashburner and E. Novitski. Academic Press, New York. [Google Scholar]
- Zitron A. E., Hawley R. S., 1989. The genetic analysis of distributive segregation in Drosophila melanogaster. I. Isolation and characterization of Aberrant X segregation (Axs), a mutation defective in chromosome partner choice. Genetics 122: 801–821 [DOI] [PMC free article] [PubMed] [Google Scholar]