Abstract
Using isotopic tracer methods, we have shown that dihydrolipoic acid (2,3-thioctic acid) acylates the distal oxygen of ferrous oxygenated Pseudomonas cytochrome P-450, forming a transient acyl peroxide intermediate that facilitates oxygen-oxygen bond cleavage. Single-turnover studies with 18O2 indicate one oxygen-18 atom incorporated into the carboxylate group of lipoic acid for each oxygen-18 inserted into the substrate, camphor, forming the product, exo-5-hydroxycamphor. Such a branching ratio for label indicates that water is initially released from an unlageled position and illustrates that the general P-450 mixed-function oxidase stoichiometry generates H218O from 18O2 only after multiple-turnover equilibration with the acylating carboxylate oxygen. Formation of an acyl peroxide state is a natural intermediate in peracid, "oxene", or radical mechanisms for methylene carbone oxygenation.
Full text
PDF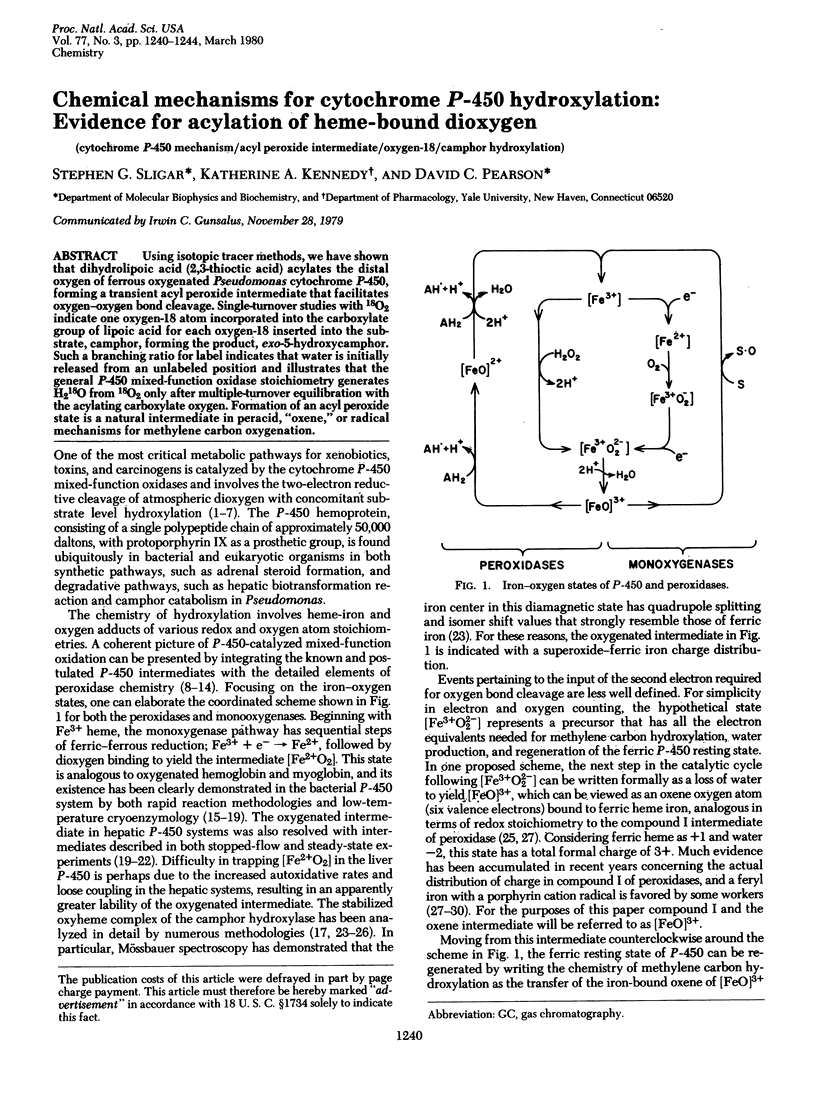

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron J., Hildebrandt A. G., Peterson J. A., Estabrook R. W. The role of oxygenated cytochrome P-450 and of cytochrome b5 in hepatic microsomal drug oxidations. Drug Metab Dispos. 1973 Jan-Feb;1(1):129–138. [PubMed] [Google Scholar]
- Champion P. M., Münck E., Debrunner P. G., Hollenberg P. F., Hager L. P. Mössbauer investigations of chloroperoxidase and its halide complexes. Biochemistry. 1973 Jan 30;12(3):426–435. doi: 10.1021/bi00727a011. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Dolphin D. Letter: Oxygen binding to mercaptide-heme complexes. Models for reduced cytochrome P-450. J Am Chem Soc. 1976 Mar 17;98(6):1607–1609. doi: 10.1021/ja00422a069. [DOI] [PubMed] [Google Scholar]
- Dawson J. H., Cramer S. P. Oxygenated cytochrome P-450cam: evidence against axial histidine ligation of iron. FEBS Lett. 1978 Apr 1;88(1):127–130. doi: 10.1016/0014-5793(78)80623-1. [DOI] [PubMed] [Google Scholar]
- Debey P., Balny C., Douzou P. Enzyme assay in microsomes below zero degrees. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2633–2636. doi: 10.1073/pnas.70.9.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debey P., Balny C., Douzou P. Low temperature studies of microsomal cytochrome P450. Release of oxidizing species. FEBS Lett. 1974 Sep 15;46(1):75–77. doi: 10.1016/0014-5793(74)80338-8. [DOI] [PubMed] [Google Scholar]
- Dolphin D., Forman A., Borg D. C., Fajer J., Felton R. H. Compounds I of catalase and horse radish peroxidase: pi-cation radicals. Proc Natl Acad Sci U S A. 1971 Mar;68(3):614–618. doi: 10.1073/pnas.68.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W., Hildebrandt A. G., Baron J., Netter K. J., Leibman K. A new spectral intermediate associated with cytochrome P-450 function in liver microsomes. Biochem Biophys Res Commun. 1971 Jan 8;42(1):132–139. doi: 10.1016/0006-291x(71)90372-x. [DOI] [PubMed] [Google Scholar]
- Groves J. T., McClusky G. A. Aliphatic hydroxylation by highly purified liver microsomal cytochrome P-450. Evidence for a carbon radical intermediate. Biochem Biophys Res Commun. 1978 Mar 15;81(1):154–160. doi: 10.1016/0006-291x(78)91643-1. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Ballou D. P., Coon M. J. Spectral intermediates in the reaction of oxygen with purified liver microsomal cytochrome P-450. Biochem Biophys Res Commun. 1976 Jun 7;70(3):951–956. doi: 10.1016/0006-291x(76)90684-7. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Pederson T. C., Sligar S. G. Oxygenase-catalyzed biological hydroxylations. Annu Rev Biochem. 1975;44:377–407. doi: 10.1146/annurev.bi.44.070175.002113. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Sligar S. G. Oxygen reduction by the P450 monoxygenase systems. Adv Enzymol Relat Areas Mol Biol. 1978;47:1–44. doi: 10.1002/9780470122921.ch1. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Wagner G. C. Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. Methods Enzymol. 1978;52:166–188. doi: 10.1016/s0076-6879(78)52019-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Hrycay E. G., Ernster L. Sodium periodate, sodium chlorite, and organic hydroperoxides as hydroxylating agents in steroid hydroxylation reactions catalyzed by adrenocortical microsomal and mitochondrial cytochrome P450. Arch Biochem Biophys. 1976 Jun;174(2):440–453. doi: 10.1016/0003-9861(76)90372-6. [DOI] [PubMed] [Google Scholar]
- Hager L. P., Doubek D. L., Silverstein R. M., Hargis J. H., Martin J. C. Chloroperoxidase. IX. The structure of compound I. J Am Chem Soc. 1972 Jun 14;94(12):4364–4366. doi: 10.1021/ja00767a068. [DOI] [PubMed] [Google Scholar]
- Hamilton G. A. Mechanisms of two- and four-electron oxidations catalyzed by some metalloenzymes. Adv Enzymol Relat Areas Mol Biol. 1969;32:55–96. doi: 10.1002/9780470122778.ch3. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., Gustafsson J. A., Ingelman-Sundberg M., Ernster L. Sodium periodate, sodium chloride, organic hydroperoxides, and H2O2 as hydroxylating agents in steroid hydroxylation reactions catalyzed by partially purified cytochrome P-450. Biochem Biophys Res Commun. 1975 Sep 2;66(1):209–216. doi: 10.1016/s0006-291x(75)80315-9. [DOI] [PubMed] [Google Scholar]
- Lichtenberger F., Nastainczyk W., Ullrich V. Cytochrome P450 as an oxene transferase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):939–946. doi: 10.1016/0006-291x(76)90682-3. [DOI] [PubMed] [Google Scholar]
- Lipscomb J. D., Sligar S. G., Namtvedt M. J., Gunsalus I. C. Autooxidation and hydroxylation reactions of oxygenated cytochrome P-450cam. J Biol Chem. 1976 Feb 25;251(4):1116–1124. [PubMed] [Google Scholar]
- Nordblom G. D., White R. E., Coon M. J. Studies on hydroperoxide-dependent substrate hydroxylation by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Ishimura Y., Griffin B. W. Pseudomonas putida cytochrome P-450: characterization of an oxygenated form of the hemoprotein. Arch Biochem Biophys. 1972 Mar;149(1):197–208. doi: 10.1016/0003-9861(72)90315-3. [DOI] [PubMed] [Google Scholar]
- Rahimtula A. D., O'Brien P. J., Hrycay E. G., Peterson J. A., Estabrook R. W. Possible higher valence states of cytochrome P-450 during oxidative reactions. Biochem Biophys Res Commun. 1974 Sep 23;60(2):695–702. doi: 10.1016/0006-291x(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Rahimtula A. D., O'Brien P. J. Hydroperoxide catalyzed liver microsomal aromatic hydroxylation reactions involving cytochrome P-450. Biochem Biophys Res Commun. 1974 Sep 9;60(1):440–447. doi: 10.1016/0006-291x(74)90223-x. [DOI] [PubMed] [Google Scholar]
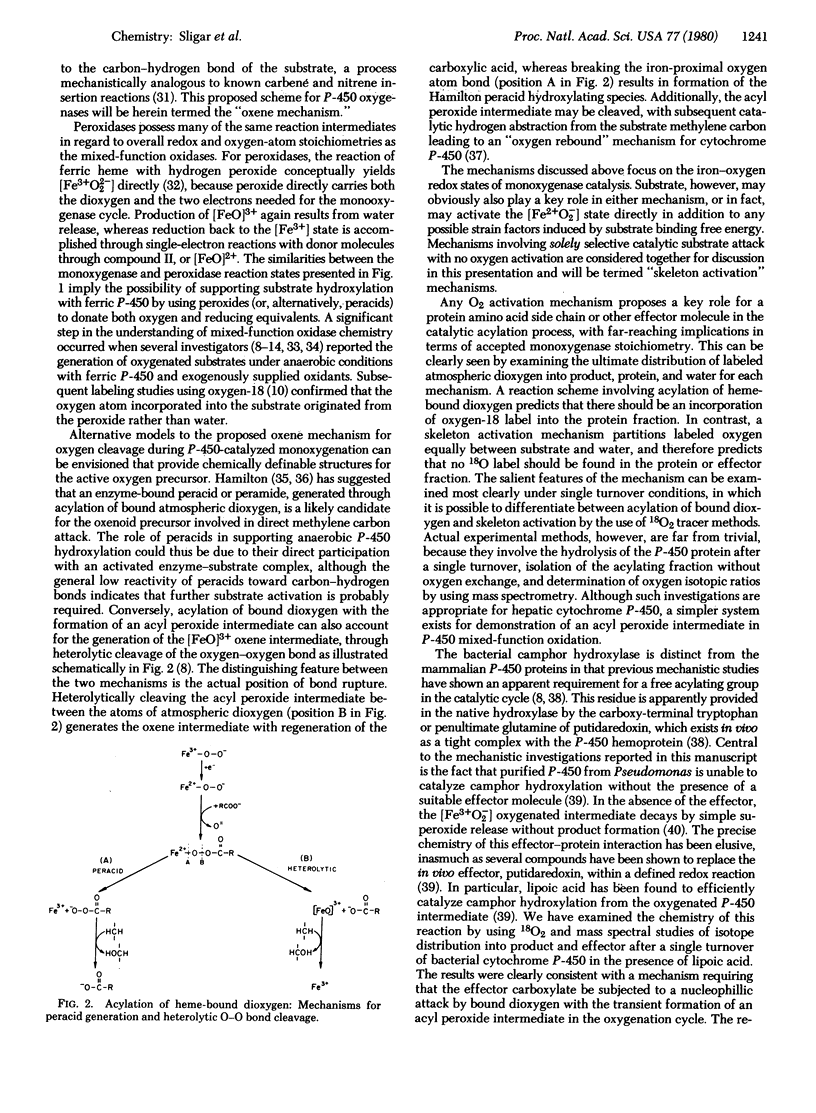
- Schonbaum G. R., Lo S. Interaction of peroxidases with aromatic peracids and alkyl peroxides. Product analysis. J Biol Chem. 1972 May 25;247(10):3353–3360. [PubMed] [Google Scholar]
- Sharrock M., Debrunner P. G., Schulz C., Lipscomb J. D., Marshall V., Gunsalus I. C. Cytochrome P450cam and its complexes. Mössbauer parameters of the heme iron. Biochim Biophys Acta. 1976 Jan 20;420(1):8–26. doi: 10.1016/0005-2795(76)90340-8. [DOI] [PubMed] [Google Scholar]
- Sharrock M., Münck E., Debrunner P. G., Marshall V., Lipscomb J. D., Gunsalus I. C. Mössbauer studies of cytochrome P-450 cam . Biochemistry. 1973 Jan 16;12(2):258–265. doi: 10.1021/bi00726a013. [DOI] [PubMed] [Google Scholar]
- Sligar S. G., Debrunner P. G., Lipscomb J. D., Namtvedt M. J., Gunsalus I. C. A role of the putidaredoxin COOH-terminus in P-450cam (cytochrome m) hydroxylations. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3906–3910. doi: 10.1073/pnas.71.10.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sligar S. G., Lipscomb J. D., Debrunner P. G., Gunsalus I. C. Superoxide anion production by the autoxidation of cytochrome P450cam. Biochem Biophys Res Commun. 1974 Nov 6;61(1):290–296. doi: 10.1016/0006-291x(74)90565-8. [DOI] [PubMed] [Google Scholar]
- Tyson C. A., Lipscomb J. D., Gunsalus I. C. The role of putidaredoxin and P450 cam in methylene hydroxylation. J Biol Chem. 1972 Sep 25;247(18):5777–5784. [PubMed] [Google Scholar]




