Abstract
AIM
To investigate the regulation of Eaf2 protein in mouse lens cells apoptosis induced by ultraviolet (UV) radiation.
METHODS
An eye of Eaf2 gene knockout mice or normal control mice was exposed to UV radiation, and the other one was non-exposed. All of lenses were analyzed by TUNEL and caspase 3 activity assays to determine the difference of the apoptosis induced by UV radiation. In addition, exposed and non-exposed lenses were analyzed by quantified p53 expression and real-time reverse transcription-polymerase chain reaction (RT-PCR) of Bax, Bid, Apaf-1, Puma and Noxa, to compare Eaf2 gene knockout mice and normal control mice.
RESULTS
UV radiation caused apoptosis of lens cells in normal control mice and Eaf2 knockout mice. Activity of caspase 3 was significantly higher in normal control mice than Eaf2 knockout mice. Expression of p53 protein was significantly higher in lenses exposed to UV radiation than nonexposed lenses, but was similar between Eaf2 gene knockout mice and normal control mice in the same UV condition. After exposing to UV radiation, the analysis of real-time RT-PCR demonstrated that mRNA levels of Puma and Noxa were significantly higher in lenses of normal control mice than Eaf2 gene knockout mice, and that mRNA levels of Bax, Bid and Apaf-1 were not significantly different between gene knockout mice and normal control mice.
CONCLUSION
Eaf2 increases lens cells apoptosis induced by ultraviolet radiation. And Eaf2 up-regulates expression of the Puma and the Noxa to act on lens cells apoptosis after UV radiation.
Keywords: Eaf2, p53, apoptosis, Noxa, Puma, ultraviolet rays
INTRODUCTION
Cataract has become a common ophthalmic diseases, and seriously impacts on the life quality of patients in the word. As we all know, ultraviolet (UV) radiation and oxidative stress leads to the formation of cataract[1],[2], therefore many related mechanisms and theories have been proposed. Generally, our lenses have a certain ability of anti-injury, but patients who undergo cataract can not defend UV radiation, which more easily induce apoptosis of lens epithelial cell and may be the cytological basis of cataract formation[3]. UV radiation induces DNA damage, including a basic site and pyrimidine dimmer, and results in interfering with DNA replication and transcription. As a result, DNA damage may either be repaired to continue through the cell cycle, or cause apoptosis to remove abnormal cells and maintain the integrity of the lens structure and function. If a large number of apoptotic cells could not be offset by the proliferation, the inherent physiological function of lens will be disordered, causing a series of pathological changes. Ultraviolet is a part of solar radiation and as an environmental factor it is also inevitable in the daily lives of people. Ultraviolet radiation is closely related to skin tumors and eye disease, therefore researching UV-induced apoptosis of lens cells is likely to provide clues to reveal the cause of cataracts.
ELL-associated factor 2 (Eaf2) is a potential tumor suppressor. It was initially discovered as a binding partner of ELL (polymerase elongation factor, 11-19 lysine rich gene) and an androgen-response gene whose expression is developmentally regulated during embryogenesis[4],[5]. The function of Eaf2 as a potential regulator of transcription has been linked to its interaction with ELL to increase RNA polymerase II elongation activity[6]. In some studies, DNA damage of cells was induced by UV radiation, and as a result Eaf2 which generally diffused in cell nuclear relocated into the nucleoli[7]. It implies that Eaf2 is likely involved in the repair of DNA damage or inducing apoptosis. Over-expression of Eaf2 induces apoptosis in prostate cancer cells lines and also suppresses xenograft tumor growth[5]. In addition, the data reported by Li et al[8], indicates that Eaf2 gene expression is detected in the developing lens, and suggests that Eaf2 may play an important role in regulating lens maturation.
So far, no experiment has described Eaf2 gene expression in the mature mouse lens, but in this present study, we found that in the mature mouse lens Eaf2 gene was transcribed at the mRNA level. It suggests Eaf2 protein may be expressed in the lens cells, and play an important physiological role. Following UV radiation, what role does Eaf2 play in lens cells apoptosis? We are trying to find out the answer in Eaf2 gene knockout mouse model.
MATERIALS AND METHODS
Materials
Gene knockout mice and normal control mice were constructed by the mice with a pure B6(C)-H2-Ab1bm12/KhEgJ background, and genotyping was determined by PCR analysis of mouse tail genomic DNA. The study protocol was approved by the authors' affiliated institution review board and animal experiments were conducted in accordance with the guideline of the local Institution Animal Care and Use Committees.
Methods
Exposure to ultraviolet radiation
An eye of every mouse was exposed to UV radiation, the other one was covered. The animal was exposed for 15 minutes to a total dose of 8kJ/m2 300nm UV-B radiation. UV radiation was generated by a UV lamp (SPECTROLINE XX-15N/F, SPECTRONICS CORPORATION, USA), and the total UV radiation dose at the corneal plane was measured with an UV radiometer (UVX RADIOMETER, UVP, USA). In our study, experimental animals were divided into 4 groups: normal-non UV, normal UV, Eaf2 K.O.-non UV, Eaf2 K.O.-UV.
Preparation of mouse lens tissues
Twenty-four hours after UV radiation, ether-anesthetized mice were sacrificed, and removed the eyes (retaining the optic nerve about 2mm). The lens of some mouse eyes were extracted under the microscope and were washed with phosphate buffer saline (PBS) for next experiments.
TUNEL assay
Whole eyes were fixed in 10% phosphate-buffered formalin at 4°C. Samples were dehydrated through a series of ethanol steps finishing with xylene and finally embedded in paraffin at 60°C. Tissues were sectioned at 5µm and first stained with HE for checking. For TUNEL assay, tissue sections were deparaffinized using xylene and rehydrated in graded ethanol series. Hydrated sections were treated with 0.1% TritonX-100 (Beijing Biosynthesis Biotechnology, Beijing, China), before incubation at 37°C for 8 minutes. Sections were washed twice with PBS, blocked with 3% H2O2, and washed twice with PBS. Sections were incubated at the controlled condition (37°C, moisturizing, and dark) for 60 minutes in the TUNEL reaction mixture which was mixed with Enzyme Solution (Roche, Penzberg, Germany) and Label Solution (Roche). Then sections were washed 3 times with PBS, and incubated at 37°C for 30 minutes in Converter-POD (Roche). Following a series of washes, sections were treated with the diaminobenzidine (DAB) substrate. Sections were counterstained with hematoxylin and coverslips were mounted using neutral balsam. The slides were air dried overnight at room temperature. Photographs were taken under the microscope.
Western blot analysis
The lenses were collected and immediately frozen at -70°C. The tissues were homogenized with lysis buffer containing 50mmol/L Tris-HCl (pH 8.0), 150mmol/L NaCl, 10% glycerol, 1% Triton X-100, 1.5mmol/L MgCl2·6H2O, 1mmol/L EGTA, 1mmol/L PMSF, 1mmol/L Na2VO4 and 100mmol/L NaF, and then ultracentrifuged at 50 000r/min for 1 hour. Protein concentration was determined by the Bradford method. Equal volumes of protein extracts were separated by 10% SDS-PAGE. The proteins were then transferred to a PVDF membrane (Millipore, Billerica, MA, USA) using a Western blotting apparatus. Membranes were blocked with 5% (w/v) non-fat dry milk and 0.1% Tween-20 in PBS, and incubated with anti-p53 (1:2000, Beijing Biosynthesis Biotechnology, Beijing, China) used as primary antibodies or anti-GAPDH (1:10000, Beijing Biosynthesis Biotechnology), overnight at 4°C. HRP-conjugated anti-mouse antibodies for p53 (1:2000, Beijing Biosynthesis Biotechnology) were used as secondary antibodies of p53. Bands detection was performed using an enhanced chemiluminescence (ECL) detection kit (Millipore, Billerica, MA, USA). To compare the relative expressions of proteins, detected bands were calculated densitometrically.
Caspase 3 activity assay
The lenses were homogenized and protein concentration was determined as described previously. Caspase 3 activity was measured using the caspase 3 activity assay kit (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) according to the manufacture's instruction. Results were analyzed with the Spectrophotometer (ELx800, Bio Tek Instruments, USA) at 405nm, and data were normalized to the sample protein concentration.
RNA isolation and real time RT-PCR
Total RNA was isolated from mouse lenses using RNAiso Plus (TaKaRa Biotechnology Co., Ltd., Dalian, China) according the manufacture's instruction, and 2µg of total RNA was reverse-transcribed with the PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa Biotechnology Co., Ltd.) for PCR. Quantitative real-time PCR was carried out in 20µL of reaction mixture containing 10µL of 2×SYBR Premix Ex TaqII, 0.8µL of forward and reverse primers, 0.4µL of Rox Reference Dye II, and 2.0µL template cDNA on ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) under the following conditions (stage 1: 95°C 30 seconds; stage 2: 95°C 5 seconds, 60°C 34 seconds, 40 cycles; 95°C 15 seconds, 60°C 1 minute, 95°C 15 seconds ). The primers were showed on the Table 1. The β-actin mRNA was simultaneously assayed as internal standard for sample normalization. All experiments were performed in triplicate and repeated at least three times. The expression of gene mRNA was calculated using the 2−ΔΔct method as described[9].
Table 1. Primers of real-time RT-PCR.
| Gene | Sequence (5′-3′) |
| p53 | Forward: TCACAGTCGGATATCAGCCT |
| Reverse: ACACTCGGAGGGCTTCACTT | |
| Caspase 3 | Forward: GGGCGTGTTTCTGTTTTGTT |
| Reverse: TTGAGGTAGCTGCACTGTGG | |
| Bax | Forward: GGAGATGAACTGGATAGCAATATGG |
| Reverse: GTTTGCTAGCAAAGTAGAAGAGGGC | |
| Bid | Forward: CCATGTAGGTGGGCTTCTGT |
| Reverse: GATCAGCCATTCGGCTTTTA | |
| Puma | Forward: AGACAAGAAGAGCAGCATCGACAC |
| Reverse: TAGGCACCTAGTTGGGCTCCATTT | |
| Noxa | Forward: GTCGGAACGCGCCAGTGAACCC |
| Reverse: TCCTTCCTGGGAGGTCCCTTCTTGC | |
| Apaf-1 | Forward: TCAGCAAACGAGAGGAAAAG |
| Reverse: CATAGGGGGAGAAGTCACAG | |
| β-actin | Forward: CATCCGTAAAGACCTCTATGCCAAC |
| Reverse: ATGGAGCCACCGATCCACA |
Statistical Analysis
All experimental data were expressed as mean ± SD and raw data were analyzed by the Independent t test using the SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
RESULTS
UV Radiation Induced Apoptosis of Lens Cells
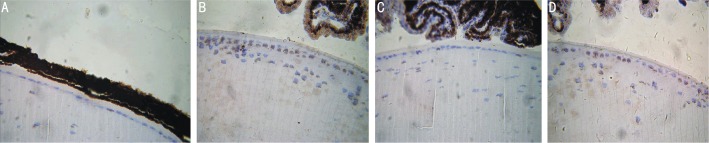
With the TUNEL assay, cells apoptosis was detected. The large dose of UV radiation induced apoptosis of lens cells in normal-UV group and Eaf2 K.O.-UV group. Samples which did not receive UV radiation, almost didn't find stained cells in normal-nonUV group and Eaf2 K.O.-nonUV group. Compared with Eaf2 K.O.-UV group, there were significantly more apoptotic cells in normal UV group (Figure 1). Previous research has also shown that exposure to UV light is an environmental factor for the lens cells apoptosis[10]. In this present study, the difference of the apoptosis level was observed between normal-UV group and Eaf2 K.O.-UV group, and the caspase 3 activity would be used to compare quantitatively.
Figure 1. TUNEL assay for apoptosis in mouse lens.
A: Lens of normal control mouse was not exposed to UVR; B: Lens of normal control mouse was exposed to UVR; C: Lens of Eaf2 knockout mouse was not exposed to UVR; D: Lens of Eaf2 knockout mouse was exposed to UVR.
Eaf2 knockout caused a reduction in caspase 3 activity induced by UV radiation in mouse lenses
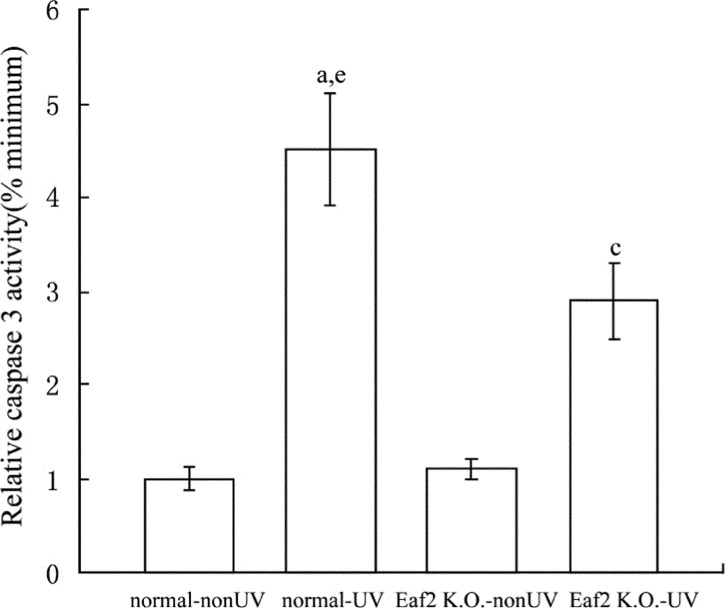
Caspase 3 activity of groups that received UV radiation, was significantly higher than groups that did not received UV radiation (P<0.05, Figure 2). And caspase 3 activity of normal-UV group was also significantly higher than Eaf2 K.O.-UV group (P<0.05).
Figure 2. Activity of caspase 3 in normal-nonUV group, normal-UV group, Eaf2 K.O.-nonUV group and Eaf2 K.O.-UV group.
aP<0.05 normal-UV group vs normal-nonUV; cP<0.05 Eaf2K.O.-UV group vs Eaf2K.O.-nonUV; eP<0.05 normal-UV vs Eaf2K.O.-UV.
UV radiation induced the increasing of p53 expression
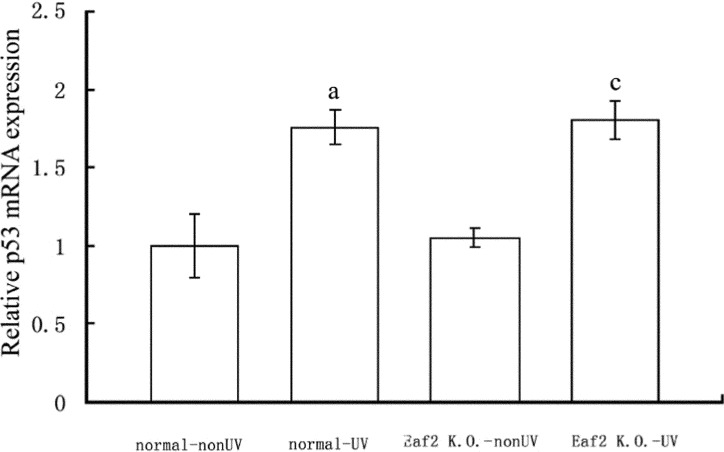
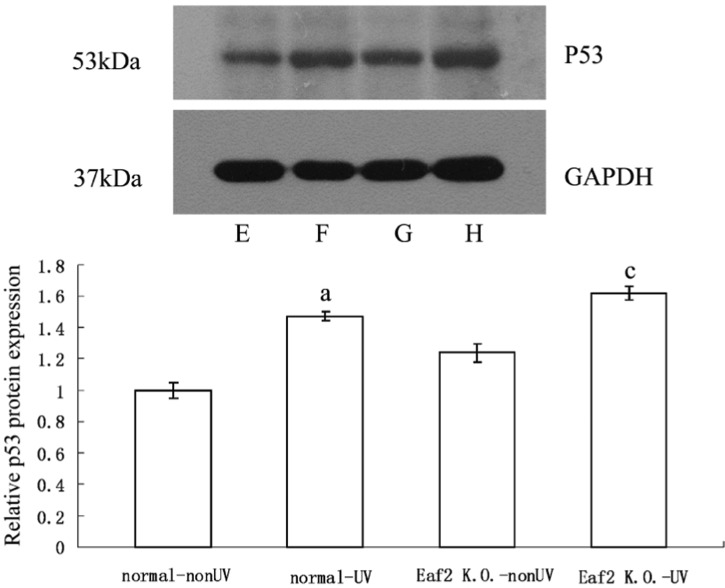
P53 mRNA levels were determined by means of real-time RT-PCR. 24 hours after UV radiation, p53 mRNA levels of normal-UV group and Eaf2 K.O.-UV group were significantly higher compared to normal-nonUV group and Eaf2 K.O.-nonUV group respectively (P<0.05, respectively, Figure 3). But between normal-UV group and Eaf2 K.O.-UV group, UV radiation did not cause significant difference in levels of p53 mRNA (P>0.05). After UV radiation, p53 protein was also significantly higher compared to the samples which did not receive UV radiation (P<0.05, Figure 4). And normal-UV group and Eaf2 K.O.-UV group had similar expression levels of p53 protein (P>0.05). Thus, UV radiation increased p53 expression at both the mRNA and protein levels, but Eaf2 did not affect the expression levels of p53 protein, after receiving UV radiation.
Figure 3. The expression of p53 mRNA in normal-nonUV group, normal-UV group, Eaf2 K.O.-nonUV group and Eaf2 K.O.-UV group.
aP<0.05 normal-UV group vs normal-nonUV; cP<0.05 Eaf2K.O.-UV group vs Eaf2K.O.-nonUV.
Figure 4. Western Blot assay of p53 protein in mouse lenses.
A: E, F, G, H were normal-nonUV group, normal-UV group, Eaf2 K.O.-nonUV group and Eaf2 K.O.-UV group, respectively; B: p53 Western blot, aP<0.05 normal-UV group vs normal-nonUV; cP<0.05 Eaf2K.O.-UV group vs Eaf2K.O.-nonUV.
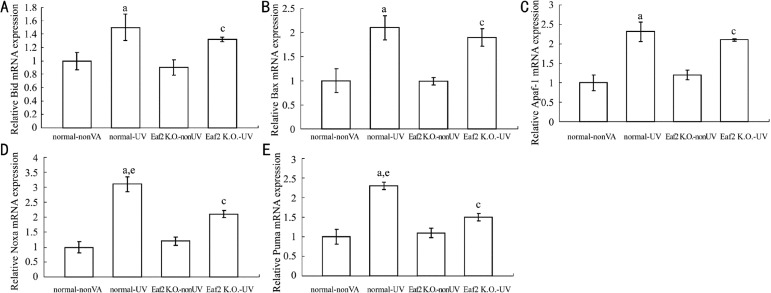
Eaf2 up-regulated the mRNA expression of Noxa and Puma
DNA damage activates p53, resulting in either cell-cycle arrest or apoptosis. p53 functions as a transcription factor and induces a variety of pro-apoptotic molecules containing Bax, Bid, Apaf-1, Puma, Noxa and so on. To investigate whether Eaf2 regulated transcriptional activation of p53 to downstream pro-apoptotic genes, we detected mRNA levels of Bax, Bid, Apaf-1, Puma and Noxa using real-time RT-PCR (Figure 5). The mRNA levels of Bax, Bid, Apaf-1, Puma and Noxa were higher in normal-UV group and Eaf2 K.O.-UV group than normal-nonUV group and Eaf2 K.O.-nonUV group respectively (P<0.05). The mRNA levels of Bax, Bid and Apaf-1 did not show significantly difference between normal-UV group and Eaf2 K.O.-UV group (P>0.05), but mRNA levels of Puma and Noxa in normal-UV group were higher than Eaf2 K.O.-UV group (P<0.05). Eaf2 up-regulated the expression of Puma and Noxa to promote the level of lens cells apoptosis induced by UV radiation.
Figure 5. mRNA expression in normal-nonUV group, normal-UV group, Eaf2 K.O.-nonUV group and Eaf2 K.O.-UV group.
A: Bid mRNA; B: Bax mRNA; C: Apaf-1 mRNA; D: Noxa mRNA; E: Puma mRNA. aP<0.05 normal-UV group vs normal-nonUV; cP<0.05 Eaf2K.O.-UV group vs Eaf2K.O.-nonUV. eP<0.05 normal-UV vs Eaf2K.O.-UV.
DISCUSSION
Godar et al[11] first defined the conception of apoptosis in 1972, and apoptosis is considered to be important physiological and pathological processes currently. It is accompanied by a reduction of the cell's specific gravity, destruction of cell membranes, condensation of chromosomes and transformation of apoptotic bodies into cysts which then undergo phagocytosis. With the rapid development of molecular biology, many studies focus on the molecular mechanisms of apoptosis. As we all know, the central mechanism of apoptosis is the activation of caspases family. DNA damage induced by UV radiation usually activates the intrinsic signaling pathway of apoptosis, and changes the mitochondrial outer-membrane permeabilization (MOMP)[12]. After MOMP happens, cytochrome c is released from mitochondrial intermembrane space and finally the executioners caspase 3 and caspase 7 are activated. Caspase 3 which is an important member of cysteine-aspartic acid protease (caspase) family can cleave and activate caspases 6, 7 and 9, and be processed by caspases 8, 9 and 10. Caspase 3 activity assay is a quantified method for the detection of apoptosis and it can accurately reflect the level of apoptosis especially in the early stages of apoptosis. Researchers find that apoptosis caused by UV radiation reaches its maximum within 24 hours of exposure[13], thus we observed lens cells apoptosis 24 hours after receiving a large dose of UV radiation in the present study. The occurrence of cataracts may be due to the lack of the defense against UV radiation and oxidative stress, and these damage factors earlily lead to lens epithelial cell apoptosis, which may become a common cellular basis of cataract formation[3]. The result of caspase 3 activity assay showed that Eaf2 knockout inhibited the increasing of caspase 3 activity induced by UV radiation and reduce the level of lens cells apoptosis. It is similar that over-expression of Eaf2 induces apoptosis in prostate cancer cells lines and also suppresses xenograft tumor growth[5], Eaf2 increases lens cells apoptosis induced by UV radiation and plays an active effect in the process of apoptosis.
The p53 protein is a transcription factor that plays an important role in apoptosis. In the present study, we found that UV radiation caused increasing of p53 expression in lens cells and triggered apoptosis. The similar results which have been reported in vivo and in vitro[10],[14] demonstrates that p53 protein is involved in lens cells apoptosis induced by UV radiation. In normal cells, low expression level of p53 is maintained by a negative feedback mechanism in which p53 promotes Mdm2 expression which, in turn, tags p53 protein for nuclear export and proteasomal degradation induced by ubiquitin[15]-[17]. After DNA damage the ATM kinase rapidly phosphorylates p53, and the serine/threonine kinase Chk2 also acts on p53. These phopshorylated site of p53 are close to the Mdm2-binding region of the protein, thereby they block the interaction with Mdm2, leading to stabilization of p53 that escapes from proteasomal degradation[18]. Such DNA damage causes p53 to accrue in the cell that received UV radiation. Generally expression of p53 is almost undetected, but some exceptional cases still appear. Researchers found that high expression of p53 was observed in the lens epithelial cells of the central and pre-equatorial zones and in the lens fiber nuclear bow[19]. The certain level of p53 expression was also detected in the lenses which did not received UV radiation in our study. It seems likely that the long-term exposure to small dose of ultraviolet radiation cause an adaptive change in mice lenses. High p53 level indicates that lens cells have the more sensitive monitoring capability of DNA damage and a more rapid response. Thus p53 expression of lens cells is stabilized at a particular level in normal condition, but large doses of ultraviolet radiation or genotoxic stress would break the balance and increase the content of p53 protein in cells.
In our study, it was found that Eaf2 gene knockout did not change the level of p53 protein expression induced by UV radiation. This hinted that Eaf2 might play an important role on the downstream of p53 dependent apoptosis signal pathway, to promote lens cells apoptosis after a large dose of UV radiation. As we know as a transcription factor, p53 activates several important genes that are crucial for the execution of the intrinsic pathway of apoptosis, including pro-apoptotic genes such as Bax, Noxa, Puma, and Apaf-1[20]. And p53 can also increase release of the cytochrome c by promoting the expression of the OKL 38 tumor suppressor gene, which localizes to mitochondria and promote cytochrome c release[21]. On the other hand, p53 also plays an important role in apoptosis with a transcription-independent manner. Researchers found that p53 can physically interact with anti-apoptosis proteins including bcl-2, bcl-Xl and mcl-1 at the mitochondrial membrane[22]. When these anti-apoptotic proteins bind p53, their ability to stabilize the mitochondrial membrane is compromised and then results in permeability change and cytochrome c release. And p53 can interact with Bak directly to induce the release of cytochrome c from the intermembrane space of mitochondrion[22]. p53 has a wide range of roles in apoptosis, thereby we focused on these molecules of the p53 downstream to investigate the mechanism of regulation in lens cells apoptosis induced by UV radiation.
We reported that Eaf2 gene knockout inhibited the increasing of Puma and Noxa expression induced by UV radiation in mouse lens cells. Does this mean that Eaf2 protein may regulate corresponding physiological functions of p53, such as promoting the specific transcriptional activation of Puma and Noxa genes? Some studies have shown that Eaf2 physically and functionally interacts with ELL[4], and ELL can also interact with p53[23]. It suggests that Eaf2 is related to p53 via their mutual-binding partners presumably. Previous research has further shown that p53 represses the expression of thrombospondin-1(TSP-1) in vitro, but Eaf2 can block this repression[24]. Additionally, researchers show Eaf2 can co-localize and co-immunoprecipitate with p53 in transfected cells[24].In summary, it is likely that Eaf2 interacts with p53 directly, and impacts on the function of the transcription factor p53. This may explain how Eaf2 regulates level of the lens cells apoptosis induced by UV radiation. Except that the Noxa expression can be activated by p53, the transcription of this pro-apoptotic protein can be promoted via p53 independent pathway[25],[26]. It is also reported that exposure of cultured human lens epithelial cell lines to ultraviolet light promotes the expression of Noxa without increasing p53 and induces apoptosis, by Kim and Koh[27]. Thus, the exact mechanism that Eaf2 increases Puma and Noxa expression in the process of apoptosis is not yet clear, and further studies are necessary.
Researches about Eaf2 have focused on its role in promoting apoptosis of tumor cells, but our studies indicate that in normal non-transformed cells Eaf2 may interact with p53 and increase the expression of p53' downstream pro-apoptotic genes, in order to regulate DNA damage-induced apoptosis of lens cells. It is likely to provide a new perspective for revealing the etiology of cataracts.
REFERENCES
- 1.Hollows F, Moran D. Cataract--the ultraviolet risk factor. Lancet. 1981;2(8258):1249–1250. doi: 10.1016/s0140-6736(81)91490-2. [DOI] [PubMed] [Google Scholar]
- 2.Varma SD, Chand D, Sharma YR, Kuck JF, Jr, Richards RD. Oxidative stress on lens and cataract formation: role of light and oxygen. Curr Eye Res. 1984;3(1):35–57. doi: 10.3109/02713688408997186. [DOI] [PubMed] [Google Scholar]
- 3.Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130(1):169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simone F, Luo RT, Polak PE, Kaberlein JJ, Thirman MJ. ELL-associated factor 2 (EAF2), a functional homolog of EAF1 with alternative ELL binding properties. Blood. 2003;101(6):2355–2362. doi: 10.1182/blood-2002-06-1664. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, Wang Z. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res. 2003;63(15):4698–4704. [PubMed] [Google Scholar]
- 6.Kong SE, Banks CA, Shilatifard A, Conaway JW, Conaway RC. ELL-associated factors 1 and 2 are positive regulators of RNA polymerase II elongation factor ELL. Proc Natl Acad Sci U S A. 2005;102(29):10094–10098. doi: 10.1073/pnas.0503017102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang F, Yen P, Zhao J, Nguyen M, Jiang M, Liu YH. Dynamic intracellular distribution of Eaf2 and its potential involvement in UV-Induced DNA damage response. DNA Cell Biol. 2008;27(12):649–656. doi: 10.1089/dna.2008.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Wu X, Zhuang F, Jiang S, Jiang M, Liu YH. Expression of murine ELL-associated factor 2 (Eaf2) is developmentally regulated. Dev Dyn. 2003;228(2):273–280. doi: 10.1002/dvdy.10367. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Ayala M, Strid H, Jacobsson U, Söderberg PG. p53 expression and apoptosis in the lens after ultraviolet radiation exposure. Invest Ophthalmol Vis Sci. 2007;48(9):4187–4191. doi: 10.1167/iovs.06-0660. [DOI] [PubMed] [Google Scholar]
- 11.Godar DE. Preprogrammed and programmed cell death mechanisms of apoptosis: UV-induced immediate and delayed apoptosis. Photochem Photobiol. 1996;63(6):825–830. doi: 10.1111/j.1751-1097.1996.tb09638.x. [DOI] [PubMed] [Google Scholar]
- 12.Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310(5745):66–67. doi: 10.1126/science.1117105. [DOI] [PubMed] [Google Scholar]
- 13.Hightower KR, Reddan JR, McCready JP, Dziedzic DC. Lens epithelium: a primary target of UVB irradiation. Exp Eye Res. 1994;59(5):557–564. doi: 10.1006/exer.1994.1141. [DOI] [PubMed] [Google Scholar]
- 14.Michael R, Vrensen GF, van Marle J, Gan L, Söderberg PG. Apoptosis in the rat lens after in vivo threshold dose ultraviolet irradiation. Invest Ophthalmol Vis Sci. 1998;39(13):2681–2687. [PubMed] [Google Scholar]
- 15.Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem. 1999;274:36031–36034. doi: 10.1074/jbc.274.51.36031. [DOI] [PubMed] [Google Scholar]
- 16.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13(3):332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 17.Kubbutat MH, Vousden KH. Keeping an old friend under control: regulation of p53 stability. Mol Med Today. 1998;4(6):250–256. doi: 10.1016/s1357-4310(98)01260-x. [DOI] [PubMed] [Google Scholar]
- 18.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268(10):2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 19.Pokroy R, Tendler Y, Pollack A, Zinder O, Weisinger G. p53 expression in the normal murine eye. Invest Ophthalmol Vis Sci. 2002;43(6):1736–1741. [PubMed] [Google Scholar]
- 20.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13(6):994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 21.Yao H, Li P, Venters BJ, Zheng S, Thompson PR, Pugh BF, Wang Y. Histone Arg modifications and p53 regulate the expression of OKL38, a mediator of apoptosis. J Biol Chem. 2008;283(29):20060–20068. doi: 10.1074/jbc.M802940200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff S, Erster S, Palacios G, Moll UM. p53's mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008;18(7):733–744. doi: 10.1038/cr.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinobu N, Maeda T, Aso T, Ito T, Kondo T, Koike K, Hatakeyama M. Physical interaction and functional antagonism between the RNA polymerase II elongation factor ELL and p53. J Biol Chem. 1999;274(24):17003–17010. doi: 10.1074/jbc.274.24.17003. [DOI] [PubMed] [Google Scholar]
- 24.Su F, Pascal LE, Xiao W, Wang Z. Tumor suppressor U19/EAF2 regulates thrombospondin-1 expression via p53. Oncogene. 2010;29(3):421–431. doi: 10.1038/onc.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jullig M, Zhang WV, Ferreira A, Stott NS. MG132 induced apoptosis is associated with p53-independent induction of pro-apoptotic Noxa and transcriptional activity of beta-catenin. Apoptosis. 2006;11(4):627–641. doi: 10.1007/s10495-006-4990-9. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong JL, Veal GJ, Redfern CP, Lovat PE. Role of Noxa in p53-independent fenretinide-induced apoptosis of neuroectodermal tumours. Apoptosis. 2007;12(3):613–622. doi: 10.1007/s10495-006-0020-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim ST, Koh JW. Mechanisms of apoptosis on human lens epithelium after ultraviolet light exposure. Korean J Ophthalmol. 2011;25(3):196–201. doi: 10.3341/kjo.2011.25.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]