Abstract
AIM
To investigate whether 15-Lipoxygenase-1 (15-LOX-1) plays an important role in the regulation of angiogenesis, inhibiting hypoxia-induced proliferation of retinal microvascular endothelial cells (RMVECs) and the underlying mechanism.
METHODS
Primary RMVECs were isolated from the retinas of C57/BL6J mice and identified by an evaluation for FITC-marked CD31. The hypoxia models were established with the Bio-bag and evaluated with a blood-gas analyzer. Experiments were performed using RMVECs treated with and without transfer Ad-15-LOX-1 or Ad-vector both under hypoxia and normoxia condition at 12, 24, 48, 72 hours. The efficacy of the gene transfer was assessed by immunofluorescence staining. Cells proliferation was evaluated by the CCK-8 method. RNA and protein expressions of 15-LOX-1, VEGF-A, VEGFR-2, eNOs and PPAR-r were analyzed by real-time reverse transcription polymerase chain reaction (RT-PCR) and Western blot.
RESULTS
Routine evaluation for FITC-marked CD31 showed that cells were pure. The results of blood-gas analysis showed that when the cultures were exposed to hypoxia for more than 2 hours, the Po2 was 4.5 to 5.4 Kpa. We verified RMVECs could be infected with Ad-15-LOX-1 or Ad-vector via Fluorescence microscopy. CCK-8 analysis revealed that the proliferative capacities of RMVECs in hypoxic group were significantly higher at each time point than they were in normoxic group (P<0.05). In a hypoxic condition, the proliferative capacities of RMVECs in 15-LOX-1 group were significantly inhibited (P<0.05). Real-time RT-PCR analysis revealed that the expressions of VEGF-A, VEGF-R2 and eNOs mRNA increased in hypoxia group compared with normoxia group (P<0.01). However, the expressions of 15-LOX-1, PPAR-r mRNA decreased in hypoxia group compared with normoxia group (P<0.01). It also showed that in a hypoxic condition, the expressions of VEGF-A, VEGF-R2 and eNOs mRNA decreased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01). However, 15-LOX-1 and PPAR-r mRNA increased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01). There was no significant difference of the mRNA expressions between vector group and hypoxia group (P>0.05). Western blot analysis revealed that the expressions of relative proteins were also ranked in that order.
CONCLUSION
Our results suggested that 15-LOX-1 and PPAR-r might act as a negative regulator of retinal angiogenesis. And the effect of 15-LOX-1 overexpression is an anti-angiogenic factor in hypoxia-induced retinal neovascularization (RNV). Overexpression 15-LOX-1 on RMVECs of hypoxia-induced RNV blocked signaling cascades by inhibiting hypoxia-induced increases in VEGF family. PPAR-r effect on VEGFR2 could be an additional mechanism whereby 15-LOX-1 inhibited the hypoxia-induced RNV.
Keywords: 15-Lipoxygenase-1, hypoxia, retinal microvascular endothelial cells, retinal neovascularization
INTRODUCTION
Retinal neovascularization (RNV) is a major cause of vision loss at all ages. It causes vision loss in retinopathy of prematurity (ROP) in children, in diabetic retinopathy (DR) in young adults, and in age-related macular degeneration (AMD) in the elderly[1],[2]. Intraocular concentrations of VEGF which is an angiogenic protein and vasopermeability factor are strongly correlated with DR, AMD and ROP[3]. VEGF has been demonstrated to play an important role in RNV[4],[5]. The inhibition of VEGF action indicated to provide a viable approach for therapeutic intervention on RNV[6]-[8]. However, the exact mechanism underlying the pathogenesis of retinal RNV is not yet well-understood.
Lipoxygenases (LOXs) constitute a heterogeneous family of lipid peroxidizing enzymes which catalyze the stereoselective dioxygenation of polyunsaturated fatty acids to their hydroperoxy derivatives[9],[10]. In mammals, LOXs are categorized with respect to their positional specificity of arachidonic acid oxygenation into 5-, 8-, 12-, and 15-LOXs[11]. Within the 15-LOXs, two isoforms have been identified[12]. 15-LOX-1 is a lipid-peroxidizing enzyme capable of introducing molecular oxygen to polyunsaturated fatty acids either in free form or in more complex compounds, such as biological membranes, phospholipids, cholesterol esters, and plasma lipoproteins[13]-[15]. 15-LOX-2 has limited tissue expression in prostate, lung, skin, and cornea[16]. In terms of enzymatic characteristics, 15-LOX-1 preferentially metabolizes linoleic acid primarily to 13-(S)-HODE also metabolizes arachidonic acid to 15-(S)-HETE[17].15-LOX-1 is involved in many pathological conditions, such as in cell differentiation, in inflammation, in atherogenesis and carcinogenesis[14]. Expression and function of 15-LOX-1 have been studied in endothelial cells, smooth muscle cells, monocytes, and 15-LOX-1 has been shown to play key roles in vascular remodeling and the progression of atherosclerosis[18]-[20]. 15-LOX-1 has also been implicated for its role in angiogenesis. However, the evidence is controversial, even contradictory. Several studies in tumor models have shown that 15-LOX-1 could promote or inhibit angiogenesis.
Angiogenesis in 2 xenograft models is inhibited in transgenic mice overexpressing 15-LOX-1 in ECs under the control of preproendothelin promoter[21]. In contrast, the PC-3 human prostate cancer cell line, which overexpresses 15-LOX-1, secretes high levels of VEGF and enhances angiogenesis as compared with the parental PC-3 cell lines[22]. Considering the angiogenic process is complex and the role of 15-LOX-1 in the development of RNV has not been well investigated, we explorethe changes in 15-LOX-1 expression on RMVECs in hypoxia condition and determine whether 15-LOX-1 activity impact on proliferation of RMVECs and the underlying mechanism in this study.
MATERIALS AND METHODS
Materials
Recombinant adenoviral vector construction
Two recombinant Adenoviral vectors based on the pDC315-EGFP vector (purchased from shanghai GeneChem Co.Ltd) were constructed, respectively, expressing the mouse 15-LOX-1 gene and the green fluorescence protein gene under the control of the mouse CMV promoter. Stock solution of Ad-15-LOX-1 and Adenoviral vector were 1.25 × 1011 and 2.50× 1011 plaque formation unit (PFU)/mL, respectively. Working solution were prepared to make 1µL of vehicle contained approximately 1.0 × 106 PFU.
RMVECs culture and identification
Primary RMVECs (MIC-CELL-0046, PriCells, China) were isolated from the retinas of C57/BL6J mice. The third generation of RMVECs was used in the experiments.
Methods
Transfection recombinant adenoviral vectors into RMVECs
RMVECs were seeded in a 6-well plate. Twenty-four hours later, RMVECs obtained a final cell density of 6×105mL−1 and changed 2mL fresh medium. Each micropipet was calibrated to deliver approximately 1.0×106 PFU of Ad-15-LOX-1 or Adenoviral vector. RMVECs were analyzed with 100× magnification by fluorescence microscopy (OLYMPUS BH2-RFL-T3) to ensure that the transfected cells express EGFP. EGFP fluorescence was detected at 510-532nm with excitation at 488nm.
In vitro hypoxia model and assessment
The experiments were divided into four groups: (1) normal control group (normoxia group), (2) untreated hypoxia group (hypoxia group), (3) hypoxia treated with Ad-15-LOX-1 group (15-LOX-1 group), and(4) hypoxia treated with Adenoviral vector group (vector group). RMVECs of hypoxia group, 15-LOX-1 group, vector group were placed inside a bio-bag (Mitsubishi Chemical Corporation, Japan) and placed in an anaero pack to induce hypoxia. Cultures were exposed in a hypoxic condition for 12, 24, 48 and 72 hours. As a control, cultures were also incubated under a normoxic condition for the same length of time. Each group was placed into an incubator at 37°C. Culture supernatants were aspirated with a blood-gas sampling needle. The Po2, Pco2, and pH values of the different hypoxia groups were measured using a blood-gas analyzer (ABL555; Radiometer, Copenhagen, Denmark).
Examination of cell proliferation by the CCK-8 method
RMVECs were seeded in a 96-well plate. Each group had 5 replicates holes. Each of the 96-well plate was located in the blank control group (added 100µL culture medium, without cell). Cell proliferation was evaluated using a CCK-8 assay according to the method of Yang et al[23]. The duration of hypoxia was 12, 24, 48 and 72 hours. As a control, cultures were also incubated in a normoxic condition for the same lengths of time. Optical density was measured with an ELISA Reader (Rayto RT-6500) at 450nm. All experiments were carried out in triplicate.
Real-time RT-PCR
Total RNA was isolated from the RMVECs of each group according to standard techniques[24]. Reverse transcription was performed according to the manufacturer's guidelines (Fermentas, Life sciences). The sequences of primers were designed using the Primer Express software (PE Biosystems). The primer sequences were 15-LOX-1: 5′- ATCCGTCCAAGATGACCAAG-3′ (forward) and 5′-AAGAGTCGAACTGGCCTGAA-3′ (reverse),VEGFA:5′-CAGGCTGCTGTAACGATGAA-3′ (forward)and5′-TTTGACCCTTTCCCTTTCCT-3′ (reverse), VEGFR2: 5′- TTCTGGACTCTCCCTGCCTA-3′ (forward)and5′-AAGGACCATCCCACTGTCTG-3′ (reverse),eNOs:5′-AGCATACCCCCACTTCTGTG-3′ (forward)and5′-GAAGATATCTCGGGCAGCAG-3′ (reverse), PPAR-r: 5′-AAGAGCTGACCCAATGGTTG-3′ (forward) and5′-ACCCTTGCATCCTTCACAAG-3′ (reverse),ß-actin:5′-CACGATGGAGGGGCCGGACTCATC-3′ (forward)and 5′-TAAAGACCTCTATGCCAACACAGT-3′ (reverse).The PCR reaction was performed in a volume of 25µL using SYBR green mix (Toyobo, Shanghai, China) on the Rotor-Gene 3000 Real-time PCR instrument (Corbett Research, Sydney, Australia). The thermal cycling program consisted of 1 min at 95°C, 40 cycles of 15 sec at 95°C, 15 sec at 58°C, 45 sec at 72°C. Relative quantification of the gene expression was performed using the 2(−ΔΔCT) method[25].All experiments were carried out in triplicate.
Western blot analysis
The RMVECs cells were homogenized by 200µL of lysis buffer (20mmol/L Tris, pH 7.4, 150mmol/L NaCl, 1mmol/L EDTA, 1mmol/l orthovanadate, 1mmol/L phenylmethylsulfonyl fluoride, 1µg/mL leupeptin and 10µg/mL aprotinin) on ice. The protein concentration of the sample was determined using the Bradford assay. Equal amounts (50µg) of proteins from each sample were loaded on SDS-PAGE and then transferred onto nitrocellulose membrane (Hybond-C, Amersham, Arlington Heights, IL) at 200mA for 1 hour. After blocking of nonspecific binding sites with 5% skim milk for 1 hour, the membrane was incubated with the primary antibodies as the following dilutions: mouse monoclonal antibody to 15-LOX-1 at 1:200 (Abcam, Cambridge, MA, USA), rabbit polyclonal antibodies to VEGFA at 1:1000 (Bioss Biotechnology, Beijing, China), rabbit polyclonal antibodies to VEGFR2 at 1:800 (Bioss Biotechnology, Beijing, China), rabbit polyclonal antibodies to eNOs at 1:500 (Bioss Biotechnology, Beijing, China) and rabbit polyclonal antibodies to PPAR-r at 1:200 (Bioss Biotechnology, Beijing, China)overnight at 4°C. Antibody dilutions were made in a solution of 5% skim milk /0.1% Tris-buffered saline Tween-20. Then, membranes were washed with TBS and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Boster Biotechnology, Wuhan, China) in blocking buffer for 2 hours at room temperature. After four washes, the proteins were detected with ECL kit (Amersham Pharmacia Biotech UK Ltd., Little Chalfont, UK). β-actin staining served as the internal standard for all membranes.
Statistical Analysis
The SPSS 13.0 software (SPSSInc, Chicago, IL, USA) was used for statistical analysis. The results are expressed as the means±standard error of mean (SEM) and were subjected to One-way analysis of variance (ANOVA). Differences between two groups were determined by Student's t test. P value of < 0.05 indicates statistical significance.
RESULTS
RMVECs Culture and Identification
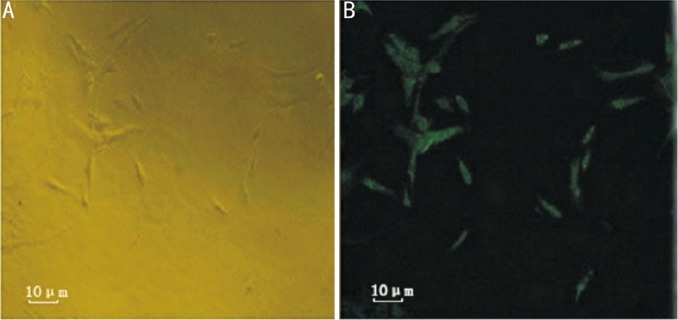
Primary RMVECs that were isolated from the retinas of C57/BL6J mice formed a colony of spindle shaped cells after 3 days in culture. Confluence of RMVECs of the primary culture exhibited contact inhabitation and presented a typical paving stone appearance. Routine evaluation for FITC-labeled CD31 showed that cells were pure (Figure 1).
Figure 1. RMVECs culture and identification.
A: RMVECs forming a colony of spindle shaped cells in primary culture (400×); B: Expression of CD31 in RMVECs with FITC staining.
Fluorescence Microscopy Identification after Transfection
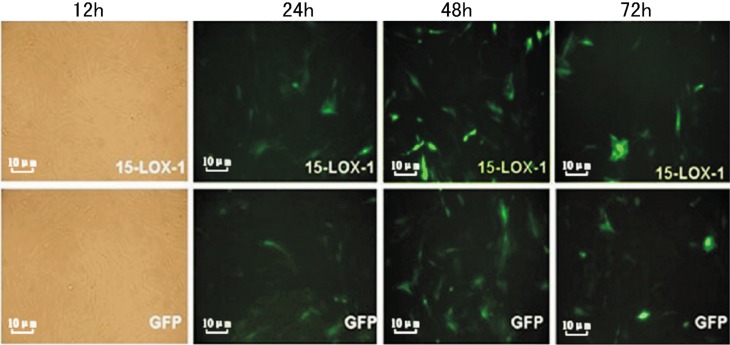
RMVECs were transfected with Ad-15-LOX-1 or Adenoviral vector. The expressions GFP were visualized by fluorescence microscopy at 12, 24, 48 and 72 hours post-transfection. Ad-15-LOX-1 or Adenoviral vector was transfected into RMVECs (Figure 2).
Figure 2. Photographs of Ad-15-LOX-1 or Ad-GFP transfected RMVECs. RMVECs were transfected with Ad-15-LOX-1 or Ad-GFP. The expressions of EGFP and GFP were visualized by fluorescence microscopy (100×) at 24, 48 and 72 hours post transfection.
In vitro Hypoxia Model and Assessment
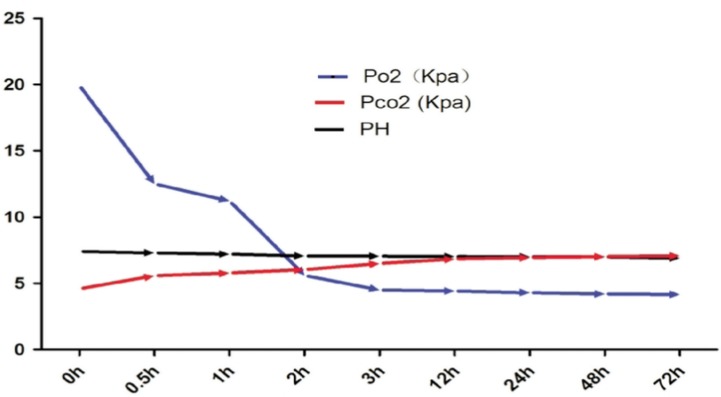
The Po2, Pco2, and pH values of the different hypoxia groups were measured with a blood-gas analyzer. Experiments showed that when the cells had been exposed to hypoxic conditions for less than 1 hour, the Po2 was 11.22 to 12.5 Kpa, the Pco2 was 4.58 to 5.57 Kpa, and the pH was 7.30 to 7.40. When the cells had been exposed to hypoxic conditions for 2 hours, the Po2 was 5.6 Kpa, the Pco2 was 6.0 Kpa, and the pH was 7.01. When the cultures had been exposed to hypoxic conditions for more than 2 hours, the Po2 was 4.5 to 5.4 Kpa, the Pco2 was 6.13 to 6.54 Kpa, and the pH was 6.8 to 7.0 (Figure 3).
Figure 3. The hypoxia model in vitro was assessed by a blood-gas analyzer. The Po2, Pco2, and pH values of the hypoxia groups were measured with a blood-gas analyzer at each time point. When the cultures had been exposed to hypoxic conditions for more than 2 hours, the Po2 was 4.5 to 5.4 Kpa, the Pco2 was 6.13 to 6.54 Kpa, and the pH was 6.8 to 7.0.
Examination of Cell Proliferation by the CCK-8 Method
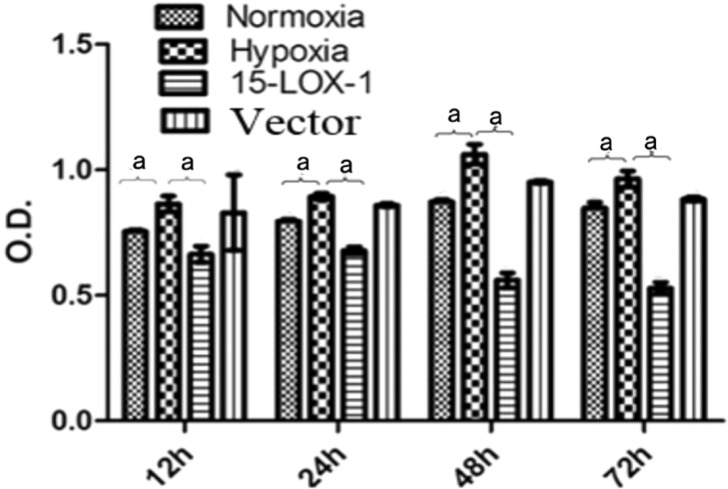
The proliferative capacities of RMVECs that had been incubated in a hypoxic environment were significantly higher at each time point than they were in cells that had been incubated in a normoxic environment (P<0.05). In a hypoxic environment, the proliferative capacities of RMVECs in 15-LOX-1 group were significantly been inhibited. The proliferative capacities of RMVECs in 15-LOX-1 group significantly decreased compared with the hypoxia group (P<0.05); No significant differences of the proliferative capacities were found between vector group and hypoxia group (P>0.05) (Figure 4).
Figure 4. The proliferative capacities of RMVECs were evaluated by CCK-8 method. The proliferative capacities of RMVECs in hypoxic group significantly increased compared with the normoxic group at each time point; the proliferative capacities of RMVECs in 15-LOX-1 group significantly decreased compared with the hypoxia group (P<0.05); no significant differences of proliferative capacities were found between GFP group and hypoxia group (P>0.05). All data represent mean±SEM (n=3); aP<0.05.
Real-time RT-PCR analysis
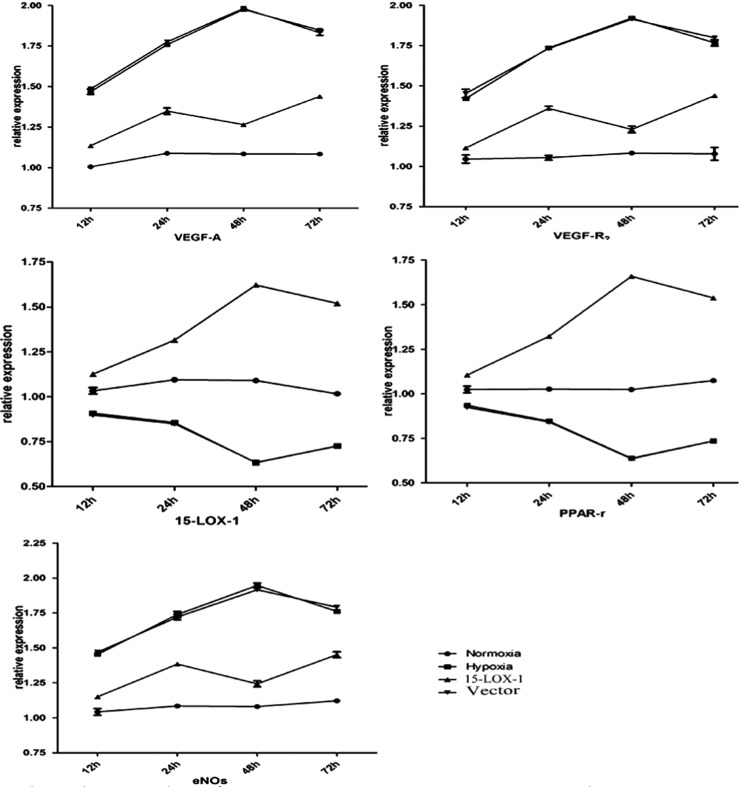
All points were measured relative to β-actin and normalized to the normoxia group. All data were presented as the means ±SE. The analysis was performed as follows: ΔCT=CT(test gene)-CT(β-actin); ΔΔCT =ΔCT(hypoxia group RNA)-ΔCT(normoxia group RNA); Relative Expression = 2−ΔΔCT. Real-time RT-PCR analysis revealed that the expressions of VEGF-A, VEGF-R2 and eNOs mRNA increased in hypoxia group compared with normoxia group (P<0.01). However, the expressions of 15-LOX-1, PPAR-r mRNA decreased in hypoxia group compared with normoxia group (P<0.01). This analysis also showed that in a hypoxic condition, the expressions of VEGF-A, VEGF-R2 and eNOs mRNA decreased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01); However, the expressions of 15-LOX-1 and PPAR-r mRNA increased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01). No significant differences of the mRNA expressions were found between vector group and hypoxia group (P>0.05) (Figure 5).
Figure 5. The expressions of 15-LOX-1, PPAR-r, VEGF-A, VEGF-R2 and eNOs mRNA at each time point were determined by real-time RT-PCR. The expressions of VEGF-A, VEGF-R2 and eNOs mRNA increased in hypoxia group compared with normoxia group (P<0.01). However, the expressions of 15-LOX-1, PPAR-r mRNA decreased in hypoxia group compared with normoxia group (P<0.01). In a hypoxic condition, the expressions of VEGF-A, VEGF-R2 and eNOs mRNA decreased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01); However, the expressions of 15-LOX-1 and PPAR-r mRNA increased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01). No significant differences of the mRNA expressions were found between vector group and hypoxia group (P>0.05). All data represent mean±SEM (n=3). bP<0.01.
Western-blotting Analysis
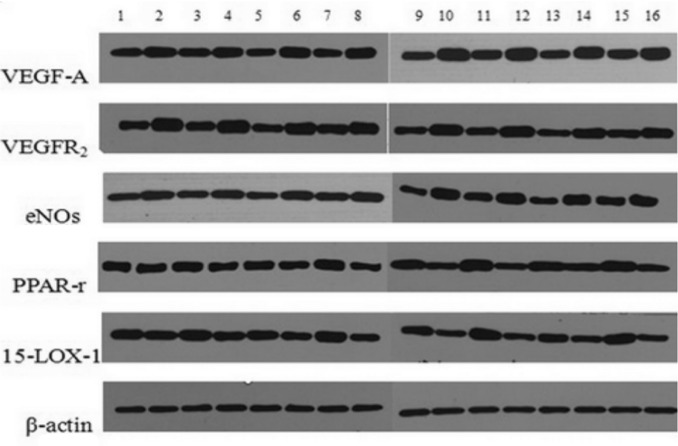
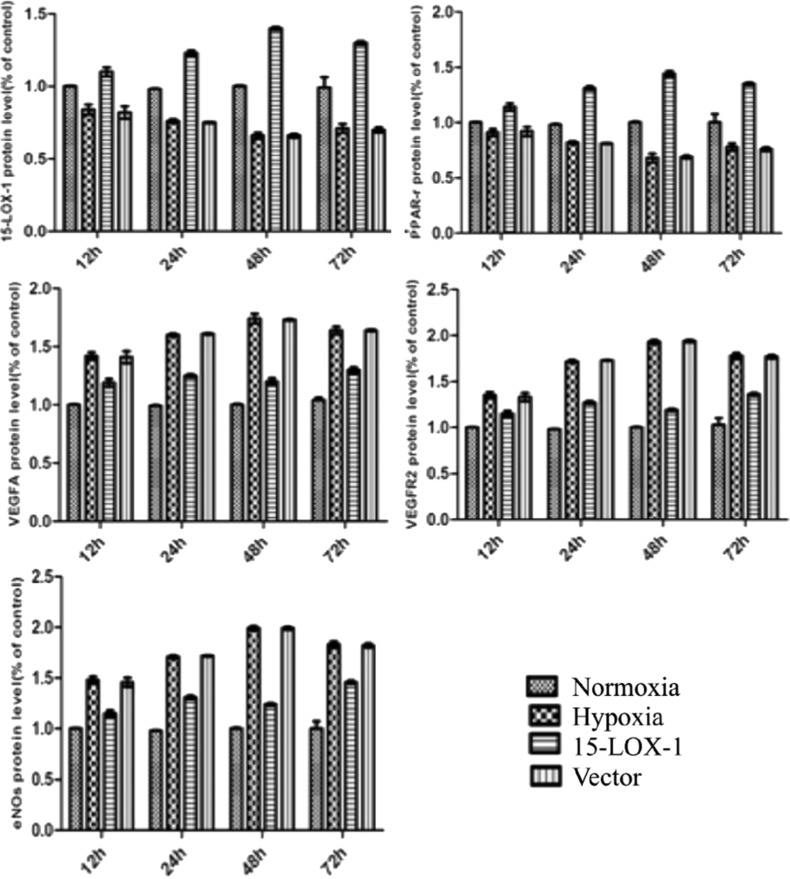
As show in Figure 6, the expressions of VEGF-A, VEGF-R2 and eNOs proteins were higher in the hypoxia group than those in normoxia group at 12, 24, 48 and 72 hours. However, the expressions of 15-LOX-1, PPAR-r proteins decreased in the hypoxia group compared with the normoxia group at each time point. This analysis also showed that in a hypoxic condition, the expressions of VEGF-A, VEGF-R2 and eNOs proteins decreased in 15-LOX-1 group compared with hypoxia group (P<0.01). In a hypoxic condition, the expressions of 15-LOX-1 and PPAR-r proteins increased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01). No significant differences of proteins expressions between vector group and hypoxia group (P>0.05). The band were quantified by densitometry and normalized byβ-actin (Figure 7).
Figure 6. Shown is the Western blot analysis of 15-LOX-1, PPAR-r, VEGF-A, VEGF-R2, eNOs and ß-actin proteins at each time point. From the left: (1-4) 12h; (5-8) 24h; (9-12) 48h; (13-16) 72h; (1, 5, 9, 13) normoxia group; (2, 6, 10, 14) hypoxia group; (3, 7, 11, 15) 15-LOX-1 group; (4, 8, 12, 16) GFP group.
Figure 7. The relative expressions of 15-LOX-1, PPAR-r, VEGF-A, VEGF-R2 and eNOs proteins at each time point by western blot. The bands were quantified by densitometry and normalized by ß-actin. The expressions of VEGF-A, VEGF-R2 and eNOs proteins increased in the hypoxia group than those in normoxia group at 12, 24, 48 and 72 hours. However, the expressions of 15-LOX-1, PPAR-r proteins decreased in the hypoxia group compared with the normoxia group at each time point. This analysis also showed that in a hypoxic condition, the expressions of VEGF-A, VEGF-R2 and eNOs proteins decreased in 15-LOX-1 group compared with hypoxia group (P<0.01). However, the expressions of 15-LOX-1 and PPAR-r proteins increased significantly in 15-LOX-1 group compared with hypoxia group (P<0.01). No significant differences of proteins expressions between GFP group and hypoxia group (P>0.05). All data represent mean ± SEM (n=3).
DISCUSSION
RNV occurs in a number of oxygen induced retinopathy (OIR) including AMD, PDR and ROP[26]. For many years, treatments for RNV in ocular diseases were limited to laser and cryosurgery of the avascular zone, which were effective for reducing severe vision loss, whereas could lead to serious side effects at the same time. It is very important to develop new therapies for the pathological angiogenesis. In recent years, progress has been made in the pharmacological inhibition of the angiogenic stimulators such as VEGF. For instance, anti-VEGF strategies have recently emerged as valuable new therapies for ocular neovascularization diseases[27]-[29].
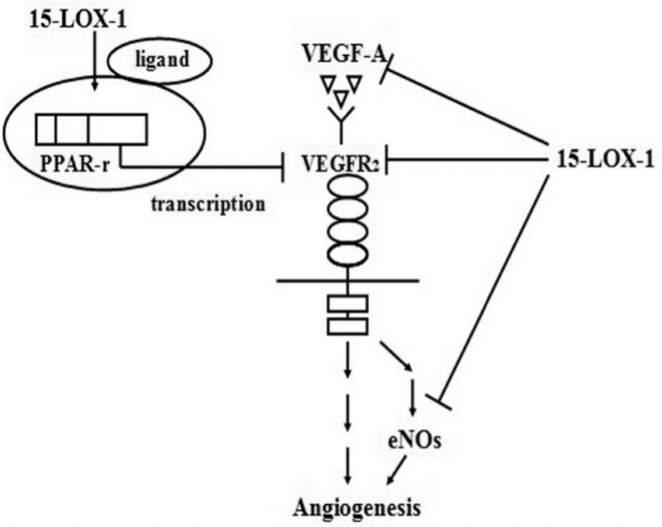
We have previously used the Bio-bag to make a hypoxia-induced RNV model of RMVECs and showed that hypoxia triggers the induction of VEGF-A expression in RMVECs, which, through an antocrine mechanism, binds to the up-regulated receptor VEGFR2 and the messenger eNOs (Figure 8), to promote the cells proliferation and ultimately lead to angiogenesis which results in retinal pathological neovascularization.
Figure 8. Proposed mechanism for the inhibitory effect on RNV by 15-LOX-1.
In recent years, progress has been made by inhibition of the angiogenic factors like VEGF.
15-LOX-1 was found being implicated in angiogenesis, but previous studies apparently contradicted on the angiogenic properties of 15-LOX-1[22], varied from promoting angiogenic effect to its suppression of angiogenesis[21],[30].The levels of 15-LOX-1 in normal and pathological conditions have not been revealed previously and the role of 15-LOX-1 in hypoxia-induced RNV needs further investigation. To the best of our knowledge, this is the first study to describe changes in the expression of 15-LOX-1 during angiogenesis model in vitro which results in retinal pathological neovascularization. The major findings of our study include (1) decreased expression of 15-LOX-1 and PPAR-r during angiogenesis model in vitro; (2) retinal 15-LOX-1 levels are negatively correlated with the proliferation of RMVECs; (3) inhibition of VEGF family expression by overexpression 15-LOX-1; (4) activation of PPAR-r effect on VEGFR2 by overexpression 15-LOX-1.
Our studies demonstrated decreased 15-LOX-1 and PPAR-r expressions in the hypoxia-induced RNV model of RMVECs. Moreover, the time course of the 15-LOX-1 change was negatively correlated with the proliferation of RMVECs. These findings suggested that 15-LOX-1 and PPAR-r may act as a negative regulator of retinal angiogenesis, and decreased 15-LOX-1 and PPAR-r levels led to RNV. However, the regulatory mechanism of 15-LOX-1 and PPAR-r expressions is presently unclear.
To evaluate the effect of 15-LOX-1 overexpression on RMVECs in the hypoxia-induced RNV model in vitro, we transferred Ad-15-LOX-1 into the RMVECs in the hypoxia environment. The CCK-8 results showed that the proliferative capacities of RMVECs in 15-LOX-1 group were significantly inhibited compared with hypoxia group. Our study presented the first evidence suggesting that overexpression of the 15-LOX-1 in the RMVECs of hypoxia-induced RNV model inhibited the proliferation of RMVECs which led to angiogenesis and then resulted in RNV ultimately. These observations further indicate that the effect of 15-LOX-1 overexpression is an anti-angiogenic factor in the RNV.
It is well-known that VEGF plays an important role in the pathogenesis of neovascularization. VEGF is a potent endothelial cell mitogen that stimulates endothelial cell over-proliferation, migration, tube formation, and ultimately leads to pathological neovascularization. But few researches have elucidated the relationship between VEGF and 15-LOX-1 on RNV. Our analysis showed that in a hypoxic condition, the expressions of VEGF-A, VEGF-R2 and eNOs mRNA decreased significantly in 15-LOX-1 group compared with hypoxia group. Our previous study displayed hypoxia triggers the induction of VEGF-A expression in RMVECs, which, through an antocrine mechanism, binds to the up-regulated receptor VEGFR2 and the messenger eNOs, to promote the cells proliferation and ultimately lead to angiogenesis which results in retinal pathological neovascularization. We hypothesized that the 15-LOX-1-mediated prevention of growth factor expression block these signaling cascades by inhibiting hypoxia-induced increasing in VEGF-A and VEGFR2 expression as well as increasing in eNOs.
The 15-LOX-1 is an endogenous ligand of PPAR-r[31]. Interestingly, it has been shown in rabbit eye model that PPAR-r binding to VEGFR2 promoter induced VEGFR2 expression, but ligand binding to PPAR-r actually resulted in an inhibition of VEGFR2 expression[32].
We hypothesized that 15-LOX-1 binding to PPAR-r in the RMVECs of hypoxia-induced RNV model could inhibit the hypoxia-induced expression of VEGFR2. Thus, PPAR-r effect on VEGFR2 could be an additional mechanism whereby 15-LOX-1 inhibits the hypoxia-induced angiogenesis.
In conclusion, we have shown that 15-LOX-1 and PPAR-r may act as a negative regulator of retinal angiogenesis, and decreased 15-LOX-1 and PPAR-r levels leading to RNV. And the effect of 15-LOX-1overexpression is an anti-angiogenic factor in hypoxia-induced RNV. Overexpression 15-LOX-1 in RMVECs of hypoxia-induced RNV blocks signaling cascades by inhibiting hypoxia-induced increases in VEGF-A and VEGFR2 expression as well as increases in eNOs. PPAR-r effect on VEGFR2 could be an additional mechanism whereby 15-LOX-1 prevents the hypoxia-induced angiogenesis.
Ranibizumab (trade name Lucentis) is the most effective treatment currently available for more damaging forms of RNV[33]. However, Ranibizumab has to be given intravitreally once a month because of short half-life (approximate 9 days)[34]. Patients have to undergo multiple rounds of intravitreal injection leading to complications like endophthalmitis, retinal detachment, and traumatic cataracts. Gene therapy may become more promising with comparable stable effect. Our study presented here showing that 15-LOX-1 gene transferred into RMVECs could also prevent hypoxia-induced RNV. For future gene therapy application in clinic, we need to make sure that the 15-LOX-1 gene introduced into RMVECs is functional and stable.
Understanding the mechanism of 15-LOX-1 pathway in inhibiting the proliferation of RMVECs may lead to developing better therapeutic approaches for the treatment of ocular neovascularization diseases.
Acknowledgments
We thank Fang Zhou, Li-Ping Wang, for technical assistance.
Footnotes
Foundation item: National Natural Science Foundation of China (No. 81000393)
REFERENCES
- 1.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438(7070):960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 2.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Wang G, Wang Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148(6):883–889. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. 2005;25(2):111–118. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Witmer AN, Vrensen G, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22(1):1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 6.Xia XB, Xiong SQ, Song WT, Luo J, Wang YK, Zhou RR. Inhibition of retinal neovascularization by sirna targeting vegf165. Mol Vis. 2008;14:1965–1973. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF, VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37(1):1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 8.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble vegf-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn H, Chaitidis P, Roffeis J, Walther M. Arachidonic acid metabolites in the cardiovascular system: The role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. J Cardiovasc Pharmacol. 2007;50(6):609–620. doi: 10.1097/FJC.0b013e318159f177. [DOI] [PubMed] [Google Scholar]
- 10.Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274(34):23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn H, Thiele BJ. The diversity of the lipoxygenase family: Many sequence data but little information on biological significance1. FEBS Lett. 1999;449(1):7–11. doi: 10.1016/s0014-5793(99)00396-8. [DOI] [PubMed] [Google Scholar]
- 12.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15s-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94(12):6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanez P, Bonnans C, Chavis C, Vachier I. 15-lipoxygenase: A janus enzyme? Am J Respir Cell Mol Biol. 2002;27(6):655–658. doi: 10.1165/rcmb.f253. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases: Structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68-69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 15.Funk CD. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta. 1996;1304(1):65–84. doi: 10.1016/s0005-2760(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 16.Shappell SB, Boeglin WE, Olson SJ, Kasper S, Brash AR. 15-lipoxygenase-2 (15-lox-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am J Pathol. 1999;155(1):235–245. doi: 10.1016/S0002-9440(10)65117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fürstenberger G, Krieg P, Müller-Decker K, Habenicht AJ. What are cyclooxygenases and lipoxygenases doing in the driver's seat of carcinogenesis? Int J Cancer. 2006;119(10):2247–2254. doi: 10.1002/ijc.22153. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn H, Römisch I, Belkner J. The role of lipoxygenase isoforms in atherogenesis. Mol Nutr Food Res. 2005;49(11):1014–1029. doi: 10.1002/mnfr.200500131. [DOI] [PubMed] [Google Scholar]
- 19.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MRF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo e-deficient mice. J Clin Invest. 1999;103(11):1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kühn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98(10):2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harats D, Ben-Shushan D, Cohen H, Gonen A, Barshack I, Goldberg I, Greenberger S, Hodish I, Harari A, Varda-Bloom N, Levanon K, Grossman E, Chaitidis P, Kühn H, Shaish A. Inhibition of carcinogenesis in transgenic mouse models over-expressing 15-lipoxygenase in the vascular wall under the control of murine preproendothelin-1 promoter. Cancer Lett. 2005;229(1):127–134. doi: 10.1016/j.canlet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in pc-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22(11):1765–1773. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, Zhou H. Grass carp transforming growth factor-beta 1 (TGF-beta 1): Molecular cloning, tissue distribution and immunobiological activity in teleost peripheral blood lymphocytes. Mol Immunol. 2008;45(6):1792–1798. doi: 10.1016/j.molimm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Godfrey TE, Kim SH, Chavira M, Ruff DW, Warren RS, Gray JW, Jensen RH. Quantitative mrna expression analysis from formalin-fixed, paraffin-embedded tissues using 5-nuclease quantitative reverse transcription-polymerase chain reaction. J Mol Diagn. 2000;2(2):84–91. doi: 10.1016/S1525-1578(10)60621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR andthe 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Bradley J, Ju M, Robinson GS. Combination therapy for the treatment of ocular neovascularization. Angiogenesis. 2007;10(1):141–148. doi: 10.1007/s10456-007-9069-x. [DOI] [PubMed] [Google Scholar]
- 27.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26(3):275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Oshima Y, Sakaguchi H, Gomi F, Tano Y. Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142(1):155–158. doi: 10.1016/j.ajo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Hernández C, Simó R. Strategies for blocking angiogenesis in diabetic retinopathy: From basic science to clinical practice. Expert Opin Investig Drugs. 2007;16(8):1206–1226. doi: 10.1517/13543784.16.8.1209. [DOI] [PubMed] [Google Scholar]
- 30.Viita H, Markkanen J, Eriksson E, Nurminen M, Kinnunen K, Babu M, Heikura T, Turpeinen S, Laidinen S, Takalo T, Ylä-Herttuala S. 15-lipoxygenase-1 prevents vascular endothelial growth factor A and placental growth factor-induced angiogenic effects in rabbit skeletal muscles via reduction in growth factor mRNA levels, NO bioactivity, and downregulation of VEGF receptor 2 expression. Circ Res. 2008;102:177–184. doi: 10.1161/CIRCRESAHA.107.155556. [DOI] [PubMed] [Google Scholar]
- 31.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 32.Sassa Y, Hata Y, Aiello LP, Taniguchi Y, Kohno K, Ishibashi T. Bifunctional properties of peroxisome proliferator-activated receptor gammal in KDR gene regulation mediated via interaction with both Sp1 and Sp3. Diabetes. 2004;53(5):1222–1229. doi: 10.2337/diabetes.53.5.1222. [DOI] [PubMed] [Google Scholar]
- 33.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian ML, Abedi G, Ness S, Ahmed E, Fenberg M, Daly MK, Houranieh A, Feinberg EB. Bevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trial. Eye. 2010;24(11):1708–1715. doi: 10.1038/eye.2010.147. [DOI] [PubMed] [Google Scholar]