Abstract
Careful analysis of the Arrhenius plot of the Na+,Mg2+-ATPase (ATP pyrophosphohydrolase, EC 3.6.1.8) activity in Acholeplasma laidlawii B membranes of varying fatty acid composition has been combined with differential thermal analysis of the membrane lipid phase transitions to evaluate the effects of membrane lipid properties on the enzyme activity. Our results indicate that the enzyme is active only in association with liquid-crystalline lipids, exhibiting a significant heat capacity of activation, delta Cp++, for the ATP hydrolytic reaction in this case. Quantitative analyses of Arrhenius plots for the enzyme activity in membranes whose lipids exhibit a gel-to-liquid-crystalline phase transition in the physiological temperature range suggest that the ATPase is inactivated when its boundary lipids undergo a phase transition that is driven by the bulk lipid phase transition but is less cooperative than the latter. Our results suggest that the familiar "biphasic linear" Arrhenius plots obtained for many membrane enzymes may in fact have a more complex shape, analysis of which can furnish useful information regarding the behavior of the enzyme molecule.
Full text
PDF

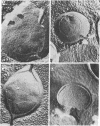
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman D., Gómez-Fernández J. C., Goñi F. M. Intrinsic protein--lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979 Feb 15;98(2):211–223. doi: 10.1016/0014-5793(79)80186-6. [DOI] [PubMed] [Google Scholar]
- Dockter M. E., Trumble W. R., Magnuson J. A. Membrane lateral phase separations and chlortetracycline transport by Bacillus megaterium. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1319–1323. doi: 10.1073/pnas.75.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. A., Glaser M., Bayer W. H., Vagelos P. R. Alteration of fatty acid composition of LM cells by lipid supplementation and temperature. Biochemistry. 1975 Jan 14;14(1):146–151. doi: 10.1021/bi00672a025. [DOI] [PubMed] [Google Scholar]
- Horwitz A. F., Wight A., Ludwig P., Cornell R. Interrelated lipid alterations and their influence on the proliferation and fusion of cultured myogenic cells. J Cell Biol. 1978 May;77(2):334–357. doi: 10.1083/jcb.77.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks D. C., Silvius J. R., McElhaney R. N. Physiological role and membrane lipid modulation of the membrane-bound (Mg2+, na+)-adenosine triphosphatase activity in Acholeplasma laidlawii. J Bacteriol. 1978 Dec;136(3):1027–1036. doi: 10.1128/jb.136.3.1027-1036.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Metcalfe J. C., Toon P. A., Warren G. B. Clusters in lipid bilayers and the interpretation of thermal effects in biological membranes. Biochemistry. 1974 Aug 27;13(18):3699–3705. doi: 10.1021/bi00715a013. [DOI] [PubMed] [Google Scholar]
- Lee M. P., Gear A. R. The effect of temperature on mitochondrial membrane-linked reactions. J Biol Chem. 1974 Dec 10;249(23):7541–7549. [PubMed] [Google Scholar]
- Marcelja S. Lipid-mediated protein interaction in membranes. Biochim Biophys Acta. 1976 Nov 11;455(1):1–7. doi: 10.1016/0005-2736(76)90149-8. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The effect of alterations in the physical state of the membrane lipids on the ability of Acholeplasma laidlawii B to grow at various temperatures. J Mol Biol. 1974 Mar 25;84(1):145–157. doi: 10.1016/0022-2836(74)90218-6. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N. The effect of membrane-lipid phase transitions on membrane structure and on the growth of Acholeplasma laidlawii B. J Supramol Struct. 1974;2(5-6):617–628. doi: 10.1002/jss.400020509. [DOI] [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. Mycoplasma membrane lipids: variations in fatty acid composition. Science. 1969 Apr 25;164(3878):433–434. doi: 10.1126/science.164.3878.433. [DOI] [PubMed] [Google Scholar]
- Mitranic M., Sturgess J. M., Moscarello M. A. The effect of temperature on the galactosyl- and sialyltransferases and on the ultrastructure of Golgi membranes. Biochim Biophys Acta. 1976 Aug 16;443(2):190–197. doi: 10.1016/0005-2736(76)90502-2. [DOI] [PubMed] [Google Scholar]
- Raison J. K. The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. J Bioenerg. 1973 Jan;4(1):285–309. doi: 10.1007/BF01516063. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., McElhaney R. N. Membrane lipid biosynthesis in Acholeplasma laidlawii B: incorporation of exogenous fatty acids into membrane glyco- and phospholipids by growing cells. J Bacteriol. 1977 Nov;132(2):485–496. doi: 10.1128/jb.132.2.485-496.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Shechter E., Letellier L., Gulik-Krzywicki G. Relations between structure and function in cytoplasmic membrane vesicles isolated from an Escherichia coli fatty-acid auxotroph. High-angle x-ray diffraction, freeze-etch electron microscopy and transport studies. Eur J Biochem. 1974 Nov 1;49(1):61–76. doi: 10.1111/j.1432-1033.1974.tb03811.x. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Growth and membrane lipid properties of Acholeplasma laidlawii B lacking fatty acid heterogeneity. Nature. 1978 Apr 13;272(5654):645–647. doi: 10.1038/272645a0. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Lipid compositional manipulation in Acholeplasma laidlawii B. Effect of exogenous fatty acids on fatty acid composition and cell growth when endogenous fatty acid production is inhibited. Can J Biochem. 1978 Jun;56(6):462–469. doi: 10.1139/o78-072. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., Read B. D., McElhaney R. N. Membrane enzymes: artifacts in Arrhenius plots due to temperature dependence of substrate-binding affinity. Science. 1978 Feb 24;199(4331):902–904. doi: 10.1126/science.146257. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Tourtellotte M. E., Reinert J. C., McElhaney R. N., Rader R. L. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci U S A. 1969 May;63(1):104–109. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant J. M., Mateo P. L. Proposed temperature-dependent conformational transition in D-amino acid oxidase: a differential scanning microcalorimetric study. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2584–2587. doi: 10.1073/pnas.75.6.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susi H., Sampugna J., Hampson J. W., Ard J. S. Laser-Raman investigation of phospholipid-polypeptide interactions in model membranes. Biochemistry. 1979 Jan 23;18(2):297–301. doi: 10.1021/bi00569a010. [DOI] [PubMed] [Google Scholar]
- Therisod H., Letellier L., Weil R., Shechter E. Functional lac carrier proteins in cytoplasmic membrane vesicles isolated from Escherichia coli. 1. Temperature dependence of dansyl galactoside binding and beta-galactoside transport. Biochemistry. 1977 Aug 23;16(17):3772–3776. doi: 10.1021/bi00636a007. [DOI] [PubMed] [Google Scholar]
- Thilo L., Träuble H., Overath P. Mechanistic interpretation of the influence of lipid phase transitions on transport functions. Biochemistry. 1977 Apr 5;16(7):1283–1290. doi: 10.1021/bi00626a007. [DOI] [PubMed] [Google Scholar]
- Ververgaert P. H., De Kruyff B., Verkleij A. J., Tocanne J. F., Van Deened L. L. Calorimetric and freeze-etch study of the influence of Mg2+ on the thermotropic behaviour of phosphatidylglycerol. Chem Phys Lipids. 1975 Feb;14(1):97–101. doi: 10.1016/0009-3084(75)90021-3. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Richards F. M., Engelman D. M. The influence of lipid state on the planar distribution of membrane proteins in Acholeplasma laidlawii. J Mol Biol. 1976 Nov 5;107(3):255–269. doi: 10.1016/s0022-2836(76)80004-6. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Parkes J. G., Huang Y. O., Fox C. F. Physical and physiological evidence for two phase transitions in cytoplasmic membranes of animal cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4381–4385. doi: 10.1073/pnas.71.11.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruyff B., van Dijck P. W., Godlbach R. W., Demel R. A., van Deenen L. L. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Dec 22;330(3):269–282. doi: 10.1016/0005-2736(73)90232-0. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L. Effect of glycophorin incorporation on the physico-chemical properties of phospholipid bilayers. Biochim Biophys Acta. 1978 Dec 4;514(1):9–24. doi: 10.1016/0005-2736(78)90073-1. [DOI] [PubMed] [Google Scholar]