Abstract
Hip fractures represent a serious health risk in the elderly, with significant associated morbidity and mortality. There is now an emerging literature that suggests that chronic hyponatremia increases the adjusted odds ratio (OR) for both falls and fractures in the elderly. Hyponatremia appears to contribute to falls and fractures by two mechanisms: (i) it produces mild cognitive impairment resulting in unsteady gait and falls and (ii) it directly contributes to osteoporosis and increased bone fragility by inducing increased bone resorption to mobilize sodium. There is debate over the effect of hyponatremia on the production of osteoporosis, as one study found decreased bone mineral density (BMD) and another did not. Should we be screening for low serum sodium in patients with osteoporosis or assessing BMD in patients with hyponatremia? The final answer is yet to come from prospective studies that allocate elderly individuals with mild hyponatremia to receive active treatment or not for hyponatremia and see if this intervention prevents gait disturbances and changes in BMD reducing fracture risk. In the meantime, physicians caring for elderly patients must be aware of the association between hyponatremia and bone problems. As serum sodium is a readily available, simple and affordable biochemical measurement, clinicians should look for hyponatremia in elderly patients who take medications that can cause hyponatremia. Also, elderly patients with unsteady gait and/or confusion should be checked for the presence of mild hyponatremia and if present it should not be ignored. Finally, elderly patients presenting with an orthopedic injury should have serum sodium checked and corrected if hyponatremia is present.
Keywords: elderly, falls, fractures, hyponatremia, risk factors
Introduction
Hyponatremia, usually defined as a serum sodium <135 mmol/L, is the most common electrolyte disorder in hospitalized patients. Its mean prevalence at admission is ∼2–4% increasing to ∼5% among inpatients [1]. Severe symptomatic hyponatremia induces a condition known as hyponatremic encephalopathy that can result in brain injury [1]. Hyponatremic encephalopathy primarily results from cerebral edema, which is caused by a flux of water into the brain down a concentration gradient [2]. This results in intracranial hypertension that can induce severe complications such as transtentorial herniation of the brainstem. Patients at high risk of developing this complication include postoperative patients, young females, children and patients with hypoxia or central nervous system disease [2, 3].
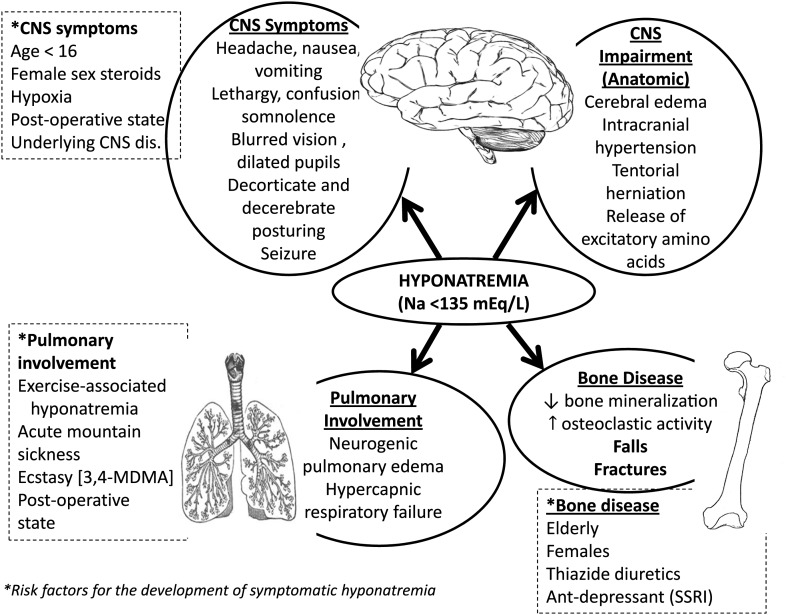
Although the brain seems to be the main target for hyponatremia, other organs can be affected by this condition (Figure 1). Recent information indicates that lung abnormalities, such as respiratory failure, can be a consequence of hyponatremic encephalopathy [4]. One distinct aspect of this condition is the so-called Ayus–Arieff syndrome: a form of non-cardiogenic pulmonary edema secondary to increased intracranial pressure from cerebral edema [5, 6].
Fig. 1.
Overview of the pathophysiology of hyponatremia associated signs and symptoms in multiple organs.
In addition to the morbidity and mortality of untreated hyponatremic encephalopathy, another rare but potentially serious complication of hyponatremia is cerebral demyelination. Cerebral demyelination is seen more commonly in patients with chronic hyponatremia with associated risk factors such as alcoholism, liver disease, malnutrition, hypokalemia and hypoxia [1, 7, 8]. In addition to these factors, the total amount of correction of serum sodium in the first 48h of treatment has been implicated, with a 25 Meq/L of sodium change in the first 48 h of treatment as a potential risk factor in the pathogenesis of this condition [7, 9, 10].
In contrast, mild-to-moderate chronic hyponatremia (120–135 mEq/L) is generally considered ‘asymptomatic’, as a result of the volume regulatory processes [1], and is believed to be without consequences. However, new information about the potential long-term adverse effects has not been carefully evaluated in controlled studies of hyponatremia in the elderly population—so-called asymptomatic hyponatremia.
Prevalence and etiology of hyponatremia in the elderly
Hyponatremia is a common disorder in the elderly, affecting ∼10% of patients living at home and 20% living in nursing homes [11]. It is estimated that as many as 50% of nursing home residents will suffer one or more episodes of hyponatremia in a 12-month period [12]. Approximately 50% of chronic hyponatremia is due to the syndrome of inappropriate antidiuretic hormone secretion (SIADH) [1]. Other causes include drugs (such as thiazide diuretics, antidepressants and antiepileptic medications) and chronic medical conditions such as hypocortisolism, hypothyroidism, congestive heart failure hepatic cirrhosis and renal disease [1] (Table 1).
Table 1.
Common drugs associated with euvolemic hyponatremias
| Diuretics: thiazides; indapamide; spironolactone |
| Antidepressants: SSRIs; venlafaxine |
| Antiepileptics: oxcarbazepine; carbamazerpine; valproate |
| Proton pump inhibitors (PPI) |
| Antipsychotics: aripiprazole; mirtazapine |
| Chemotherapeutics: cisplatin; Trimethoprim-sulfamethoxazole; Voriconazole |
| Recreational party drugs: N-benzylpiperazine; 3,4-methylenedioxymethamphetamine or ecstasy. |
The importance of thiazide-induced hyponatremia (TIH) is re-emerging because thiazide diuretic prescription seems to be increasing after the guidelines recommending thiazides as first-line treatment of essential hypertension have been introduced [13]. In a recent retrospective cohort study of adult outpatients treated for hypertension, ∼3 in 10 patients exposed to thiazides who continue to take them develop hyponatremia. The risk factors predisposing to TIH are old age, female gender, reduced body mass and concurrent use of other medications that impair water excretion [1]. While taking thiazides, the elderly may have a greater defect in water excretion after water load compared with young subjects [1].
Among antidepressants, selective serotonin re-uptake inhibitors (SSRIs) are probably the drugs that more frequently induce antidiuretic hormone release and SIADH [14–16]. Its precise prevalence and incidence in the elderly are hard to determine because of confounding factors including other prescribed medications and medical conditions. Although hyponatremia has been reported with all SSRIs and venlafaxine, most studies are small, retrospective, limited by confounding variables or are individual case reports. The risk of developing hyponatremia while on an SSRI seems to increase with age, female sex, previous history of hyponatremia and the concomitant use of other medications known to induce hyponatremia [14]. The sodium concentrations of most patients with SSRI-associated hyponatremia return to normal within days to weeks of SSRI withdrawal. A few cases of SSRI rechallenge indicate that hyponatremia may sometimes be a transient effect with tolerance developing over time [14].
Another group of drugs that induce hyponatremia are antiepileptics. The frequency of hyponatremia is approximately double among oxcarbazepine-treated patients compared with carbamazerpine-treated patients and its severity is also greater. Again, advanced age was a risk factor for its development [17].
Risk factors for hip fractures in the elderly
Hip fractures represent a serious health risk in the elderly, with a significant associated morbidity and mortality [18]. Approximately 13.5% of those who suffer a hip fracture die within 6 months and 24% within 1 year of the episode [19]. Among those who survive to 6 months, only 50% recover their ability to perform activities of daily living and only 25% recover their ability to perform instrumental activities of daily living [20]. The primary identifiable risk factors for hip fracture are advanced age, female sex, osteoporosis, low vitamin D levels and calcium intake, physical inactivity, dizziness and balance disorders, and prior history of fractures [18]. Bone mineral density (BMD) is a strong predictor of fracture, yet most fractures occur in individuals without osteoporosis by BMD criteria. To improve fracture risk prediction, the World Health Organization recently developed a country-specific fracture risk index of clinical risk factors (FRAX) that estimates 10-year probabilities of hip and major osteoporotic fracture [21]. The included risk factors comprise femoral neck bone mineral density, prior fractures, parental hip fracture history, age, gender, body mass index, ethnicity, smoking, alcohol use, glucocorticoid use, rheumatoid arthritis and secondary osteoporosis (Table 2). Other important clinical risk factors (such as falls) have not yet been included in the FRAX algorithms because they have not been validated in prospective cohorts. Thus, there is a continuous search for new risk factors that could increase risk fracture prediction.
Table 2.
Risk factors for hip fractures
| Age | Falls |
| Gender | Low vitamin D levels |
| Body mass index | Low calcium intake |
| Ethnicity | Prolonged immobilization |
| Femoral neck BMD | Early menopause (<40 years) |
| Previous fractures | Surgical menopause (<45 years) |
| Parent fractured hip | Organ transplantation |
| Smoking | Diabetes |
| Alcohol use | Chronic renal failure |
| Glucocorticoid use | Use of thiazolidinediones |
| Rheumatoid arthritis | Use of PPI |
| Secondary osteoporosis | Hyponatremia |
Validated (FRAXR) not yet validated.
Can bone abnormalities be a consequence of chronic hyponatremia?
In 1999, our group first described that an orthopedic injury was a frequent initial manifestation of chronic hyponatremic encephalopathy in elderly women [22]. There is now an emerging literature that suggests that chronic hyponatremia may be an independent risk factor for both falls and fractures in the elderly.
Epidemiologic association between fractures and chronic hyponatremia
Studies have revealed that the incidence of hyponatremia is significantly higher in elderly patients presenting with a fracture than those without (Table 3). Gankam Kengne et al. [23] evaluated the incidence of hyponatremia (Na <135 mmol/L) in 513 elderly patients presenting to the emergency department with a bone fracture and compared them with a sex- and age-matched control group of ambulatory patients. Patients with bone fracture had a significantly higher incidence of hyponatremia than controls (13% versus 3.9%), with an adjusted odds ratio (OR) of 4.16 for bone fracture associated with hyponatremia. Sandhu et al. [24] conducted a similar study comparing the incidence of hyponatremia in 364 elderly patients presenting to an emergency department with and without a fracture. The incidence of hyponatremia was significantly higher in the fracture group (9.1 versus 4.1%), with a mean serum sodium of the entire fracture group of 131 ± 2 mmol/L. Tolouian et al. [25] identified 249 patients ≥65 years of age who were admitted to a hospital with the diagnosis of hip fracture secondary to a fall, during a 3-year period and compared their serum Na levels on admission with those of 44 ambulatory patients admitted for elective hip or knee replacement surgery during the same time frame. The prevalence of hyponatremia in cases was 16.9%, versus 4.6% in controls (P = 0.03). Using a lower cutoff value for hyponatremia that eliminated the milder cases (<130 mmol/L), McPherson and Dunsmuir found in patients with hip fractures an incidence of preoperative and postoperative hyponatremia of only 2.8% [26].
Table 3.
Association between hyponatremia, falls and fractures in the elderly patients
| Author | Study design | Mean serum Na in HyNa group | N | Outcome |
|---|---|---|---|---|
| Ayus and Arieff [22] | Prospective study of postmenopausal women with chronic symptomatic HyNa (Na <130 mmol/L) | 111 ± 12 | 53 | 19% presented with orthopedic injury. |
| McPherson and Dunsmuir [26] | Retrospective study; incidence of moderate HyNa (Na <130 mmol/L) in patients with hip fractures. | N/A | 107 | 2.8% incidence of HyNa at presentation. |
| Renneboog et al. [30] | Case–control study; prevalence of falls in patients with chronic asymptomatic HyNa (Na <133 mmol/L) versus normonatremic controls. | 126 ± 5 | 244 | 21.3% incidence of falls in HyNa group versus 5.3% controls. |
| Renneboog et al. [30] | Prospective study evaluating gait disorders in patients with chronic asymptomatic HyNa (Na <132 mmol/L). | 128 ± 3 | 16 | Significant disorders in gait and attention. |
| Gankam Kengne et al. [23] | Case–control study; prevalence of HyNa in elderly patients (>65 years) presenting with and without bone fracture. | 131 ± 3 | 1026 | 13% incidence of HyNa in fracture patients versus 3.9% in controls. |
| Sandhu et al. [24] | Case-control study comparing the incidence of mild HyNa (Na <135 mmol/L) in elderly patients (>65 years) with and without large bone fracture. | 131 ± 2 | 728 | 9.1% incidence of HyNa in patients with fractures versus 4.1% in controls. |
| Verbalis et al. [33] | Cross-sectional cohort study; evaluation of BMD in patients >50 years with HyNa (Na <135 mmol/L) versus normonatremic controls in the NHANES III | 133 ± 2 | N/A | Adjusted OR of osteoporosis in HyNa adults was 2.87 times that among controls. |
| Kinsella et al. [27] | Cross-sectional cohort study; incidence of HyNa (Na <135 mmol/L) in women with and without a fracture who underwent previous bone densitometry measurement. | 132.2 ± 1.8 | 1408 | 8.7% incidence of HyNa in women with fracture versus 3.2% in those without. |
| Hoorn et al. [28] | Cross-sectional cohort study; incidence of falls and fractures in an elderly population with and without HyNa (Na <136 mmol/L) | 133.4 ± 2 | 5208 | 23.8% incidence of falls in HyNa versus 16.4% in those without. OR of V/non-V fracture in HyNa 1.39 and 1.78, respectively. |
| Tolouian et al. [25] | Case–control study; patients admitted for hip fracture secondary to fall compared to patients admitted for elective hip or knee replacement | 131 ± 2 | 249 | Prevalence of HyNa 16.9% in cases versus 4.65 in controls (P = 0.03). OR = 4.80;P = 0.04 |
HyNa: hyponatremic; V/non V: vertebral and non-vertebral.
Two recent studies have demonstrated that hyponatremia is associated with bone fracture. Kinsella et al. [27] analyzed data for 1408 consecutive women who underwent bone densitometry. Fractures were found in 18% of study patients and hyponatremia in 4.2%. Patients with fractures had a significantly higher incidence of hyponatremia than those without (8.7 versus 3.2%), with hyponatremic patients having a 2.25-fold increased adjusted OR for sustaining a fracture. Hoorn et al. [28] similarly evaluated 5208 elderly patients, of whom 7.7% were hyponatremic. Hyponatremic patients had an increased adjusted OR for vertebral and non-vertebral fractures of 1.34 and 1.61, respectively. Thus, the literature clearly suggests an epidemiologic association between chronic hyponatremia and bone fractures in the elderly.
Hyponatremia, falls and gait disturbance
Hip fractures in the elderly are usually associated with simple falls. These falls are frequently produced by gait disturbances that are a common medical problem in old age. A population-based study has shown a 35% prevalence of gait disorders among persons over age 70 [29]. Gait is mainly controlled by the premotor and motor areas of the frontal cortex; these areas project fibers to the basal ganglia and onward to the locomotor centers of the brainstem and cerebellum, which, in turn, control the spinal generators. Several types of gait disturbances are particularly common among elderly patients: (i) sensory (e.g. polyneuropathy); (ii) hypokinetic (e.g. Parkinson's disease); (iii) ataxic (e.g. degenerative cerebellar atrophy) and (iv) anxiety-related (e.g. fear of falling).
Recent data have now revealed that mild chronic hyponatremia is associated with unsteady gait and falls. Renneboog et al. [30] evaluated the incidence of falls among 122 patients admitted to the emergency department (mean age 72 years) with asymptomatic chronic hyponatremia (mean serum sodium 126 mmol/L) compared with 244 normonatremic matched controls. The incidence of falls was 21.3% in the hyponatremic group compared with 5.3% in the controls, with an adjusted OR of falls in patients with hyponatremia of 67 (95% confidence interval 7.5–607). To evaluate the mechanism of falls in hyponatremic patients, the authors performed eight attention tests and a gait test consisting of three steps ‘in tandem,’ in which they measured the ‘total traveled way’ by the center of pressure or the total traveled way in 16 adults with chronic asymptomatic hyponatremia (mean sodium 128 mmol/L) and tested them again following the correction of hyponatremia. The hyponatremic group demonstrated highly unstable gait and attention impairment, more severe than that observed in subjects with moderate alcohol consumption. The attention and gait abnormalities completely reversed following the correction of hyponatremia. Therefore, mild hyponatremia in and of itself can result in unsteady gait, cognitive impairment and falls by inducing subtle neurologic changes.
The adaptive process of the brain to chronic hyponatremia initially involves cellular loss of sodium, followed by potassium. If hyponatremia persists, the next stage of brain adaptation consists, in part, in loss of organic compounds, largely amino acids [31], as glutamine, an important neurotransmitter. This loss of cellular glutamine may contribute to some of the physical findings present in chronic hyponatremia, such as unsteady gait and falls [32].
Mechanism of bone disease induced by hyponatremia
There are new data to suggest that hyponatremia can directly contribute to osteoporosis and increased bone frangibility (see Figure 2). Hyponatremia appears to contribute both to worsening osteoporosis and to an unsteady gait that leads to falls. Two studies have demonstrated an association between hyponatremia and osteoporosis in humans, and this association has been confirmed in the animal model. Verbalis et al. [33] evaluated the contribution of hyponatremia to the development of osteoporosis in adults by analyzing the data from the Third National Health and Nutrition Examination Survey (NHANES III). After adjustments, they found that the OR for the development of osteoporosis was 2.87 times greater among adults with mild hyponatremia (mean serum sodium 133 mEq/L) than among those without. There was also a positive linear association between serum sodium and femoral neck bone mineral density in patients with hyponatremia. Kinsella [27] et al. also found in their study that hyponatremic individuals had lower bone mineral density and an increased prevalence of osteoporosis. Despite this, in the more recent prospective study in elderly subjects, hyponatremia was associated with an increased risk of vertebral fractures and incident nonvertebral fractures but not with decreased BMD [28].
Fig. 2.
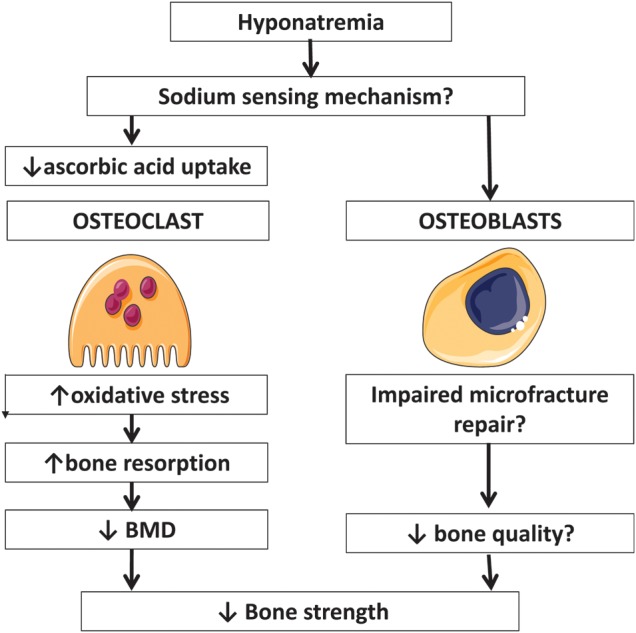
Mechanism of bone disease induced by hyponatremia.
Verbalis et al. [31] were able to produce similar results to those seen in humans in an animal model of hyponatremia. They found that rats with induced hyponatremia for 3 months had a 30% reduction in bone mineral density as measured by dual X-ray absorptiometry when compared with controls. It is important to note that the degree of hyponatremia in the animal model was much more severe than in the population studies. Bone histomorphology was highly abnormal, with a reduction in both trabecular and cortical bone contents and an increase in the number of osteoclasts per bone area. The rats also had decreased serum concentrations of osteocalcin. Further studies by this group demonstrated similar findings in cell culture, with low extracellular sodium directly stimulating osteoclastogenesis and bone resorptive activity [34]. Through cellular and molecular approaches they showed that chronic reduction of [Na(+)] dose-dependently decreased intracellular calcium without depleting endoplasmic reticulum calcium stores. Moreover, they found that reduction of [Na(+)] dose-dependently decreased cellular uptake of radiolabeled ascorbic acid, and reduction of ascorbic acid in the culture medium mimicked the osteoclastogenic effect of low [Na(+)]. They also detected downstream effects of reduced ascorbic acid uptake, namely evidence of hyponatremia-induced oxidative stress. This was manifested by increased intracellular-free oxygen radical accumulation and proportional changes in protein expression and phosphorylation. These results, therefore, reveal a novel sodium signaling mechanism in osteoclasts that may serve to mobilize sodium from bone stores during prolonged hyponatremia [34] These findings are supported by older studies [35, 36], demonstrating that one-third of total body sodium resides in bone. Forty percent of bone Na is exchangeable with the serum sodium, supporting the notion that chronic sodium depletion could lead to sodium loss from the bone with consequent bone demineralization.
An important question posed by this evidence is how bone cells sense the changes in extracellular sodium concentration and/or extracelluar osmolality. A high salt diet (HSD) triggers oxidative stress and kidney injury in salt sensitive hypertension. However, the mechanism for sensing increased extracellular Na(+) concentration [Na(+) ] has remained unclear. A Na(+) -activated Na(+) channel (Na sensor) has been described in the brain, and operates as a sensor of extracellular fluid [Na(+)]. It has been recently shown that rat kidney epithelial cells of the thick ascending limb and principal cells of the collecting duct possess this Na sensor that is upregulated by HSD, suggesting an important role in monitoring changes in tubular fluid [Na(+)] [37]. We still do not know if this sodium-activated sodium channel is present in bone cells. Another explanation proposed is that voltage-gated sodium channels, which are highly expressed in bone, could be sensitive to changes in extracellular tonicity or function as a sodium sensor in bone [38]. These voltage-gated sodium channels, which were previously only implicated in the propagation of action potentials, are also crucial in mammalian repair mechanisms [39]. It remains to be shown that sodium currents through these channels can favor bone microfracture repair. If hyponatremia impairs these mechanisms, bone fragility could be increased through a decrease in bone quality.
Perspective
What does this mean for the practicing clinician? Should serum sodium be viewed as a novel risk factor for gait disturbances and bone disease in the elderly which need to be monitored and corrected, similar to vitamin D?
From the discussion above, the following conclusions can be drawn. First, observational studies suggest that hyponatremia is associated with gait disturbances, falls, osteoporosis and fractures in elderly patients. There is still some debate over the association of hyponatremia with osteoporosis as low BMD was found in one study but not in another. Hyponatremia could lead to fractures through other effects on bone, such as decreased bone quality, which is not captured by BMD. Second, one translational study has demonstrated that hyponatremia decreases bone mass by stimulating bone resorption in a rat model of chronic hyponatremia. Third, one in vitro study has shown a plausible mechanism by which hyponatremia increases bone resorption that is reduced ascorbic acid uptake inducing oxidative stress. However, a separate factor could cause hyponatremia, osteoporosis and fractures; in this scenario, hyponatremia should be viewed as a surrogate marker. No studies have measured bone parameters before and after correction of hyponatremia. Although the animal study suggests a direct relationship of hyponatremia with bone mass and bone turnover, it does not completely exclude the possibility that factors associated with hyponatremia, such as a lower extracellular osmolality, can play a role. On the other hand, the observation that a low sodium level but not a low osmolality activates osteoclasts in vitro argues against the importance of osmolality. Prospective studies demonstrating that correction of hyponatremia affects clinical outcomes are lacking. Patients with mild chronic hyponatremia should receive active treatment and be examined for whether this intervention can change BMD and/ or reduce fracture risk. Thus, hyponatremia cannot yet qualify as a risk factor for fracture in the elderly. There is a clear need for additional research.
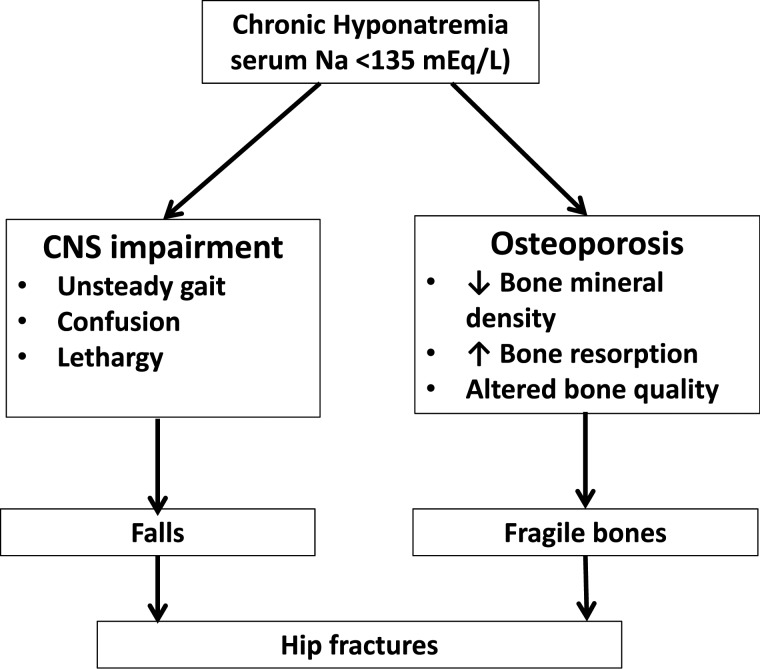
In the meantime, physicians caring for elderly patients must be aware of the association between hyponatremia and bone problems. We now know that mild hyponatremia can contribute to bone fractures in the elderly by two separate mechanisms (Figure 3) [40]. Hyponatremia can impair cognitive function and result in unsteady gait and falls. It can also produce bone impairment through osteoporosis (due to increased bone resorption in order to mobilize sodium from the bone) or altered bone quality (through decreased bone microfracture repair). As serum sodium is a readily available, simple and affordable biochemical measurement, clinicians should look for hyponatremia in elderly patients who take medications that can cause hyponatremia, namely diuretics, antidepressants and anticonvulsants. Thiazides should be avoided in frail elderly patients with chronically high water intake or in others who depend on the excretion of maximally dilute urine to maintain fluid balance, such as patients with psychogenic polydipsia or heavy beer drinking. Inadvertent rapid correction of hyponatremia is common in TIH because the ability to dilute the urine is restored when the diuretic is discontinued and volume deficits are repaired [41]. Also, elderly patients with unsteady gait and/or confusion should be checked for the presence of mild hyponatremia (Na <135 mEq/L) and if present, it should not be ignored. A thorough investigation should be conducted to determine the etiology, and the underlying cause should be treated. If the patient is asymptomatic, then medications which could cause hyponatremia should be withdrawn.
Fig. 3.
Mechanisms of hip fracture associated with chronic hyponatremia in the elderly [40].
Should we treat mild hyponatremia? Fluid restriction in patients with symptomatic hyponatremia is ineffective and associated with high morbidity and mortality as has been recently shown by our group [22]. Recently, a new class of drugs, vasopressin antagonists (vaptans), have been approved to treat hyponatremia. Two vaptans are now in the market for the treatment of euvolemic (Europe) or euvolemic and hypervolemic (USA) hyponatremia: conivaptan for intravenous use and tolvaptan for oral application. Although their specificity and effectiveness are considered established [42, 43], their indications are not. At present, we do not know which symptoms of hyponatremia and which degree of hyponatremia should serve as indications for vaptans. A single dose may be all that is needed to correct mild asymptomatic hyponatremia, while chronic administration could be of use if there is congestive heart failure, liver disease, SIADH or a medication that cannot be safely discontinued. Despite this, there are several points of uncertainty relating their use. The optimal vaptan regimen (dose, timing of controls) to treat SIADH is currently not established, as is the procedure to be recommended in a highly rapid correction rate of chronic hyponatremia. There are only few data regarding their safety and long-term side effects. In the SALTWATER study, the most common adverse effects attributed to tolvaptan were pollakiuria, thirst, fatigue, dry mouth, polydipsia and polyuria but only six drug-related adverse effects led to study discontinuation [44]. We do not know if vaptans will decrease the high mortality associated with hyponatremia or if the cost of chronic vaptan therapy will be justified [45]. Until these requirements are met by additional studies, we are hesitant to consider vaptans a treatment of choice for the appropriate hyponatremias.
Finally, if unsteady gait or confusion is found in an elderly patient with hyponatremia, active treatment with 100 mL of intravenous bolus with 3% sodium chloride (Na+ 513 Meq/L) should be considered to acutely reverse neurologic symptoms and get the patient out of harm's way, as recently proposed by our group [46]. Elderly patients presenting with an orthopedic injury should have serum sodium checked and corrected if hyponatremia is present.
Funding
K.K.Z. is supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01-DK078106 and K24-DK091419) and by a philanthropic grant from Mr. Harold Simmons.
Conflict of interest statement
None declared.
References
- 1.Moritz ML, Ayus JC. The pathophysiology and treatment of hyponatraemic encephalopathy: an update. Nephrol Dial Transplant. 2003;18:2486–2491. doi: 10.1093/ndt/gfg394. [DOI] [PubMed] [Google Scholar]
- 2.Ayus JC, Achinger SG, Arief A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol. 2008;295:F619–F624. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 3.Ayus JC, Wheeler JM, Arief AI. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med. 1992;117:891–897. doi: 10.7326/0003-4819-117-11-891. [DOI] [PubMed] [Google Scholar]
- 4.Ayus JC, Arieff AI. Pulmonary complications of hyponatremic encephalopathy: noncardiogenic pulmonary edema and hypercapnic respiratory failure. Chest. 1995;107:517–522. doi: 10.1378/chest.107.2.517. [DOI] [PubMed] [Google Scholar]
- 5.Ayus JC, Varon J, Arieff AI. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann Intern Med. 2000;132:711–714. doi: 10.7326/0003-4819-132-9-200005020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Nuguyen MK, Chang R, et al. Fatal hyponatremia in a young woman after ecstasy ingestion. Nat Clin Pract Nephrol. 2006;2:283–288. doi: 10.1038/ncpneph0167. [DOI] [PubMed] [Google Scholar]
- 7.Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317:1190–1195. doi: 10.1056/NEJM198711053171905. [DOI] [PubMed] [Google Scholar]
- 8.Ayus JC, Armstrong D, Arieff AI. Hyponatremia with hypoxia: effects on brain adaptation, perfusion, and histology in rodents. Kidney Int. 2006;69:1319–1325. doi: 10.1038/sj.ki.5000187. [DOI] [PubMed] [Google Scholar]
- 9.Ayus JC, Krothapalli RK, Armstrong DL, et al. Symptomatic hyponatremia in rats: effect of treatment on mortality and brain lesions. Am J Physiol. 1989;257(1 Pt 2):F18–F22. doi: 10.1152/ajprenal.1989.257.1.F18. [DOI] [PubMed] [Google Scholar]
- 10.Ayus JC, Krothapalli RK, Armstrong DL. Rapid correction of severe hyponatremia in the rat: histopathological changes in the brain. Am J Physiol. 1985;248:F711–F19. doi: 10.1152/ajprenal.1985.248.5.F711. [DOI] [PubMed] [Google Scholar]
- 11.Miller M. Hyponatremia and arginine vasopressin dysregulation: mechanisms, clinical consequences, and management. J Am Geriatr Soc. 2006;54:345–53. doi: 10.1111/j.1532-5415.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller M, Morley JE, Rubenstein LZ. Hyponatremia in a nursing home population. J Am Geriatr Soc. 1995;43:1410–3. doi: 10.1111/j.1532-5415.1995.tb06623.x. [DOI] [PubMed] [Google Scholar]
- 13.Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361:2153–2164. doi: 10.1056/NEJMra0907219. [DOI] [PubMed] [Google Scholar]
- 14.Bigaillon C, El Jahiri Y, Garcia C, et al. Inappropriate ADH secretion-induced hyponatremia associated with paroxetine use. Rev Med Interne. 2007;28:642–644. doi: 10.1016/j.revmed.2007.03.336. [DOI] [PubMed] [Google Scholar]
- 15.Nirmalani A, Stock SL, Catalano G. Syndrome of inappropriate antidiuretic hormone associated with escitalopram therapy. CNS Spect. 2006;11:429–432. doi: 10.1017/s1092852900014620. [DOI] [PubMed] [Google Scholar]
- 16.Fonzo-Christe C, Vogt N. Susceptibility of the elderly patient to hyponatremia induced by selective serotonin reuptake inhibitors. Therapie. 2000;55:597–604. [PubMed] [Google Scholar]
- 17.Dong X, Leppik IE, White J, et al. Hyponatremia from oxcarbazepine and carbamazepine. Neurology. 2005;65:1976–1978. doi: 10.1212/01.wnl.0000188819.45330.90. [DOI] [PubMed] [Google Scholar]
- 18.Porto Carreiro F, Christmas C. Hip Fracture. Ann Inter Med. 2011;155:ITC6-1–ITC6-15. doi: 10.7326/0003-4819-155-11-201112060-01006. [DOI] [PubMed] [Google Scholar]
- 19.Hannan EL, Magziner J, Wang JJ, et al. Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted outcomes. JAMA. 2001;285:2736–2742. doi: 10.1001/jama.285.21.2736. [DOI] [PubMed] [Google Scholar]
- 20.Magaziner J, Simonsick EM, Kashner TM, et al. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45:M101–M107. doi: 10.1093/geronj/45.3.m101. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, McCloskey EV, Johansson H, et al. Development and use of FRAX® in osteoporosis. Osteoporos Int. 2010;21(Suppl 2):S407–S413. doi: 10.1007/s00198-010-1253-y. [DOI] [PubMed] [Google Scholar]
- 22.Ayus JC, Arieff AL. Chronic hyponatremic encephalopathy in postmenopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281:2299–2304. doi: 10.1001/jama.281.24.2299. [DOI] [PubMed] [Google Scholar]
- 23.Gankam Kengne F, Andres C, Sattar L, et al. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM. 2008;101:583–588. doi: 10.1093/qjmed/hcn061. [DOI] [PubMed] [Google Scholar]
- 24.Sandhu HS, Gilles E, DeVita MV, et al. Hyponatremia associated with large-bone fracture in elderly patients. Int Urol Nephrol. 2009;41:733–737. doi: 10.1007/s11255-009-9585-2. [DOI] [PubMed] [Google Scholar]
- 25.Tolouian R, Alhamad T, Farazmand M, et al. The correlation of hip fracture and hyponatremia in the elderly. J Nephrol. 2011 doi: 10.5301/jn.5000064. 0 doi: 10.5301/jn.5000064 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.McPherson E, Dunsmuir RA. Hyponatraemia in hip fracture patients. Scott Med J. 2002;47:115–116. doi: 10.1177/003693300204700506. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella S, Moran S, Sullivan MO, et al. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5:275–280. doi: 10.2215/CJN.06120809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoorn EJ, Rivadeneira F, van Meurs JB, et al. Mild hyponatremia as a risk factor for fractures: The Rotterdam Study. J Bone Miner Res. 2011;26:1822–1828. doi: 10.1002/jbmr.380. [DOI] [PubMed] [Google Scholar]
- 29.Verghese J, Levalley A, Hall CB, et al. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54:255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renneboog B, Musch W, Vandemergel X, et al. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71 e1–e8. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Lien YH, Shapiro JI, Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. J Clin Invest. 1991;88:303–309. doi: 10.1172/JCI115292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. New Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 33.Verbalis JG, Barsony J, Sugimura Y, et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25:54–63. doi: 10.1359/jbmr.090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2011;286:10864–10875. doi: 10.1074/jbc.M110.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergstrom WH, Wallace WM. Bone as a sodium and potassium reservoir. J Clin Invest. 1954;33:867–873. doi: 10.1172/JCI102959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergstrom WH. The participation of bone in total body sodium metabolism in the rat. J Clin Invest. 1955;34:97–1004. doi: 10.1172/JCI103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lara LS, Sato R, Bourgeois CR, et al. The sodium-activated sodium 565 channel is expressed in the rat kidney thick ascending limb and collecting duct cells and is upregulated during high salt intake. Am J Physiol Renal Physiol. 2012;303:F105–F109. doi: 10.1152/ajprenal.00490.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoorn EJ, Liamis G, Zietse R, et al. Hyponatremia and bone: an emerging relationship. Nat Rev Endocrinol. 2011;8:33–39. doi: 10.1038/nrendo.2011.173. [DOI] [PubMed] [Google Scholar]
- 39.Tseng AS, Beane WS, Lemire JM, et al. Induction of vertebrate regeneration by transient sodium current. J Neurosci. 2010;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayus JC, Moritz ML. Bone disease as a new complication of hyponatremia: moving beyond brain injury. Clin J Am Soc Nephro. 2010;l5:167–178. doi: 10.2215/CJN.09281209. [DOI] [PubMed] [Google Scholar]
- 41.Hix JK, Silver S, Sterns RH. Diuretic-associated hyponatremia. Semin Nephrol. 2011;31:553–66. doi: 10.1016/j.semnephrol.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–712. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeltser D, Rosansky S, van Rensburg H, et al. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27:447–457. doi: 10.1159/000106456. [DOI] [PubMed] [Google Scholar]
- 44.Berl T, Quittnat-Pelletier F, Verbalis JG, et al. SALTWATER Investigators. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–712. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross PA, Wagner A, Decaux G. Vaptans are not the mainstay of treatment in hyponatremia: perhaps not yet. Kidney Int. 2011;80:594–600. doi: 10.1038/ki.2011.78. [DOI] [PubMed] [Google Scholar]
- 46.Ayus JC, Arieff A, Moritz ML. Hyponatremia in marathon runners. N Engl J Med. 2005;353:427–428. [PubMed] [Google Scholar]