Abstract
Background
Few longitudinal studies of children have taken place in the developing world, despite child mortality being concentrated there. This review summarises the methodologies and main outcomes of longitudinal studies of pre-school children (0 to 59 months) in the World Health Organization’s South East Asia (SEA) and Eastern Mediterranean (EM) Regions.
Methods
A systematic search of literature using pre-defined criteria revealed 7863 papers. After application of quality criteria, 120 studies were selected for analysis.
Results
The search revealed 83 studies in the SEA region and 37 in the EM region, of which 92 were community-based and 8 facility-based. Objectives were diverse but topics included growth (n = 49 studies), mortality (n = 28), nutrition (n = 24), and infectious diseases (n = 33). Only 12 studies focused on non-communicable diseases. Duration ranged from 7 to 384 months. Measurements included anthropometric (n = 56 studies), socioeconomic (n = 50) and biological sampling (n = 25), but only one study was DNA-based.
Conclusion
Biobanks have emerged as the most successful approach to generating knowledge about disease causes and mechanisms. Little of this is possible to undertake in the in SEA or EM regions, however. Further longitudinal studies of young children with DNA sampling should be set up to better understand determinants of diseases in low-income countries.
Very few longitudinal studies of children have been conducted in the developing world, despite the global burden of child morbidity and mortality being centred there (1,2). A history of birth cohort studies in the UK has outlined the important determinants of individual well-being and established these studies as invaluable in planning services (3-7). Examining genetic and environmental determinants of child health and disease is fundamental in reducing child mortality, to achieve the Millennium Development Goal 4 – “to reduce child mortality by two thirds by 2015” (8), Health determinants in low and middle-income countries are somewhat different to those studied in the UK and developed world, however. The most taxing diseases in the developing world are those that affect mothers and children and continue to cause high morbidity and mortality (9). Infections are still of greatest concern (including acute respiratory infections, diarrhoea, malaria, HIV/AIDS, tuberculosis and maternal tetanus), followed by neonatal issues (including birth asphyxia or preterm birth) (2,9,10). Further birth cohort studies may better establish the determinants of such diseases and inform health expenditures. Understanding the epidemiology of those diseases has important policy implications. There has been some concern within the scientific community for example, toward the movements against a HiB-vaccine programme in India (11).
A birth cohort study with genetic samples could considerably advance the understanding of the influence of genetics and epigenetics on disease burden, particularly in developing countries. This is because the natural selection imposed by infectious diseases through child mortality has been shaping the human genome for hundreds of thousands of years (12). Large biobanks have emerged as the most successful way of harnessing the new health research technologies available and generating new knowledge about disease causes and mechanisms. Moreover, storage of samples may enable testing of future hypotheses, although the screening of biobanks for research purposes does not need to be hypothesis-driven, and the whole of the genome may be screened in search for genetic associations. There has been a surge in global interest towards birth cohort studies in recent years, as shown by the trend in global publications (Figure 1). It is essential however that this interest be directed toward those in greatest need in the future, thus reducing inequalities in global health research.
Figure 1.
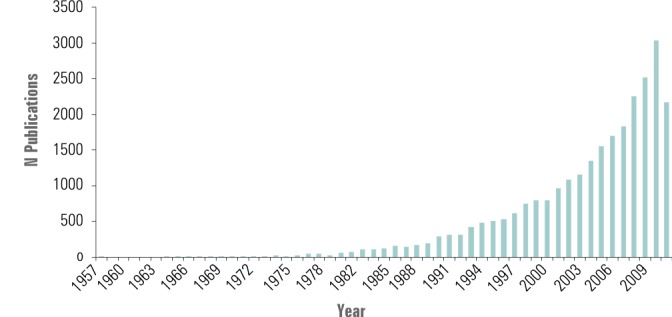
Global number of publications per year when ‘birth* cohort*’ was used as a search term in ISI Web of Knowledge database on 27 September 2010; no language or other restrictions.
The aims of the present paper were:
1. to produce a systematic review of birth cohort studies from the World Health Organisation’s South East Asia (SEA) and Eastern Mediterranean (EM) regions;
2. to examine the methodology, data collected from, and strengths / weaknesses of these studies and
3. to offer recommendations on the feasibility and sustainability of this area of research based on the findings of this review and in the context of the current literature.
METHODS
Search strategy
An initial scoping exercise was conducted to identify key words and MeSH headings, and the final search terms agreed with a librarian (Table 1). Systematic searches were run across the following electronic databases via Ovid (Figure 2): Medline (1950-onwards) on 30 July 2010 and Embase (1980-onwards) on 7 July 2010.
Table 1.
Search strategy for Medline and Embase*
|
Birth Cohort / Longitudinal Study
1. Longitudinal Studies/
2. (birth* adj3 longitudinal).ti,ab.
3. (birth* adj3 cohort*).ti,ab.
4. (pregnan* adj3 cohort*).ti,ab.
5. famil* cohort*.ti,ab.
6. cohort* survey*.ti,ab.
7. panel stud*.ti,ab.
8. panel survey*.ti,ab. |
| AND |
| Developing Country 1. Developing Countries/ 2. low income countr*.tw. 3. middle income countr*.tw. 4. (low adj2 middle income countr*).tw. 5. africa/ or africa, northern/ or algeria/ or egypt/ or libya/ or morocco/ or tunisia/ or “africa south of the sahara”/ or africa, central/ or cameroon/ or central african republic/ or chad/ or congo/ or “democratic republic of the congo”/ or equatorial guinea/ or gabon/ or africa, eastern/ or burundi/ or djibouti/ or eritrea/ or ethiopia/ or kenya/ or rwanda/ or somalia/ or sudan/ or tanzania/ or uganda/ or africa, southern/ or angola/ or botswana/ or lesotho/ or malawi/ or mozambique/ or namibia/ or south africa/ or swaziland/ or zambia/ or zimbabwe/ or africa, western/ or benin/ or burkina faso/ or cape verde/ or cote d'ivoire/ or gambia/ or ghana/ or guinea/ or guinea-bissau/ or liberia/ or mali/ or mauritania/ or niger/ or nigeria/ or senegal/ or sierra leone/ or togo/ or “antigua and barbuda”/ or cuba/ or dominica/ or dominican republic/ or grenada/ or guadeloupe/ or haiti/ or jamaica/ or “saint kitts and nevis”/ or saint lucia/ or “saint vincent and the grenadines”/ or central america/ or belize/ or costa rica/ or el salvador/ or guatemala/ or honduras/ or nicaragua/ or panama/ or panama canal zone/ or mexico/ or argentina/ or bolivia/ or brazil/ or chile/ or colombia/ or ecuador/ or guyana/ or paraguay/ or peru/ or suriname/ or uruguay/ or venezuela/ or asia, central/ or kazakhstan/ or kyrgyzstan/ or tajikistan/ or turkmenistan/ or uzbekistan/ or cambodia/ or east timor/ or indonesia/ or laos/ or malaysia/ or myanmar/ or philippines/ or thailand/ or vietnam/ or asia, western/ or bangladesh/ or bhutan/ or india/ or sikkim/ or afghanistan/ or iran/ or iraq/ or jordan/ or lebanon/ or syria/ or turkey/ or yemen/ or nepal/ or pakistan/ or sri lanka/ or exp china/ or exp japan/ or korea/ or “democratic people's republic of korea”/ or “republic of korea”/ or mongolia/ or albania/ or lithuania/ or bosnia-herzegovina/ or bulgaria/ or byelarus/ or “macedonia (republic)”/ or moldova/ or montenegro/ or romania/ or russia/ or serbia/ or ukraine/ or yugoslavia/ or exp transcaucasia/ or armenia/ or azerbaijan/ or “georgia (republic)”/ or comoros/ or madagascar/ or mauritius/ or seychelles/ or fiji/ or papua new guinea/ or vanuatu/ or palau/ or samoa/ or tonga/ |
*Within a box, all terms were combined with Boolean operator OR.
Figure 2.
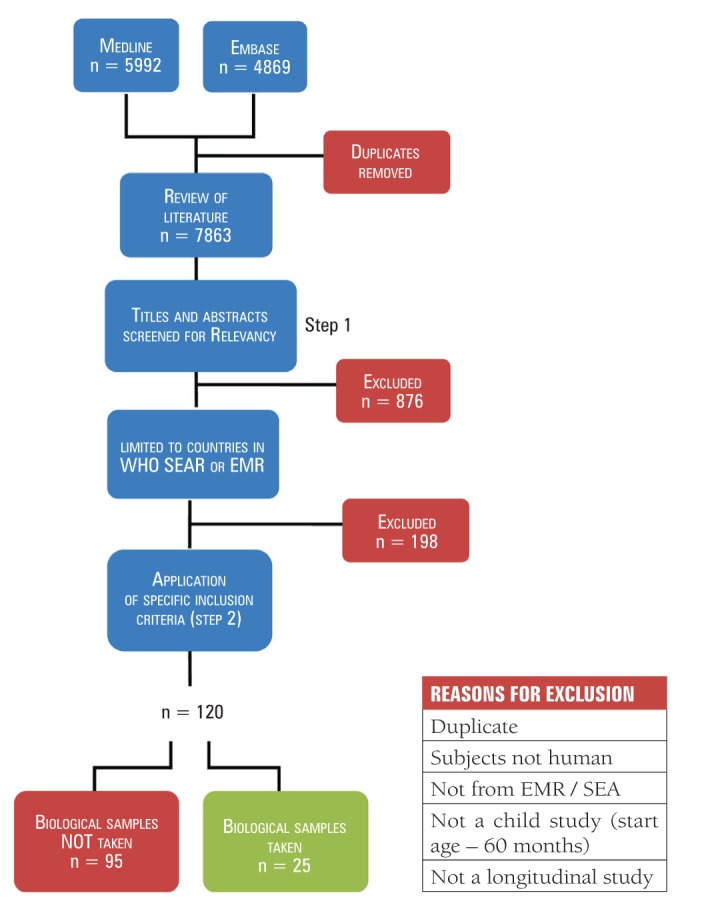
Summary of the literature search. SEAR – South East Asian Region, EMR – Eastern Mediterranean Region of the World Health Organisation (WHO).
Specific countries were those identified by the World Bank list of economies (July 2011) as being lower or middle income countries. There were no additional limitations placed on publication date, type or language.
Inclusion and exclusion criteria
All titles, abstracts, then full papers were screened for relevance (Table 2).
Table 2.
Inclusion steps
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Stage 1 – Titles
• Primary data OR methods from a birth cohort study / child longitudinal study (including cross-sectional data)
• Meta-analysis of several birth cohort / child longitudinal studies |
Stage 1
• Not from a developing country (12) |
| Stage 2 – Abstracts and Full Papers • Country in WHO South East Asian or Eastern Mediterranean regions. | Stage 2 • Start age <60 mo. |
Data extraction
Data were extracted from abstracts and entered into Microsoft Excel sheets. Where more than one paper referred to a single study, the first chronologically published paper was entered into the database and any additional data from other paper(s) imputed into relevant cells. Noted study characteristics include: country, city/state, urban/rural, year of start, recruitment strategy, start number, start age, proportion of population, specific population characteristics, measurements and observations, measurement frequency, family inclusion, end age, duration, attrition, study aims, funding.
Data analysis
Measurements were categorized into anthropometric, socioeconomic, and biological samples / measurements. Study aims and/or outcomes were characterised into topics. These included infectious diseases (respiratory tract infections (RTIs), diarrhoea, other), non-communicable diseases, nutrition, growth, socioeconomic factors, and mortality. Some studies were relevant to ≥1 topic. To provide an overview of studies, data types were counted. Where possible, summations and calculations were done with Excel formulae to minimise human error. A specialist programme (StatPlanet MapMaker, developed by Frank van Cappelle, 2011; http://www.sacmeq.org/statplanet) was used to display the geographical spread of studies.
RESULTS
Study characteristics
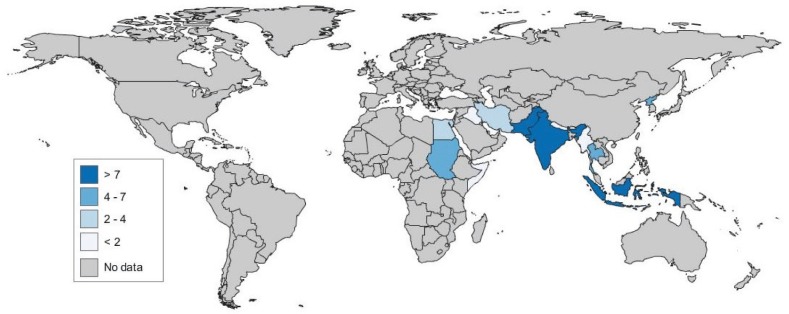
We analyzed 120 studies. There were only a small number of papers being published each year on birth cohort studies in the SEA or EM regions (with a maximum of 10 in total) and little change in trend in the past 20 years (Figure 3). In total, there were more than twice the number of studies in the SEA region (n = 83) compared to the EM region (n = 37) and these are most concentrated in India and Bangladesh and in Pakistan, respectively (Figure 4).
Figure 3.
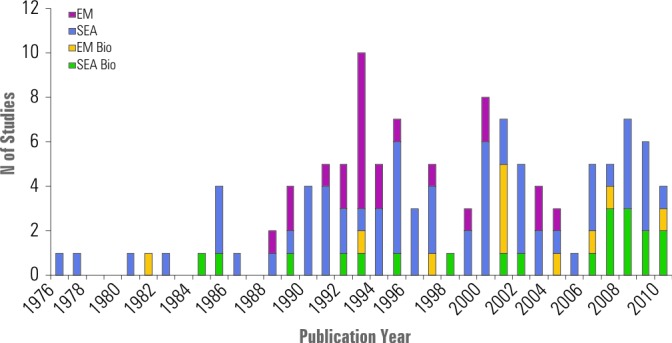
Number of studies published each year (n = 120). EM – Eastern Mediterranean, SEA – South East Asia, Bio – countries in the region taking biological samples (Bio).
Figure 4.
Geographical spread of all birth cohort studies in South East Asia (SEA) and Eastern Mediterranean (EM) regions (n = 120).
An overview of studies (Table 3, Supplementary Web Table 1)(Supplementary Web Table 1) revealed large diversity in their methodologies.
Table 3.
Characteristics of all studies in South East Asia (SEA) and Eastern Mediterranean (EM) regions (n = 120)
| Characteristic | WHO SEA | WHO EMR | EMR or SEA (% 120) | |
|---|---|---|---|---|
| Year |
Pre 1980 |
1 |
1 |
2 (1.7) |
| 1980–1989 |
9 |
5 |
14 (11.7) |
|
| 1990–1999 |
31 |
16 |
47 (39.2) |
|
| 2000–2010 |
42 |
15 |
57 (47.5) |
|
| Number of subjects at start |
<100 |
4 |
2 |
6 (4.2) |
| 100–499 |
25 |
6 |
31 (25.8) |
|
| 500–999 |
13 |
5 |
18 (15.0) |
|
| ≥1000 |
22 |
11 |
33 (27.5) |
|
| No data* |
19 |
13 |
32 (26.7) |
|
| Max age at start (months) |
<0 |
5 |
24 |
29 (24.2) |
| 0–3 |
28 |
1 |
29 (24.2) |
|
| 3–6 |
0 |
0 |
0 (0.0) |
|
| 6–12 |
1 |
0 |
1 (0.0) |
|
| 12–59 |
14 |
1 |
15 (12.5) |
|
| No data* |
35 |
11 |
46 (38.3) |
|
| Duration of follow up (months) |
<12 |
1 |
0 |
1 (0.8) |
| 12– 24 |
16 |
13 |
29 (24.2) |
|
| 25–60 |
15 |
3 |
18 (15.0) |
|
| >60 |
3 |
0 |
3 (2.5) |
|
| No data* |
48 |
21 |
69 (57.5) |
|
| Measurements / Samples (≥1 possible per study) |
Anthropometric |
40 |
16 |
56 (46.7) |
| Socioeconomic |
28 |
22 |
50 (41.7) |
|
| Biological |
17 |
8 |
25 (20.8) |
|
| DNA |
0 |
1 |
1 (0.0) |
|
| Family included* |
Yes |
11 |
7 |
18 (15.0) |
| No |
40 |
13 |
53 (44.2) |
|
| No data* |
32 |
17 |
49 (40.8) |
|
| Setting |
Community-based |
65 |
27 |
92 (76.6) |
| Facility-based |
5 |
3 |
8 (6.6) |
|
| No data |
13 |
7 |
20 (16.7) |
|
| Topic (≥1 possible per study) |
Growth |
31 |
18 |
49 (40.8) |
| Nutrition |
21 |
3 |
24 (20.0) |
|
| Infectious diseases |
24 |
9 |
33 (27.5) |
|
| Non-communicable diseases |
6 |
6 |
12 (10.0) |
|
| Socioeconomic effects |
13 |
7 |
20 (16.7) |
|
| Mortality | 25 | 3 | 28 (23.3) | |
*Some papers did not include data searched for. Moreover, some full papers (n=22) were not obtained and details not presented in abstract.
The number of children enrolled ranged from 22 (13) to 5 711 337 (14). The latter were recruited retrospectively in Korea using nationally linked birth and death certificates, but made no active measurements. The largest prospective study was a cohort of 3729 in rural Bangladesh followed from birth for 36 months to examine the effect of birth spacing on mortality (15). In most studies (58), subjects were <3 months of age at the start, with many studies recruiting mothers during pregnancy. A number of the studies presented recruited older children (Table 3, Supplementary Web Table 1)(Supplementary Web Table 1). Studies tended to be of short duration (12–24 months), but at least three of them followed up their subjects for >60 months. The Mysore Parthenon cohort (16), in which maternal serum folate, B12 and homocysteine concentrations were measured during pregnancy and child cognition (measured at 9 years) was a positive example. An even better established cohort is a New-Delhi cohort contacted at mean 32 years, with birth weight and adult glucose metabolism examined (17). Most studies tended to record socioeconomic measurements. These included parental age, education and occupation, and often indicators of household wealth (including monthly income, land size, possession of animals, and possession of transportation). Living circumstances (number of rooms: family size ratio, water supply, proximity of latrine) were also measured occasionally. Other observations commonly made in retained studies included family size and spacing and nutrition practices (prelactal feeds, exclusivity of breastfeeding, specific supplements given). Finally, exposures (eg, specific occupational hazards, household cigarettes or fuel-burning), utilisation of health services and mental health were also noted. There were only 25 studies taking biological samples (Figure 5) and their characteristics are presented in Tables 4–5.
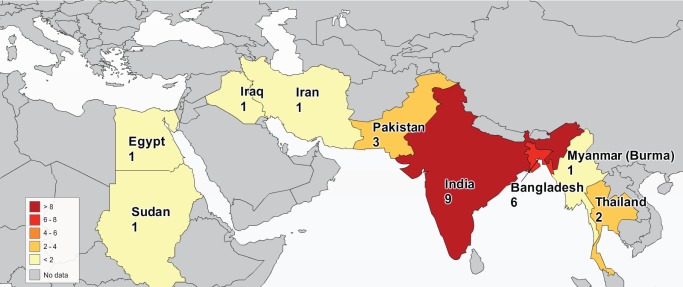
Figure 5.
Geographical spread of birth cohort studies in the Eastern Mediterranean (EM) and South East Asia (SEA) regions taking biological samples (n = 25).
Table 4.
Studies in South East Asia (SEA) and Eastern Mediterranean (EM) regions taking biological samples (n = 25)
| Ref | Author | Country | Location | Publication year | Study type | Number subjects enrolled (attrition %) | Max age at enrolment (months) | Duration of follow up (months) | Frequency of follow up |
|---|---|---|---|---|---|---|---|---|---|
| 58 |
Bhuiyan et al. |
Bangladesh |
Dhaka |
2009 |
prospective |
238 (25.9) |
0 |
24 |
Monthly |
| 34 |
Rousham et al. |
Bangladesh |
Jalampur |
1998 |
prospective |
117 (4.9) |
59 |
12 |
0, 2, 4, 6, 8, 12 months |
| 61 |
Black et al. |
Bangladesh |
Matlab |
1984 |
prospective |
197 (–*) |
48 |
12 |
Every second day |
| 129 |
Raqib et al. |
Bangladesh |
Matlab |
2007 |
retrospective |
132 (0) |
0 |
60 |
0, 60 months |
| 35 |
Hasan et al. |
Bangladesh |
Mirzapur |
2006 |
prospective |
252 (3.2) |
0 |
24 |
Twice weekly |
| 77 |
Granat et al. |
Bangladesh |
Svar |
2007 |
prospective |
99 (3.0) |
0 |
24 |
Fortnightly |
| 55 |
Bhan et al. |
India |
Anapur-Palla |
1989 |
prospective |
452 (0) |
35 |
13 |
Weekly |
| 17 |
Fall et al. |
India |
Dehli |
2008 |
retrospective |
1492 (0) |
0 |
384 |
6 monthly |
| 76 |
Gladstone et al. |
India |
Haryana |
2007 |
prospective |
281 (7.3) |
10 |
20 |
Weekly |
| 119 |
Mathur et al. |
India |
Hyderabad |
1985 |
prospective |
721 (–*) |
59 |
12 |
* |
| 63 |
Broor et al. |
India |
Mysore |
2010 |
cross-sectional |
663 (19.2) |
<0 |
120 |
Annually till 5 years, 6 months thereafter |
| 146 |
Yajnik et al. |
India |
Pune |
1995 |
retrospective |
379 (–*) |
0 |
48 |
* |
| 68 |
Coles et al. |
India |
Tamilnadu |
2001 |
prospective |
539 (13.9) |
0 |
6 |
2 monthly |
| 51 |
Banerjee et al. |
India |
Vellore |
2006 |
prospective |
452 (–*) |
0 |
36 |
Twice weekly |
| 126 |
Raghupathy et al. |
India |
Vellore |
2010 |
retrospective |
2218 (13.8) |
0 |
336 |
0, 3 mo, every 6 months until 14 year, mean 336 months |
| 100 |
Khin-Maung-U et al. |
Myanmar |
Intakaw |
1990 |
prospective |
75 (0) |
59 |
6 |
Monthly |
| 148 |
Burke et al. |
Thailand |
Bangkok |
1988 |
retrospective |
218† (–*) |
48 |
8 |
Daily |
| 132 |
Ruangkan-Chanasetr et al. |
Thailand |
Bangkok |
2002 |
prospective |
84 (–*) |
0 |
72 |
Twice yearly till 2 years, 6 years |
| 149 |
Bassily et al. |
Egypt |
Alexandria |
1999 |
prospective |
169 (–*) |
<0 |
18 |
At 8, 18 months |
| 98 |
Kelishadi et al. |
Iran |
Isfahan |
2007 |
prospective |
442 (0) |
0 |
0, samples stored for future |
At birth, 72h |
| 13 |
Amin-Zak et al. |
Iraq |
Baghdad |
1981 |
retrospective |
32 (0) |
12 |
66 |
At 18, 42, 66 months |
| 99 |
Khalil et al. |
Pakistan |
Lahore |
1991 |
prospective |
1476 (–*) |
0 |
24 |
* |
| 115 |
Lone et al. |
Pakistan |
Lahore |
2004 |
prospective |
629 (–*) |
<0 |
1 |
At 1 months |
| 142 |
Tikmani et al. |
Pakistan |
Karachi |
2010 |
prospective |
1690 (–*) |
0 |
2 |
Weekly |
| 88 | Ibrahim et al. | Sudan | Khartoum | 2006 | prospective | 205 (16.1) | 0 | 24 | 6 monthly |
*Not stated.
†Branch (aged 4 years) of a larger study (aged 4 – 16 years).
Table 5.
An overview of studies taking blood samples
| Reference | Blood sample | Analysis | Frequency | ||
|---|---|---|---|---|---|
| Subject | Cord | Maternal | |||
| 58 |
Y |
Y |
N |
ELISA – ABO & Rh group; Anti-H Pylori IgA and IgG. (Samples stored at –70°C) |
Every 3 months |
| 34 |
Y (fi ngerprick) |
N |
N |
Serum albumin, IgA, alpha-1-antichymotripsinogen |
Every 2 months |
| 61 |
Y |
N |
N |
T-cell telomere length, CD3 concentration, plasma interleukin 7 concentration; serum stored at –70°C and PBMCs stored for DNA. |
Once (60.8±3.2 months) |
| 17 |
Y |
N |
N |
Serum glucose, lipids, insulin (fasting and after oral load) |
Once (25–32 years) |
| 63 |
N |
N |
Y |
Hb, B12, folate, homocysteine |
Once (30±2 weeks gestation) |
| 146 |
Y |
N |
N |
Glucose (0, 30 min after oral load) |
Once (48 months) |
| 126 |
Y |
N |
N |
Fasting glucose, fastinginsulin |
Once (mean 28 years) |
| 148 |
Y |
N |
N |
Anti-dengue antibodies – haemagluttinin inhibition methods |
At 0 and 8 months |
| 132 |
Y |
N |
N |
Serum lead |
Biannually |
| 149 |
Y |
N |
Y |
Maternal anti-H. Pylori IgG in 3rd trimester; subject anti-H. Pylori (Pylori-stat ELISA) |
3rd trimester, At 7–9 and 18 months |
| 98 |
N |
Y |
N |
Triglyceride, LDL-c, HDL-c, apo-A, apoB, Lpa; frozen plasma sample stored |
Once (birth) (stored for future study) |
| 13 |
N |
N |
Y |
Mercury |
At 0–18 months |
| 115 |
N |
N |
Y |
Hb |
Once (3rd trimester) |
| 142 |
Y |
N |
N |
bilirubin |
If jaundiced (assessed weekly) |
| 88 |
Y (heel prick) |
Y |
N |
ELISA and immunofluorescence for measles IgG |
At 6, 12, 24 months |
| Total = 15 | 11 | 1 | 4 | ||
Y – yes, N – no
Biological sampling
We found a total of 25 studies in this region which took and stored biological samples (9 of them conducted in India, 6 in Bangladesh and 3 in Pakistan) (Figure 5). Their characteristics are shown in Table 4. The samples collected in a total of fifteen studies that stored blood samples are shown in Table 5.
DISCUSSION
A review of birth cohort studies in the SEA and EM regions has identified some positive examples of research. There is a distinct lack of studies addressing infectious diseases however, especially respiratory tract infections (RTI). Diarrhoea and RTI are a major cause of child morbidity and yet these topics receive little proportionate funding in research (18). Furthermore, biobanking has emerged as the most efficient way of studying the mechanisms of major diseases. However, there was only a single study from the SEA and EM regions in which DNA has been stored, in contrast to the numerous studies in the developed world benefitting from the Human Genome Project (19). A new birth cohort study and biobank in the SEA or EM regions would require significant planning, but such research must be directed to these areas of high child mortality in order to ensure future equity in health research.
In addition to infections, the incidence of non-communicable diseases such as cardiovascular disease, diabetes, cancers, cognitive defects and mental illness, are also increasing in low and middle-income countries (2,10). Thus, the developed world faces a dual burden of communicable and non-communicable disease. The diseases of adulthood have known associations with risk factors in earlier life. A life-course perspective of health determinants is now well outlined with understanding being drawn from previous longitudinal studies. Data from the UK NCDS study (3) for example outlined the association between maternal smoking and low birth weight (20). Risk factors even for the same non-communicable diseases are likely to differ somewhat between developed and developing countries. Therefore, any future birth cohort studies in the developing world would be more informative than those in the developed world.
Environmental exposures may differ in addition to different cultural manifestations. Breastfeeding, for example, is more prevalent in affluent members of developed countries in contrast to an association with poor socioeconomic status in low-income countries (21). Breastfeeding in the developed world is linked to obesity; however this may be socially patterned with high income (21). Comparative trends between countries with thus the removal of confounding factors may lead to a better understanding of causality of disease. Furthermore, the influence of the environment on child development has been better explored with new technologies made available by the Human Genome Project (22). Following the association of maternal smoking with low birth weight as outlined in the NCDS study (3), specific polymorphisms and their associations with maternal smoking, birth weight and adult cardiovascular disease have been examined (23).
Limitations
The search for studies was systematic, explicit and designed to have high sensitivity. The databases searched are extensive and thus it is unlikely that any studies of interest were missed. In the future, however, further databases could be searched, including IndMed and the Global Health Library. More studies may also be found by screening the cited papers. Due to the small number of studies, no further quality criteria were set, such as minimum number of subjects or minimum duration. This ensured a valid overview. Application of further exclusion criteria could highlight higher quality studies for analysis. Moreover, no distinction was made in this review between prospective and retrospective studies (with individually linked data from cohort at <60 months). Detailed analysis of only prospective studies may better inform the methodological considerations of a future prospective birth cohort study. In addition, analysis of more variables could be useful. Frequency of measurements, although documented for individual studies, was not compared across studies for example. One author (24) noted that more frequent anthropometric measurements resulted in faster recognition of reduced growth in infants such that therapeutic interventions (eg, food supplements) could be directed to individuals more quickly but that it resulted in more false reports of reduced growth at younger ages. Moreover, attrition data was noted for individual studies but no comparison made between studies, including for example between geographic areas (rural vs. urban), setting (community-based vs. facility-based), start number, study duration, measurement types. Again, this may be informative when planning a new study. Finally, whilst the end date of studies was noted where possible, there was often no indication in papers as to whether or not the cohort may be followed up later.
Study quality and comparison to international birth cohort studies
A comparison with birth cohorts in other low-income regions (including Sub-Saharan Africa) would be interesting, as well as to cohorts in middle-income (25,26) and developed countries. The cohorts examined in the SEA and EM regions are generally of a far smaller start number than those in the developed world. The National Child Development Study (3), British Cohort Study (4) and Millennium Cohort Study (5) for example initially recruited 16 634, 17 287 and 18 818 babies, respectively. Studies in the SEA and EM region are also generally of shorter duration than such studies. Finally, only a single study stored DNA from index children for later analysis, and no studies took genetic material from family members. By contrast, for example, the Avon Longitudinal Study of Parents and Children (ALSPAC) in the UK included 10 000 mothers and children for with consent for genetic sample storage and future analysis. Cohorts in middle-income countries, however, including the Pelotas study (25,26) and Birth-to-Twenty (27) recruited a large number of children for genetic analysis, providing a positive example.
Funding
Few studies have detailed their source of funding, and this should be further examined. Other publications, however have described the main sources of funding in health research (18) including governments, NGOs and private organisations.
Recommendations for future cohort studies
Considerable planning would be needed before a birth cohort study is undertaken in SEA or EM regions. A series of papers have been published detailing the steps towards a 2012 British Birth Cohort (28,29) and this review highlights some of the considerations of a study in the SEA or EM regions. Clear aims and objectives should be agreed in order to facilitate direction and efficient methodology. Data may be used to test future hypotheses, and so as much information should be gathered as concisely as possible. The Aberdeen cohort (30) has been criticised for having no information on smoking in households, despite making detailed social observations, since this was before the association between maternal smoking and low birth weight had been established (31). The study must be feasible for staff, as well as not too invasive to subjects. Studies should be sensitive to local cultures, with adversities to certain measurements considered (32). Moreover, consent for future analysis of measurements should be obtained from subjects and family (33). In some studies (34) fingerprints of parents were used to sign documents where literacy rates were low.
A large number of examinees should be enrolled in the cohort from an early age, so that conclusions can be drawn on the whole lifecourse perspective. Pregnant mothers could be sourced through prenatal services wherever possible, or through door-to-door interviews, aided by a registry of women of child-bearing age (eg, census) if available. Attrition could be minimised by following a population with a low migration rate, since this is a major reason for loss of follow-up. Mothers may also be offered incentive, for example meals at test facility (34) or health care for the duration of the study (35). One group described possible bias created by mothers attending facilities only to seek care (34). Moreover, number at follow up could be maximised through efficient planning, for example by utilising scheduled vaccination clinics or at entry to compulsory military service (20,26). Also, care should be taken to avoid recall errors. One study team gave calendars to mothers to record daily symptoms and treatment, thus reducing recall bias (36).
Local capacity must be developed and ensured before executing such a study. Laboratory staff should receive training and local field workers should be recruited. A number of sampling types towards the building of a biobank have been described including blood, saliva, and hair (37). Suitable media and storage should be further considered. The full list of studies retained for analysis in this paper is given at the end of our reference list (38–149), and their description in Supplementary Web Table 1. None of the present studies have included DNA from family members. Family members can act as proxies of exposure and can help identify parent-of-origin effects and de novo mutations and can help control for population stratification effects and should be included wherever possible (31).
Acknowledgements
We would like to thank Sheila Fisken, librarian, for her advice on databases and refining of search terms and Edinburgh medical students Prasad Velu, Tom Roberts, and Alasdair Campbell for their consultation on various aspects of the project.
Funding: None.
Ethical approval: Not required.
Authorship declaration: Both authors designed and conducted the study and wrote the paper.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Additional Material
References
- 1.World Health Organization. World Health Statistics 2010. Geneva: WHO. Available at: http://www.who.int/whosis/whostat/EN_WHS10_Full.pdf Accessed: 20 April 2011.
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study). Int J Epidemiol. 2006;35:34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- 4.Elliott J, Shepherd P. Cohort profile:1970 British Birth Cohort (BCS70). Int J Epidemiol. 2006;35:836–843. doi: 10.1093/ije/dyl174. [DOI] [PubMed] [Google Scholar]
- 5.Smith K, Joshi H. The Millennium Cohort Study. Popul Trends. 2002;30–34. Available at: http://www.esds.ac.uk/findingData/mcs.asp Accessed: 15 April 2011. [PubMed]
- 6.Golding J. Determinants of child health and development: the contribution of ALSPAC – a personal view of the birth cohort study. Arch Dis Child. 2010;95:319–322. doi: 10.1136/adc.2009.178954. [DOI] [PubMed] [Google Scholar]
- 7.Elliot J. Now we are 50. Key findings from the National Child Development Study. London: Centre for Longitudinal Studies, 2008. Available at: http://www.cls.ioe.ac.uk/news.asp?section=000100010003&item=449=449. Accessed: 15 April 2011.
- 8.United Nations. The Millennium Development Goals report 2010. New York: UN. Available at: http://www.un.org/millenniumgoals/pdf/MDG%20Report%202010%20En%20r15%20-low%20res%2020100615%20-.pdf#page=28 Accessed 20 April 2011.
- 9.World Health Organization. The World Health Report 2005: Make every mother and child count. Geneva: WHO. Available at: http://www.who.int/whr/2005/en/index.html Accessed: 15 April 2011.
- 10.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 11.Mudur G. Anti-vaccine Lobby Resists Introduction of Hib Vaccine in India. BMJ. 2010;340:c3508. doi: 10.1136/bmj.c3508. [DOI] [PubMed] [Google Scholar]
- 12.The World Bank. Countries and economies. Income levels. Available at: http://data.worldbank.org/country Accessed: 20 April 2011.
- 13.Amin-Zak L, Majeed MA, Greenwood L, Elhassani SB, Clarkson TW, Doherty RA. Methylmercury poisoning in the Iraqi suckling infant: a longitudinal study over five years. J Applied Toxicol. 1981;1:210–214. doi: 10.1002/jat.2550010405. [DOI] [PubMed] [Google Scholar]
- 14.Son M, Oh J, Choi YJ, Kong JO, Choi J, Jin E, et al. The effects of the parents’ social class on infant and child death among 1995–2004 birth cohort in Korea. J Prev Med Public Health. 2006;39:469–476. [PubMed] [Google Scholar]
- 15.Alam N. Birth spacing and infant and early childhood mortality in a high fertility area of Bangladesh: Age-dependent and interactive effects. J Biosoc Sci. 1995;27:393–404. doi: 10.1017/S0021932000023002. [DOI] [PubMed] [Google Scholar]
- 16.Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Muthayya S, Kurpad AV, et al. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in south India. J Nutr. 2010;140:1014–1022. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fall CH, Sachdev HS, Osmond C, Lakshmy R, Biswas SD, Prabhakaran D. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: data from the New Delhi birth cohort. Diabetes Care. 2008;31:2349–2356. doi: 10.2337/dc08-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enserink M. Global health. Some neglected diseases are more neglected than others. Science. 2009;324:700. doi: 10.1126/science.323.5915.700. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg NA, Huang L, Jewett EM, Szpiech ZA, Jankovic I, Boehnke M. Genome-wide association studies in diverse populations. Nat Rev Genet. 2010;11:356–366. doi: 10.1038/nrg2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batty GD, Alves JG, Correia J, Lawlor DA. Examining life-course influences on chronic disease: the importance of birth cohort studies from low- and middle-income countries. An overview. Braz J Med Biol Res. 2007;40:1277–1286. doi: 10.1590/S0100-879X2007000900015. [DOI] [PubMed] [Google Scholar]
- 22.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of human genome. Science. 2001;291:1304. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 23.Morales E, Sunyer J, Julvez J, Castro-Giner F, Estivill X, Torrent M, et al. GSTM1 polymorphisms modify the effect of maternal smoking during pregnancy on cognitive functioning in preschoolers. Int J Epidemiol. 2009;38:690–697. doi: 10.1093/ije/dyp141. [DOI] [PubMed] [Google Scholar]
- 24.Zumrawi FY, Dimond H. Determinants of growth in the first 6 months of life among the urban poor in Sudan. J Trop Med Hyg. 1988;91:139–146. [PubMed] [Google Scholar]
- 25.Araujo CL, Victora CG, Hallal PC, Gigante DP. Breastfeeding and overweight in childhood: evidence from the Pelotas 1993 birth cohort study. Int J Obes (Lond) 2006;30:500–506. doi: 10.1038/sj.ijo.0803160. [DOI] [PubMed] [Google Scholar]
- 26.Hallal PC, Wells JC, Reichert FF, Anselmi L, Victora CG. Early determinants of physical activity in adolescence: prospective birth cohort study. BMJ. 2006;332:1002–1007. doi: 10.1136/bmj.38776.434560.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter L, Norris S, Pettifor J, Yach D, Cameron N. Cohort profile: Mandela’s children: the 1990 birth to twenty study in South Africa. Int J Epidemiol. 2007;36:504–511. doi: 10.1093/ije/dym016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bynner J, Wadsworth M, Goldstein H, Maughan B, Purdon S, Michael R, et al. Scientific case for a new birth cohort study. Report to the research resources board of the Economic and Research Council. London: Longview, 2007. Available at: http://www.longviewuk.com/pages/documents/FINALREPORTSCNCS.pdf Accessed: 10 April 2011.
- 29.Bynner J, Wadsworth M, Goldstein H, Maughan B, Leesof C, Michael R. Options for the design of the 2012 birth cohort study. Report to the Research Resources Board of the Economic and Social Research Council and Appendices. London: Longview, 2009. Available at: http://www.longviewuk.com/pages/documents/NewCohort.pdf Accessed: 10 April 2011.
- 30.Leon DA, Lawlor DA, Clark H, MacIntyre S. Cohort profile: the Aberdeen children of the 1950s study. Int J Epidemiol. 2006;35:549–552. doi: 10.1093/ije/dyi319. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DA, Andersen AM, Batty GD. Birth cohort studies: past, present and future. Int J Epidemiol. 2009;38:897–902. doi: 10.1093/ije/dyp240. [DOI] [PubMed] [Google Scholar]
- 32.Abel R. Traditional beliefs against weighing children regularly. Indian J Pediatr. 1994;31:377–413. doi: 10.1177/004947558601600119. [DOI] [PubMed] [Google Scholar]
- 33.Sleeboom-Faulkner M, editor. Human genetic biobanks in Asia. Politics of trust and scientific advancement. 1st ed. Oxford: Routledge, 2009. [Google Scholar]
- 34.Rousham EK, Northrop-Clewes CA, Lunn PG.Maternal reports of child illness and the biochemical status of the child: the use of morbidity interviews in rural Bangladesh. Br J Nutr 199880451–456.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 35.Hasan KZ, Pathela P, Alam K, Podder G, Faruque SM, Roy E, et al. Aetiology of diarrhoea in a birth cohort of children aged 0–2 year(s) in rural Mirzapur, Bangladesh. J Health Popul Nutr 20062425–35.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 36.Gryboski KL. Maternal and non-maternal time-allocation to infant care, and care during infant illness in rural Java, Indonesia. Soc Sci Med. 1996;43:209–219. doi: 10.1016/0277-9536(95)00363-0. [DOI] [PubMed] [Google Scholar]
- 37.European Commission, The Wellcome Trust. From biobanks to biomarkers: translating the potential of human population research to improve the quality of health of the EU citizens. Proceedings of a conference held at the Wellcome Trust Conference Centre, Hinxton, Cambridge, 20–22 September 2005. Available at: http://www.wellcome.ac.uk/stellent/groups/corporatesite/@sitestudioobjects/documents/web_document/ wtx032086.pdf Accessed: 10 April 2011.
- 38.Agnihotri B, Antonisamy B, Priya G, Fall CH, Raghupathy P. Trends in human birth weight across two successive generations. Indian J Pediatr. 2008;75:111–117. doi: 10.1007/s12098-008-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed NU, Zeitlin MF, Beiser AS, Super CM, Gershoff SN. A longitudinal study of the impact of behavioural change intervention on cleanliness, diarrhoeal morbidity and growth of children in rural Bangladesh. Soc Sci Med. 1993;37:159–171. doi: 10.1016/0277-9536(93)90452-A. [DOI] [PubMed] [Google Scholar]
- 40.Akram DS, Agboatwala M. Growth parameters of Pakistani children. Indian J Pediatr. 1991;58:825–832. doi: 10.1007/BF02825444. [DOI] [PubMed] [Google Scholar]
- 41.Akram DS, Agboatwalla M, Bharmal FY. Community growth monitoring. J Pak Med Assoc. 2000;50:188–191. [PubMed] [Google Scholar]
- 42.Alam DS, Marks GC, Baqui AH, Yunus M, Fuchs GJ. Association between clinical type of diarrhoea and growth of children under 5 years in rural Bangladesh. Int J Epidemiol. 2000;29:916–921. doi: 10.1093/ije/29.5.916. [DOI] [PubMed] [Google Scholar]
- 43.Alam N, David PH. Infant and child mortality in Bangladesh: age-specific effects of previous child’s death. J Biosoc Sci. 1998;30:333–348. doi: 10.1017/S0021932098003332. [DOI] [PubMed] [Google Scholar]
- 44.Alam N, Saha SK, Razzaque A, van Ginneken JK. The effect of divorce on infant mortality in a remote area of Bangladesh. J Biosoc Sci. 2001;33:271–278. doi: 10.1017/S0021932001002711. [DOI] [PubMed] [Google Scholar]
- 45.Amin S. The effect of women’s status on sex differentials in infant and child mortality in South Asia. Genus. 1990;46:55–69. [PubMed] [Google Scholar]
- 46.Arifeen S, Black RE, Antelman G, Baqui A, Caulfield L, Becker S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108:E67. doi: 10.1542/peds.108.4.e67. [DOI] [PubMed] [Google Scholar]
- 47.Ashraf RN, Jalil F, Khan SR, Zaman S, Karlberg J, Lindblad BS. Early child health in Lahore, Pakistan:V. Feeding patterns. Acta Paediatr Suppl. 1993;82(Suppl 390):47–61. doi: 10.1111/j.1651-2227.1993.tb12906.x. [DOI] [PubMed] [Google Scholar]
- 48.Avsm YS, Gandhi N, Tandon BN, Krishnamurthy KS. Integrated child development services scheme and nutritional status of Indian children. J Trop Pediatr. 1995;41:123–128. doi: 10.1093/tropej/41.2.123. [DOI] [PubMed] [Google Scholar]
- 49.Ayatollahi SM, Ahmadi K. Infants’ growth charts for southern Iran. Ann Hum Biol. 2001;28:337–345. doi: 10.1080/030144601300119142. [DOI] [PubMed] [Google Scholar]
- 50.Badari S, Gopal YS, Devaramani SC. Infant mortality, its components and correlates: findings from a longitudinal study in rural Karnataka, India. Genus. 1991;47:89–108. [PubMed] [Google Scholar]
- 51.Banerjee I, Ramani S, Primrose B, Moses P, Iturriza-Gomara M, Gray JJ, et al. Comparative study of the epidemiology of rotavirus in children from a community-based birth cohort and a hospital in South India. J Clin Microbiol. 2006;44:2468–2474. doi: 10.1128/JCM.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baqui AH, Black RE, Sack RB, Chowdhury HR, Yunus M, Siddique AK.Malnutrition, cell-mediated immune deficiency, and diarrhea: a community-based longitudinal study in rural Bangladeshi children. Am J Epidemiol 1993137355–365.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 53.Bavdekar AR, Vaidya UV, Bhave SA, Pandit AN.Catch up growth and its determinants in low birth weight babies: a study using z-scores. Indian Pediatr 1994311483–1490.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 54.Bhalla AK. Longitudinal growth of arm circumference in Punjabi infants. Indian Pediatr. 1999;36:257–262. [PubMed] [Google Scholar]
- 55.Bhan MK, Raj P, Levine MM, Kaper JB, Bhandari N, Srivastava R, et al. Enteroaggregative Escherichia Coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 56.Bhandari N, Bahl R, Taneja S, Martines J, Bhan MK. Pathways to infant mortality in urban slums of Delhi, India: implications for improving the quality of community- and hospital-based programmes. J Health Popul Nutr. 2002;20:148–155. [PubMed] [Google Scholar]
- 57.Bhardwaj N, Hasan SB, Zaheer M. Maternal care receptivity and its relation to perinatal and neonatal mortality. A rural study. Indian Pediatr. 1995;32:416–423. [PubMed] [Google Scholar]
- 58.Bhuiyan TR, Qadri F, Saha A, Svennerholm AM. Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. Pediatr Infect Dis J. 2009;28:79–85. doi: 10.1097/INF.0b013e31818a5d9d. [DOI] [PubMed] [Google Scholar]
- 59.Bhutta ZA, Yusuf K. Early-onset neonatal sepsis in Pakistan: a case control study of risk factors in a birth cohort. Am J Perinatol. 1997;14:577–581. doi: 10.1055/s-2007-994338. [DOI] [PubMed] [Google Scholar]
- 60.Bishai D, Koenig M, Ali Khan M. Measles vaccination improves the equity of health outcomes: evidence from Bangladesh. Health Econ. 2003;12:415–419. doi: 10.1002/hec.732. [DOI] [PubMed] [Google Scholar]
- 61.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 62.Bosch AM, Baqui AH, van Ginneken JK. Early-life determinants of stunted adolescent girls and boys in Mat-lab, Bangladesh. J Health Popul Nutr. 2008;26:189–199. [PMC free article] [PubMed] [Google Scholar]
- 63.Broor S, Parveen S, Bharaj P, Prasad VS, Srinivasulu KN, Sumanth KM, et al. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS ONE. 2007;2:e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brush G, Harrison GA. Components of length growth variation in infants from the same population but different environments. Am J Hum Biol. 2001;13:197–203. doi: 10.1002/1520-6300(200102/03)13:2<197::AID-AJHB1029>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 65.Brush G, Harrison GA, Zumrawi FY. A path analysis of some determinants of infant growth in Khartoum. Ann Hum Biol. 1993;20:381–387. doi: 10.1080/03014469300002782. [DOI] [PubMed] [Google Scholar]
- 66.Cheung YB, Yip PS, Karlberg JP. Fetal growth, early postnatal growth and motor development in Pakistani infants. Int J Epidemiol. 2001;30:66–72. doi: 10.1093/ije/30.1.66. [DOI] [PubMed] [Google Scholar]
- 67.Chowdhury AK. Education and infant survival in rural Bangladesh. Health Policy Educ. 1982;2:369–374. doi: 10.1016/0165-2281(82)90017-0. [DOI] [PubMed] [Google Scholar]
- 68.Coles CL, Kanungo R, Rahmathullah L, Thulasiraj RD, Katz J, Santosham M, et al. Pneumococcal nasopharyngeal colonization in young south Indian infants. Pediatr Infect Dis J. 2001;20:289–295. doi: 10.1097/00006454-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Drewett R, Wolke D, Asefa M, Kaba M, Tessema F. Malnutrition and mental development: is there a sensitive period? A nested case-control study. J Child Psychol Psychiatry. 2001;42:181–187. doi: 10.1111/1469-7610.00709. [DOI] [PubMed] [Google Scholar]
- 70.Dutt D, Srinivasa DK.Impact of maternal and child health strategy on child survival in a rural community of Pondicherry. Indian Pediatr 199734785–792.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 71.Fikree FF, Rahbar MH, Berendes HW.Risk factors for stunting and wasting at age six, twelve and twenty-four months for squatter children of Karachi, Pakistan. J Pak Med Assoc 200050341–8.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 72.Fikree FF, Rahbar MH, Berendes HW. Role of intrauterine growth retardation on physical growth of Pakistani squatter children from birth to 2 years of age. J Trop Pediatr. 1999;45:338–344. doi: 10.1093/tropej/45.6.338. [DOI] [PubMed] [Google Scholar]
- 73.Ghosh R, Mascie-Taylor CG, Rosetta L. Longitudinal study of the frequency and duration of breastfeeding in rural Bangladeshi women. Am J Hum Biol. 2006;18:630–638. doi: 10.1002/ajhb.20533. [DOI] [PubMed] [Google Scholar]
- 74.Ghosh S, Gidwani S, Mittal SK, Verma RK. Socio-cultural factors affecting breast feeding and other infant feeding practices in an urban community. Indian Pediatr. 1976;13:827–832. [PubMed] [Google Scholar]
- 75.Ghosh S, Kilaru A, Ganapathy S. Nutrition education and infant growth in rural Indian infants: narrowing the gender gap? J Indian Med Assoc. 2002;100:483–484. [PubMed] [Google Scholar]
- 76.Gladstone BP, Muliyil JP, Jaffar S, Wheeler JG, Le Fevre A, Iturriza-Gomara M, et al. Infant morbidity in an Indian slum birth cohort. Arch Dis Child. 2008;93:479–484. doi: 10.1136/adc.2006.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Granat SM, Mia Z, Ollgren J, Herva E, Das M, Piirainen L, et al. Longitudinal study on pneumococcal carriage during the first year of life in Bangladesh. Pediatr Infect Dis J. 2007;26:319–324. doi: 10.1097/01.inf.0000257425.24492.11. [DOI] [PubMed] [Google Scholar]
- 78.Gubhaju BB. The effect of previous child death on infant and child mortality in rural Nepal. Stud Fam Plann. 1985;16:231–236. doi: 10.2307/1967085. [DOI] [PubMed] [Google Scholar]
- 79.Hafeman D, Factor-Litvak P, Cheng Z, van Geen A, Ahsan H. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ Health Perspect. 2007;115:1107–1112. doi: 10.1289/ehp.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagekull B, Nazir R, Jalil F, Karlberg J. Early child health in Lahore, Pakistan: iii. Maternal and family situation. Acta Paediatr Suppl. 1993;82(Suppl 390):27–37. doi: 10.1111/j.1651-2227.1993.tb12904.x. [DOI] [PubMed] [Google Scholar]
- 81.Harrison GA, Brush G, Zumrawi FY. Comparative length and weight growth in Khartoum infants. Ann Hum Biol. 1994;21:399–405. doi: 10.1080/03014469400003412. [DOI] [PubMed] [Google Scholar]
- 82.Harrison GA, Brush G, Zumrawi FY.Interrelations between growth, weaning and disease experience in Khartoum infants. Eur J Clin Nutr 199246273–278.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 83.Hauspie RC, Das SR, Preece MA, Tanner JM. A longitudinal study of the growth in height of boys and girls of west Bengal (India) aged six months of 20 years. Ann Hum Biol. 1980;7:429–440. doi: 10.1080/03014468000004541. [DOI] [PubMed] [Google Scholar]
- 84.Hebert JR. Relationship of vegetarianism to child growth in South India. Am J Clin Nutr. 1985;42:1246–1254. doi: 10.1093/ajcn/42.6.1246. [DOI] [PubMed] [Google Scholar]
- 85.Hirve SS, Ganatra BR.Determinants of low birth weight: a community based prospective cohort study. Indian Pediatr 1994311221–1225.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 86.Huttly SR, Hoque BA, Aziz KM, Hasan KZ, Patwary MY, Rahaman MM, et al. Persistent diarrhoea in a rural area of Bangladesh: a community-based longitudinal study. Int J Epidemiol. 1989;18:964–969. doi: 10.1093/ije/18.4.964. [DOI] [PubMed] [Google Scholar]
- 87.Ibrahim MM, Aden AS, Omar HM, Wall S, Persson LA.Diarrhoea among children in rural Somalia. Maternal perceptions, management and mortality. Ann Trop Paediatr 199414215–222.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 88.Ibrahim SA, Abdallah A, Saleh EA, Osterhaus AD, de Swart RL. Measles virus-specific antibody levels in Sudanese infants: a prospective study using filter-paper blood samples. Epidemiol Infect. 2006;134:79–85. doi: 10.1017/S0950268805004620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jahari AB, Saco-Pollitt C, Husaini MA, Pollitt E. Effects of an energy and micronutrient supplement on motor development and motor activity in undernourished children in Indonesia. Eur J Clin Nutr. 2000;54(Suppl 2):S60–68. doi: 10.1038/sj.ejcn.1601006. [DOI] [PubMed] [Google Scholar]
- 90.Jalil F, Karlberg J, Hanson LA, Lindblad BS. Growth disturbance in an urban area of Lahore, Pakistan related to feeding patterns, infections and age, sex, socio-economic factors and seasons. Acta Paediatr Scand Suppl. 1989;350:44–54. doi: 10.1111/j.1651-2227.1989.tb11197.x. [DOI] [PubMed] [Google Scholar]
- 91.Jalil F, Lindblad BS, Hanson LA, Khan SR, Ashraf RN, Carlsson B, et al. Early child health in Lahore, Pakistan: i. Study design. Acta Paediatr Suppl. 1993;82(Suppl 390):3–16. doi: 10.1111/j.1651-2227.1993.tb12902.x. [DOI] [PubMed] [Google Scholar]
- 92.Jeon BP, Berger MC. The demographic cycle and optimal schooling choices. South Econ J. 1996;63:301–311. doi: 10.2307/1061169. [DOI] [PubMed] [Google Scholar]
- 93.Jirapinyo P, Densupsoontorn N, Chinrungrueng D, Wongarn R, Thamonsiri N. Relative risks of becoming overweight and obese in children after 6 years in secondary school. J Med Assoc Thai. 2005;88:651–654. [PubMed] [Google Scholar]
- 94.Kabir M, Chowdhury RI, Amin R. Infant and child mortality levels and trends in Bangladesh. J Biosoc Sci. 1995;27:179–192. doi: 10.1017/S0021932000022689. [DOI] [PubMed] [Google Scholar]
- 95.Kabir Z, Long J. Child mortality rates in rural India: an experience from the Ballabgarh project. J Trop Pediatr. 2002;48:178–180. doi: 10.1093/tropej/48.3.178. [DOI] [PubMed] [Google Scholar]
- 96.Kabir Z, Long J, Reddaiah VP, Kevany J, Kapoor SK. Non-specific effect of measles vaccination on overall child mortality in an area of rural India with high vaccination coverage: a population-based case-control study. Bull World Health Organ. 2003;81:244–250. [PMC free article] [PubMed] [Google Scholar]
- 97.Kardjati S, Kusin JA, de With C, Renqvist UH. Low birth weight babies under village conditions: feeding pattern, growth and motor development. Paediatr Indones. 1991;31:84–98. [PubMed] [Google Scholar]
- 98.Kelishadi R, Badiee Z, Adeli K. Cord blood lipid profile and associated factors: baseline data of a birth cohort study. Paediatr Perinat Epidemiol. 2007;21:518–524. doi: 10.1111/j.1365-3016.2007.00870.x. [DOI] [PubMed] [Google Scholar]
- 99.Khalil K, Lindblom GB, Mazhar K, Khan SR, Kajiser B. Early child health in Lahore, Pakistan: viii. Microbiology. Acta Paediatr Suppl. 1993;82(Suppl 390):87–94. doi: 10.1111/j.1651-2227.1993.tb12909.x. [DOI] [PubMed] [Google Scholar]
- 100.Khin-Maung-U, Pereira SP, Bolin TD, Duncombe VM, Myo-Khin, Nyunt-Nyunt-Waiet alMalabsorption of carbohydrate from rice and child growth: a longitudinal study with the breath-hydrogen test in Burmese village children. Am J Clin Nutr 199052348–352. [DOI] [PubMed] [Google Scholar]
- 101.Kim J, Son M, Kawachi I, Oh J. The extent and distribution of inequalities in childhood mortality by cause of death according to parental socioeconomic positions: a birth cohort study in South Korea. Soc Sci Med. 2009;69:1116–1126. doi: 10.1016/j.socscimed.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 102.Kim TH. Changing determinants of infant and child mortality: on the basis of the Korean experience, 1955–73. J Biosoc Sci. 1988;20:345–355. doi: 10.1017/S0021932000006684. [DOI] [PubMed] [Google Scholar]
- 103.Kirksey A, Rahmanifar A, Wachs TD, McCabe GP, Bassily NS, Bishry Z, et al. Determinants of pregnancy outcome and newborn behavior of a semirural Egyptian population. Am J Clin Nutr 199154657–667.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 104.Koenig MA, Khan MA, Wojtyniak B, Clemens JD, Chakraborty J, Fauveau V, et al. Impact of measles vaccination on childhood mortality in rural Bangladesh. Bull World Health Organ 199068441–447.[REMOVED HYPERLINK FIELD] [PMC free article] [PubMed] [Google Scholar]
- 105.Koenig MA, Phillips JF, Campbell OM, d’Souza S. Birth intervals and childhood mortality in rural Bangladesh. Demography. 1990;27:251–265. doi: 10.2307/2061452. [DOI] [PubMed] [Google Scholar]
- 106.Kolsteren PW, Kusin JA, Kardjati S.Morbidity and growth performance of infants in Madura, Indonesia. Ann Trop Paediatr 199717201–208.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 107.Kolsteren PW, Kusin JA, Kardjati S. Pattern of linear growth velocities of infants from birth to 12 months in Madura, Indonesia. Trop Med Int Health. 1997;2:291–301. doi: 10.1046/j.1365-3156.1997.d01-262.x. [DOI] [PubMed] [Google Scholar]
- 108.Kongsomboon K, Singhasivanon P, Kaewkungwal J, Nimmannitya S, Mammen MP, Jr, Nisalak A, et al. Temporal trends of dengue fever/dengue hemorrhagic fever in Bangkok, Thailand from 1981 to 2000: an age-period-cohort analysis. Southeast Asian J Trop Med Public Health 200435913–917.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 109.Krishnaswamy K, Naidu AN, Prasad MP, Reddy GA. Fetal malnutrition and adult chronic disease. Nutr Rev. 2002;60:S35–39. doi: 10.1301/00296640260130713. [DOI] [PubMed] [Google Scholar]
- 110.Kulsoom U, Saeed A.Breast feeding practices and beliefs about weaning among mothers of infants aged 0–12 months. J Pak Med Assoc. 19974754–60.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 111.Kusin JA, Kardjati S, de With C, Rengvist UH.When does growth faltering start? Paediatr Indones 19913126–40.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 112.Kusin JA, Kardjati S, Houtkooper JM, Renqvist UH. Energy supplementation during pregnancy and postnatal growth. Lancet. 1992;340:623–626. doi: 10.1016/0140-6736(92)92168-F. [DOI] [PubMed] [Google Scholar]
- 113.Kusin JA, Kardjati S, van Steenbergen WM, Renqvist UH.Nutritional transition during infancy in East Java, Indonesia:2. A longitudinal study of growth in relation to the intake of breast milk and additional foods. Eur J Clin Nutr 19914577–84.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 114.Launer LJ, Habicht JP, Kardjati S.Breast feeding protects infants in Indonesia against illness and weight loss due to illness. Am J Epidemiol 1990131322–331.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 115.Lone FW, Qureshi RN, Emmanuel F.Maternal anaemia and its impact on perinatal outcome in a tertiary care hospital in Pakistan. East Mediterr Health J 200410801–807.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 116.Mahmud A, Jalil F, Karlberg J, Lindblad BS. Early child health in Lahore, Pakistan: vii. Diarrhoea. Acta Paediatr Suppl. 1993;82(Suppl 390):79–85. doi: 10.1111/j.1651-2227.1993.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 117.Mahmud MA, Chappell C, Hossain MM, Habib M, Dupont HL. Risk factors for development of first symptomatic Giardia infection among infants of a birth cohort in rural Egypt. Am J Trop Med Hyg. 1995;53:84–88. [PubMed] [Google Scholar]
- 118.Mathur R, Reddy V, Naidu AN, Ravikumar N, Krishnamachari KA. Nutritional status and diarrhoeal morbidity:a longitudinal study in rural Indian preschool children development. Hum Nutr Clin Nutr. 1985;39:447–54. [PubMed] [Google Scholar]
- 119.Meguid NA, El-Kotoury AI, Abdel-Salam GM, el-Ruby MO, Afifi HH. Growth charts of Egyptian children with Down syndrome (0–36 months). East Mediterr Health J. 2004;10:106–115. [PubMed] [Google Scholar]
- 120.Mehta S, Nath N, Thukural S, Pasricha S. Growth profile of preschool children from an urban low socioeconomic community in India. Trop Geograph Med. 1977;29:177–186. [PubMed] [Google Scholar]
- 121.Mo-suwan L, Tongkumchum P, Puetpaiboon A. Determinants of overweight tracking from childhood to adolescence: a 5 year follow-up study of Hat Yai schoolchildren. Int J Obes Relat Metab Disord. 2000;24:1642–1647. doi: 10.1038/sj.ijo.0801432. [DOI] [PubMed] [Google Scholar]
- 122.Nagra SA.Body weight of Pakistani infants reared on different feeding regimes. Arch Latinoam Nutr 1989399–16.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 123.Pandey A, Chakraborty AK.Undernutrition, vitamin A deficiency and ARI morbidity in underfives. Indian J Public Health 19964013–16.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 124.Paul B, Saha I, Dasgupta A, Chaudhuri RN.A study on catch up growth among low birth weight infants in an urban slum of Kolkata. Indian J Public Health 20085216–20.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 125.Raghupathy P, Antonisamy B, Geethanjali FS, Saperia J, Leary SD, Priya G. Glucose tolerance, insulin resistance and insulin secretion in young south Indian adults: relationships to parental size, neonatal size and childhood body mass index. Diabetes Res Clin Pract. 2010;87:283–292. doi: 10.1016/j.diabres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rao S, Joshi S, Kanade A. Growth in some physical dimensions in relation to adolescent growth spurt among rural Indian children. Ann Hum Biol. 2000;27:127–138. doi: 10.1080/030144600282244. [DOI] [PubMed] [Google Scholar]
- 127.Rao S, Joshi SB, Kelkar RS. Changes in nutritional status and morbidity over time among pre-school children from slums in Pune, India. Indian Pediatr. 2000;37:1060–1071. [PubMed] [Google Scholar]
- 128.Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am J Clin Nutr. 2007;85:845–852. doi: 10.1093/ajcn/85.3.845. [DOI] [PubMed] [Google Scholar]
- 129.Ravikumara M, Bhat BV. Early neonatal mortality in an intramural birth cohort at a tertiary care hospital. Indian J Pediatr. 1996;63:785–789. doi: 10.1007/BF02730930. [DOI] [PubMed] [Google Scholar]
- 130.Rehman AM, Gladstone BP, Verghese VP, Muliyil J, Jaffar S, Kang G. Chronic growth faltering amongst a birth cohort of Indian children begins prior to weaning and is highly prevalent at three years of age. Nutr J. 2009;8:44. doi: 10.1186/1475-2891-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ruangkanchanasetr S, Suepiantham J. Risk factors of high lead level in Bangkok children. J Med Assoc Thai. 2002;85(Suppl 4):s1049–s1058. [PubMed] [Google Scholar]
- 132.Saha KK, Frongillo EA, Alam DS, Arifeen SE, Persson LA, Rasmussen KM.Use of the new World Health Organization child growth standards to describe longitudinal growth of breastfed rural Bangladeshi infants and young children. Food Nutr Bull 200930137–144.[REMOVED HYPERLINK FIELD] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saleemi MA, Ashraf RN, Mellander L, Zaman S. Determinants of stunting at 6, 12, 24 and 60 months and postnatal linear growth in Pakistani children. Acta Paediatr. 2001;90:1304–1308. doi: 10.1111/j.1651-2227.2001.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 134.Saleemi MA, Zaman S, Akhtar HZ, Jalil F, Ashraf RN, Hanson LA. Feeding patterns, diarrhoeal illness and linear growth in 0–24-month-old children. J Trop Pediatr. 2004;50:164–169. doi: 10.1093/tropej/50.3.164. [DOI] [PubMed] [Google Scholar]
- 135.Satyanarayana K, Prasanna Krishna T, Narasinga Rao BS. Effect of early childhood undernutrition and child labour on growth and adult nutritional status of rural Indian boys around Hyderabad. Hum Nutr Clin Nutr. 1986;40:131–139. [PubMed] [Google Scholar]
- 136.Satyanarayana L, Indrayan A, Sachdev HP, Gupta SM. A comprehensive index for longitudinal monitoring of child health status. Indian Pediatr. 1995;32:443–452. [PubMed] [Google Scholar]
- 137.Schwekendiek D, Pak S, Kim HK. Variations in the birth-season effects on height attainment in the two Koreas. Ann Hum Biol. 2009;36:421–430. doi: 10.1080/03014460902905920. [DOI] [PubMed] [Google Scholar]
- 138.Shahidullah M. Breast-feeding and child survival in Matlab, Bangladesh. J Biosoc Sci. 1994;26:143–154. doi: 10.1017/S0021932000021180. [DOI] [PubMed] [Google Scholar]
- 139.Soudarssanane MB, Srinivasa DK, Narayan KA, Ramalingam G.Infant mortality in Pondicherry – an analysis of a cohort of 8185 births. Indian Pediatr 1992291379–1384.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 140.Thitasomakul S, Thearmontree A, Piwat S, Chankanka O, Pithpornchaiyakul W, Teanpaisan R, et al. A longitudinal study of early childhood caries in 9- to 18-month-old Thai infants. Community Dent Oral Epidemiol. 2006;34:429–436. doi: 10.1111/j.1600-0528.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 141.Tikmani SS, Warraich HJ, Abbasi F, Rizvi A, Darmstadt GL, Zaidi AK. Incidence of neonatal hyperbilirubinemia: a population-based prospective study in Pakistan. Trop Med Int Health. 2010;15:502–507. doi: 10.1111/j.1365-3156.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 142.Tripathy R, Das RN, Das MM, Parija AC.Growth in the first year in children following IAP policy on infant feeding. Indian Pediatr 2000371051–1059.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 143.Tuntiseranee P, Olsen J, Chongsuvivatwong V, Limbutara S. Socioeconomic and work related determinants of pregnancy outcome in southern Thailand. J Epidemiol Community Health. 1999;53:624–629. doi: 10.1136/jech.53.10.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Hill JC, Kurpad AV, et al. Infant feeding practice and childhood cognitive performance in South India. Arch Dis Child. 2010;95:347–354. doi: 10.1136/adc.2009.165159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yajnik CS, Fall CH, Vaidya U, Pandit AN, Bavdekar A, Bhat DS, et al. Fetal growth and glucose and insulin metabolism in four-year-old Indian children. Diabetic Med. 1995;12:330–336. doi: 10.1111/j.1464-5491.1995.tb00487.x. [DOI] [PubMed] [Google Scholar]
- 146.Zaman S, Jalil F, Karlberg J. Early child health in Lahore, Pakistan:iv. Child care practices. Acta Paediatr Suppl. 1993;82(Suppl 390):39–46. doi: 10.1111/j.1651-2227.1993.tb12905.x. [DOI] [PubMed] [Google Scholar]
- 147.Zaman S, Jalil F, Karlberg J, Hanson LA. Early child health in Lahore, Pakistan:vi. Morbidity. Acta Paediatr Suppl. 1993;82(Suppl 390):63–78. doi: 10.1111/j.1651-2227.1993.tb12907.x. [DOI] [PubMed] [Google Scholar]
- 148.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 149.Bassily S, Frenck RW, Mohareb EW, Wierzba T, Savarino S, Hall E, et al. Seroprevalence of Helicobacter pylori among Egyptian newborns and their mothers: a preliminary report. Am J Trop Med Hyg 19996137–140.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.