Abstract
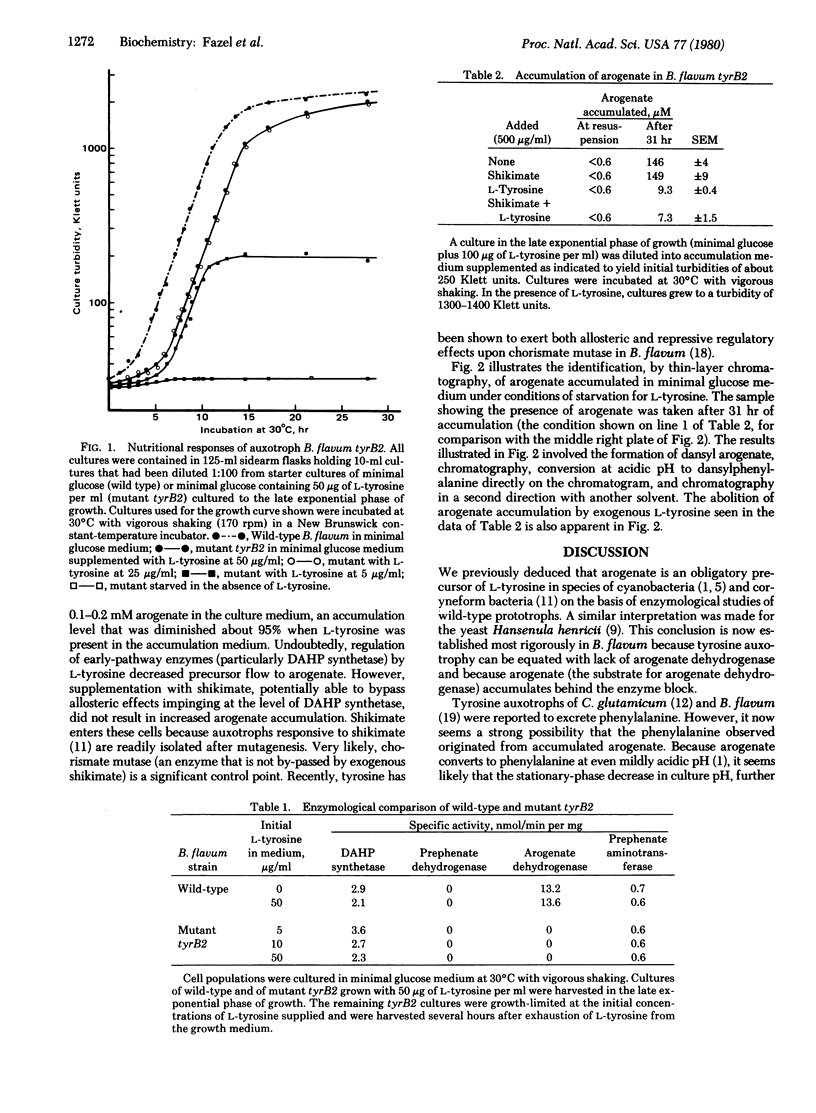
Wild-type Brevibacterium flavum has been shown to possess arogenate dehydrogenase activity and to lack prephenate dehydrogenase, thereby providing presumptive evidence that arogenate (previously named "pretyrosine") is an obligatory intermediate of L-tyrosine biosynthesis. A similar enzymological pattern has been discerned in extracts made from wild-type cultures of various species of cyanobacteria. Application of rigorous molecular genetic criteria in confirmation of the exclusive role of arogenate in L-tyrosine synthesis was made possible by the isolation of an auxotrophic mutant exhibiting a nutritional requirement for L-tyrosine. The mutant was found to lack activity for arogenate dehydrogenase and to accumulate substantial amounts of arogenate behind the mutant block during starvation for L-tyrosine.
Full text
PDF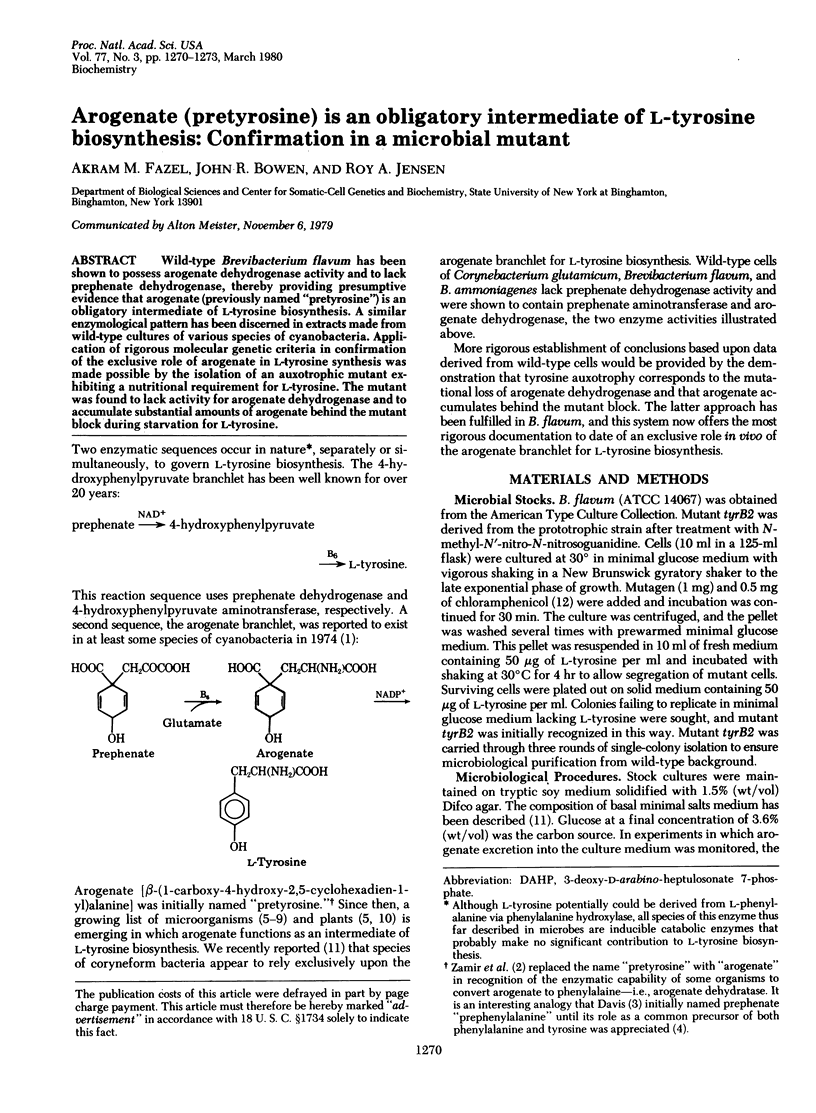


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks C. J., DeBusk B. G., DeBusk A. G. Cellular compartmentation of aromatic amino acids in Neurospora crassa. II. Synthesis and misplaced accumulation of phenylalanine in phen-2 auxotrophs. Biochem Genet. 1973 Oct;10(2):105–120. doi: 10.1007/BF00485759. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D. Autocatalytic growth of a mutant due to accumulation of unstable phenylalanine precursor. Science. 1953 Aug 28;118(3061):251–252. doi: 10.1126/science.118.3061.251. [DOI] [PubMed] [Google Scholar]
- Fazel A. M., Jensen R. A. Aromatic aminotransferases in coryneform bacteria. J Bacteriol. 1979 Nov;140(2):580–587. doi: 10.1128/jb.140.2.580-587.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel A. M., Jensen R. A. Obligatory biosynthesis of L-tyrosine via the pretyrosine branchlet in coryneform bacteria. J Bacteriol. 1979 Jun;138(3):805–815. doi: 10.1128/jb.138.3.805-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. II. The enzymology of feedback inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3373–3380. [PubMed] [Google Scholar]
- Jensen R. A., Pierson D. L. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature. 1975 Apr 24;254(5502):667–671. doi: 10.1038/254667a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Patel N., Stenmark-Cox S. L., Jensen R. A. Enzymological basis of reluctant auxotrophy for phenylalanine and tyrosine in Pseudomonas aeruginosa. J Biol Chem. 1978 May 10;253(9):2972–2978. [PubMed] [Google Scholar]
- Rubin J. L., Jensen R. A. Enzymology of l-Tyrosine Biosynthesis in Mung Bean (Vigna radiata [L.] Wilczek). Plant Physiol. 1979 Nov;64(5):727–734. doi: 10.1104/pp.64.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. The control of 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthesis by phenylalanine and tyrosine. J Biol Chem. 1962 Nov;237:3566–3570. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Shiio I., Sugimoto S. Two components of chorismate mutase in Brevibacterium flavum. J Biochem. 1979 Jul;86(1):17–25. [PubMed] [Google Scholar]
- Stenmark S. L., Pierson D. L., Jensen R. A., Glover G. I. Blue-green bacteria synthesise L-tyrosine by the pretyrosine pathway. Nature. 1974 Feb 1;247(5439):290–292. doi: 10.1038/247290a0. [DOI] [PubMed] [Google Scholar]
- WEISS U., GILVARG C., MINGIOLI E. S., DAVID B. D. Aromatic biosynthesis. XI. The aromatization step in the synthesis of phenylalanine. Science. 1954 May 28;119(3100):774–775. doi: 10.1126/science.119.3100.774. [DOI] [PubMed] [Google Scholar]