Abstract
In the past few years, the transdisciplinary field of HIV prevention has reached several milestones. Topically applied tenofovir gel provided significant protection from sexual transmission of HIV in a large-scale clinical trial and oral Truvada (emtricitabine/tenofovir disoproxil fumarate) was recently approved for preexposure prophylaxis (PrEP) following two successful clinical trials in men and women. These achievements are tempered by the disappointing results of other clinical trials, which highlight the complexities of prevention research. In this perspective, we discuss scientific and developmental gaps for topical chemoprophylaxis of the sexual transmission of HIV, which depends on the complex interactions between the pharmacokinetics and pharmacodynamics of drugs, formulation and delivery systems, anatomic site of transmission, and host mucosal immune defenses. Despite the considerable time and resources devoted to unraveling the initial steps in sexual transmission of HIV, current knowledge is based on animal models and human explanted tissue, which may not fully recapitulate what happens clinically. Understanding these events, including the role that sex hormones, semen, and mucosal secretions play in transmission, and the interplay between innate immunity, the mucosal environment, and drug efficacy is paramount. This drives some of the most pressing questions in the field.
Introduction
In the past year, the HIV prevention field has witnessed the first clinical successes with oral and topical preexposure prophylaxis (PrEP) in combatting sexual transmission of HIV-1.1–3 This represents a major advancement, as until now, the only available technology to prevent transmission was the 500-year-old condom. The recent successes are the result of decades of study of HIV transmission complemented by an increasing number of antiretroviral drugs (ARV), recognition of the need for compartment-specific formulations, and improved clinical trial design. Together these and other advances in pharmacology, behavioral research, and formulation science form the foundation for the emergence of HIV prevention as a transdisciplinary field in its own right. The achievements generate new questions highlighting knowledge gaps, and suggest new directions for scientific investigation and funding priorities for the HIV prevention field.
Success in HIV prevention, defined as having a significant impact on the global pandemic, is, however, still tenuous. Only oral PrEP with the combination drug Truvada (tenofovir disoproxil fumarate and emtricitabine) has been approved by the FDA in high-risk populations and other modalities have yet to meet clinical endpoints for registration.2 A number of critical scientific, pharmacological, and practical hurdles must be addressed if we are to achieve our goal. We have little understanding of the mechanisms of how sexual transmission and dissemination occur and there are likely important differences depending on the anatomic site of exposure (cervicovaginal, rectal, and penile).
The viral inoculum needed for infection, the relative contribution of cell-free or cell-associated virus, the importance of the epithelial barrier and mucosal immunity, and the role semen plays in augmenting or interfering with transmission represent major knowledge gaps. Other unanswered questions include the following: What cells are infected first and how do they disseminate? Should prevention target infection of the first cells, virus dissemination after the founder population is established, or more likely, will highly effective prevention modalities require strategies that target both? Vaginal transmission is further complicated by endogenous and exogenous hormones, which modulate the epithelial, cellular, and soluble mucosal immune environment and possibly impact drug pharmacokinetics (PK) and pharmacodynamics (PD). The importance of exogenous hormones is highlighted by studies suggesting that women on medroxyprogesterone are more likely both to acquire and to transmit HIV compared to women not on hormonal contraception.4 The presence of other sexually transmitted infections (STI) impacts the risk of transmission and acquisition in both men and women and is likely a factor in all anatomic sites, although this has not been well studied in the rectal compartment.
Just as each anatomic compartment is unique with respect to mechanisms of transmission, each pharmacological agent differs in its site (luminal, cell surface, or intracellular) and mechanism of action (entry, reverse transcriptase, integrase, or protease inhibitor), ability to penetrate tissue, and, for intracellularly active drugs such as the reverse transcriptase inhibitors (RTI), kinetics and mechanisms of intracellular transport, metabolism, and intracellular retention. The potential efficacy of each candidate will derive not only from these properties of the drug substance, but also from the delivery system (oral, topical gel, film or ring, and implant), and the ability to achieve sufficient concentration of bioactive drug at the right site and right time. Finally, we must not forget that understanding all of this will not make people use prevention technology. If adherence, cost, and other social issues are not addressed, even if the product shows efficacy within the confined boundaries of a controlled clinical trial, real world effectiveness will not be achieved and HIV prevention will be nothing more than an academic exercise that produced some niche products with little global impact. The men and women who are exposed to HIV every day demand that we do better than this. This perspective highlights some of the gaps and barriers to our scientific understanding and identifies next steps for translational research and development in the field.
Drugs for Topical PrEP
The challenges in advancing a drug from preclinical discovery through successful clinical trials is enormous as evidenced by the large number of drugs that never reach the market. The challenge is far greater for prevention (compared to treatment) where safety is paramount, as the goal is to prescribe a product to a relatively healthy population for potentially prolonged time periods to prevent a relatively uncommon endpoint (HIV acquisition). Topical HIV and STI prevention is further hampered by the absence of validated preclinical models and the lack of a gold standard of success.
The selection and development of topical agents for HIV prevention have changed considerably in the past few years with the current focus on potent HIV-specific agents.5 The field learned the hard way, by conducting expensive clinical trials, that the first generation of nonspecific agents, including surfactants [Nonoxynol-9 and C31G (Savvy)], pH buffering agents (Acidform and Buffergel), and sulfated/sulfonated polymers (PRO 2000, cellulose sulfate, and Carraguard), did not prevent HIV or other STI. Low potency, interference with antiviral activity by seminal plasma, and changes in PK following sex likely contributed to the reduced efficacy observed with sulfonated polymers.6–8 In some cases, repeated gel exposures were associated with an unanticipated increase in HIV susceptibility, presumably due to inflammatory responses (nonoxynol-9)9,10 and disruption of the genital tract epithelial barrier (surfactants and cellulose sulfate).11–13
Together these disappointing clinical trial results highlight the need for more predictive preclinical models of efficacy and safety and the need to conduct expanded Phase 1/2 clinical studies that include measures of drug PK/PD following barrier unprotected sex prior to embarking on large-scale Phase 3 clinical efficacy trials. Two PK/PD postcoital studies with tenofovir gel are only now underway (conducted by CONRAD and the MTN) and may provide insights into the discrepant results obtained in the 1% tenofovir (TFV) gel efficacy trials. In the first efficacy study, CAPRISA 004, a 39% (95% CI 6–61%) reduction in HIV acquisition was observed in women who applied 1% TFV gel before and after sex.3 In contrast, no protection was observed in a coitally independent daily dosing study of the same product (MTN 003) and the study was prematurely halted by the Data Safety Monitoring Board for futility.14 Whether the different outcomes in these two studies reflect altered PK/PD related to the timing of dosing relative to HIV exposure, other characteristics of participating populations (such as the use of hormonal contraception, which was a requirement for MTN 003) that might modulate drug PK/PD by impacting intracellular transporters and drug metabolism (see below), differences in sexual practices (frequency of rectal versus vaginal intercourse), adherence, or unrecognized toxicities with daily dosing that overcame the protective effects of the product are currently the focus of intense investigations. These contradictory results highlight the complexities of prevention science.
Articles in this issue of AIDS Research and Human Retroviruses describe a range of pharmacological agents and formulations that are at different stages of development as candidates for topical PrEP. These can be broadly categorized into three groups based on the need for preclinical and regulatory development. The first are clinically approved ARV that are currently used to treat infected individuals. Development of these products requires optimization of formulations and safety studies related to topical application. This category includes tenofovir, which, in addition to ongoing studies with the original 1% gel formulation, is now being evaluated as a reduced glycerin gel formulation15 that may be better tolerated, particularly when applied rectally. Other tenofovir formulations in development include a film, tablet, and intravaginal ring (IVR). Other drugs in this first category include tenofovir disoproxil fumarate, the more potent prodrug of tenofovir that exhibits greater tissue penetration and cellular uptake, which has recently been formulated as an IVR and is currently being evaluated in nonhuman primates16; and maraviroc, a CCR5 coreceptor antagonist, which has been formulated as an IVR and is being evaluated in clinical trials alone and in combination with the nonnucleoside RTI (NNRTI), dapivirine. In addition, integrase inhibitors such as elvitegravir and protease inhibitors such as saquinivir17 are being explored for topical formulations.
The second group includes ARV whose development as a therapeutic was discontinued for reasons such as suboptimal oral bioavailability. Many of these have preexisting INDs simplifying the regulatory path needed to bring topical formulations into the clinic. This group includes the NNRTIs dapivirine (currently in clinical trials as IVR formulation but also developed as a gel)18,19 and MIV-150 (formulated with carrageenan as a gel).20,21
The final group contains more novel products that have not been previously tested as therapeutics and thus require a much more extensive and costly regulatory evaluation before they can be advanced into the clinic. Importantly, some of these are intended only for topical PrEP and block HIV infection by unique mechanisms. This may prove advantageous, as these products may be less likely to select for resistant HIV isolates. Selection of resistant isolates is a concern when drugs still considered first line for treatment are used as prevention, as selection of resistant variants may limit future treatment options.22 This third category includes agents such as the dual acting entry and NNRTI drug IQP-052823 (in development formulated as both a gel and ring alone and in combination with tenofovir disoproxil fumarate), Ncp7 maturation inhibitors,24–26 entry inhibitors such as griffithsin27 (film and gel), and PSC-RANTES (nanoparticles)28 and the gp41 inhibitor PIE12-trimer.29 Despite their potential, the scarcity of resources coupled with the urgency in developing effective prevention strategies restricts the development of compounds from this last group. The field can afford to invest time and money in the development of novel compounds only if they offer clear advantages with respect to potency, lack of toxicity, PK/PD, or formulation potential compared to what is already available.
Initial Events in Sexual Transmission
For sexual transmission to occur, infectious viral HIV-1 particles must either cross the mucosal epithelial barrier, which, in healthy vaginal tissue, is composed of a multilayered squamous epithelium, or be captured by epithelial or immune cells [immature dendritic cells (DCs) or Langerhans cells] and subsequently transferred to target cells that support viral replication. The latter include resident or recruited submucosal CD4+ T cells, macrophages, and DCs. The mechanism and extent to which virus directly crosses the epithelium or is captured by immune cells remain controversial and likely vary at different anatomic sites.30–35 The small infected founder population, which in macaque models is composed mainly of resting CD4 T cells,36,37 expands locally, resulting in dissemination of virus to draining lymph nodes and subsequently through the bloodstream to establish infection in secondary lymphoid organs. Precisely how virus (cell-free or cell-associated) reaches submucosal immune target cells and the inoculum required are all areas of intense investigation. There is also controversy regarding the primary site of the initial foci of infection with some studies implicating the cervix and others suggesting that viral particles are percolating through the vaginal and penile epithelium and coming in contact with immune system cells in the epithelia. It is likely that all of these mechanisms contribute to infection and thus an effective prevention strategy will require drugs and drug delivery systems that generate inhibitory concentrations of antiretrovirals within the vaginal, penile, and rectal epithelium, in the submucosa, and perhaps at the draining lymph nodes.
Right Place and Right Time
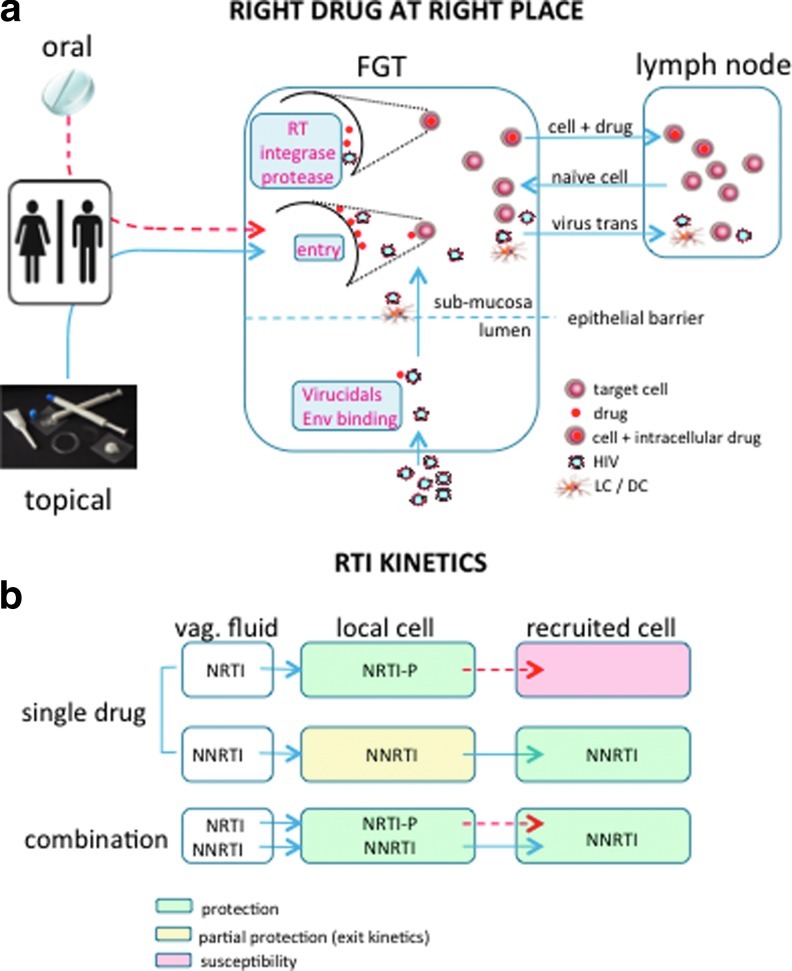
Drugs that act luminally and directly target incoming viral particles must be present at sufficiently high concentrations within genital tract secretions following intercourse to block infection. For these drugs, a topical controlled delivery system in which the highest concentrations are local may be ideal. In contrast, drugs that target cell structures (CCR5 coreceptor) or intracellular processes (reverse transcription, integration, or the proteolytic cleavage of viral proteins) must be present at the cell surface or retained intracellularly within HIV target cells and thus, if applied topically, must cross the epithelial barrier to reach submucosal immune cells and, possibly, reach draining lymph nodes (Fig. 1a). These drugs must also be accessible to immune cells recruited into the genital tract in response to chemokines and other inflammatory signals.
FIG. 1.
(a) Right drug at the right place. The amount of drug available at sites of exposure to HIV (vaginal, rectal, and penile) depends on a suitable delivery system. Drug levels in the female genital tract following oral dosage are significantly lower than what can be achieved using topical delivery systems (depicted by dashed red line). Drugs may act on the virus (virucidals and binding agents), at the cell surface (entry inhibitors), or intracellularly (inhibitors of reverse transcriptase, integrase, and protease) and must be present in sufficient amount at the appropriate site depending on the mode of action. In addition to protecting local immune cells, these drugs must be able to protect target cells recruited into the mucosa in response to inflammatory signals and have the potential to reach cell reservoirs to achieve protection. (b) Reverse transcriptase inhibitor (RTI) kinetics. In the absence of topical sustained delivery, limited local amounts of nucleoside reverse transcriptase inhibitor (NRTI) and nonnucleoside reverse transcriptase inhibitor (NNRTI) drugs may fail to protect both local and recruited target cells. Flux of phosphorylated NRTIs from intracellular to extracellular space is reduced, therefore recruited drug-naive target cells will have limited exposure to drug and remain susceptible to infection. The ability of NNRTIs to be easily transported in and out of cells makes these drugs more available to recruited immune cells, but also subject to being “flushed” out of local cells, for example, in response to higher volumes of genital tract secretions/semen following sex. This could render local cells susceptible. An ideal approach may therefore consist of an NRTI/NNRTI combination that would provide enough drug to be retained in local cells but also be available to recruited cells. Extensive and reduced drug fluxes are represented by full and dashed arrows, respectively.
Recent approaches to model the compartmentalization of drugs are in development.38,39 For example, we found that coculture of drug-naive T cells (representing newly recruited immune cells) with minced explant tissue that had been pretreated with tenofovir failed to protect the T cells from HIV infection, presumably because tenofovir has been metabolized to its active form, tenofovir-diphosphate, which is retained intracellularly. Thus there was insufficient drug released from the tissue to protect the cocultured T cells (Fig. 1b). The explanted tissue itself was protected from direct viral challenge in the absence of coculture. These observations may provide some insights into the discrepant results obtained in CAPRISA 004 and MTN 003 with tenofovir gel. Possibly, application of drug close to the time of sex (and HIV exposure) is necessary to protect immune cells that are recruited into the genital tract in response to sexual intercourse.
Consistent with this notion are the observations from several studies that there is an increase in immune cells in cervical biopsies or cytobrush samples following barrier unprotected sexual intercourse compared to samples obtained from abstinent women.40–42 Thus, coitally independent gel dosing, which may not achieve high enough drug levels in draining lymph nodes, may fail to protect immune cells recruited into the genital tract following sex or in response to other inflammatory stimuli. Whether there is a similar cellular response to sex in the rectal compartment has not been evaluated.
Unlike tenofovir, NNRTIs such as dapivirine and IQP-0528, which are rapidly transported in and out of cells, were transferred from the treated tissue to the cocultured T cells, which became protected from HIV challenge in this model system (Fig. 1, lower panel). However, because these drugs are not retained intracellularly, they may be “washed away” following sex thus limiting their efficacy. Sustained IVR drug delivery43 may overcome this PK property. In summary, an ideal prevention strategy will probably require sustained delivery of combinations of drugs that target different steps in the HIV life cycle and/or possess complementary PK properties so that protection is achieved in all anatomic compartments at all possible times of exposure. For example, in the case of an NRTI/NNRTI combination, resident immune cell populations may be protected by retaining sufficient intracellular NRTI and newly recruited immune cells by taking up the rapidly diffusing NNRTI. Optimization and validation of models that address the complexities of drug PK/PD are a scientific priority for the prevention field.
Inflammation, Hormones, and Drug PK/PD
Clinical trial results and other observations suggest that multiple factors may act on the genital tract mucosal environment to alter virus transport, density of immune system cells, and drug transport and thus shift the balance from protection to infection.44 Hormonal changes may play an important role in determining the risk of HIV infection and the efficacy of topical PrEP. The vaginal epithelia in humans and primates undergo cyclical changes in its structure, which in primates is associated with increased susceptibility to infection in the late luteal phase.45 Similar changes may contribute to increased susceptibility in humans during normal cycling, pregnancy, or in the setting of systemic hormonal contraception, although further studies are needed. These same structural changes may also alter the absorption and PK of topical microbicides.
Inflammatory conditions, which include adolescence,46 bacterial vaginosis, and other STI47–49 and the response to semen40–42 may not only recruit new immune cells (as noted above) but enhance susceptibility to HIV by disrupting tight junctions and facilitating viral access to submucosal immune cells and directly augmenting HIV replication through activation of the viral promoter.50 Inflammatory responses also induce T cell activation, which impacts PK/PD, particularly in the case of tenofovir. For example, the in vitro half-life of TFV-DP is 12 to 15 h in activated lymphocytes, but 40 to 60 h in resting lymphocytes.51,52 Moreover, activation may increase the intracellular concentrations of 2'-deoxy adenosine triphosphate (dATP), leading to reduced efficacy since TFV-DP competes with dATP for incorporation into viral DNA during reverse transcription.53,54 The efficacy of maraviroc may also be altered by inflammatory responses, which result in increased CCR5 expression. In addition, inflammation possibly affects the expression of cellular transporters that are responsible for the uptake of drugs such as tenofovir. The transporters involved in tenofovir uptake in the kidney have been well characterized and primarily include the family of organic anionic transporters (OAT).55 Far less is known about the expression and regulation of these transporters in the female or male genital tract, within the rectal mucosa, or on immune target cells. Possibly, differences in the expression of transporters or the enzymes required for phosphorylation of tenofovir to its active metabolite contribute to the variable protection observed with tenofovir gel. The notion that inflammation modulates the efficacy of tenofovir is supported by a nested case control substudy of participants in the CAPRISA 004 trial in which higher levels of systemic inflammatory immune mediators were associated with increased risk of HIV acquisition, independent of tenofovir gel use.44
Endogenous or exogenous reproductive hormones have pleiotropic effects on the mucosal immune environment and likely also impact drug PK/PD and therefore could impact efficacy. Specific effects of medroxyprogesterone, which remains the primary contraceptive method in much of the developing world, on immune cell populations and their relative activation status, expression of drug transporters, intracellular dATP concentrations, and enzymes involved in the phosphorylation or dephosphorylation of NRTI have not been evaluated. These are important gaps in our knowledge that must be addressed if we are to advance the field and optimize prevention strategies.
Future Directions
The tenuous progress of the field does not yet add up to success. The emphasis on approved ARVs for prevention signals an evolution from a focus on product discovery to one of formulation and delivery. This change, which hopefully will rapidly advance safe and effective products into the clinic, highlights the need to prioritize research to better understand drug PK/PD and safety in a complex and rapidly changing mucosal environment. The cervicovaginal and rectal mucosa are impacted by a dynamic microbiome and modulated by hormones, sex, semen, STI, and sexual behaviors; all of these may modulate drug efficacy and safety. There are as yet no validated animal models that simulate these complexities, although there have been significant advances particularly with nonhuman primates. Thus, a key research priority for the field is to develop and optimize animal and ex vivo models (see Anton et al. in this issue) with biological samples predictive of drug efficacy and safety. We need to optimize and expand Phase1/2 clinical trial designs to address the complexities of the field and evaluate formulations in multiple relevant anatomical compartments. These should include earlier studies of PK/PD and safety in different populations of sexually active men and women, adolescents, women on different hormones and menopausal women, and postcoital studies. This will require the development and validation of biomarkers predictive of efficacy, safety, and adherence. Predictive biomarkers will become an even greater priority as we enter an era in which partially protective interventions such as oral Truvada become the standard of care, which will preclude inclusion of a placebo arm in large-scale efficacy clinical trials. If we meet these scientific challenges, we will be able to advance the best products forward.
Acknowledgment
This work was supported by an award from NIH U19AI076980.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Baeten JM. Donnell D. Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxmen A. Wary approval for drug to prevent HIV. Nature. 2012;487(7407):283. doi: 10.1038/487283a. [DOI] [PubMed] [Google Scholar]
- 3.Abdool Karim Q. Abdool Karim SS. Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heffron R. Donnell D. Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: A prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewi P. Heeres J. Arien K, et al. Reverse transcriptase inhibitors as microbicides. Curr HIV Res. 2012;10(1):27–35. doi: 10.2174/157016212799304643. [DOI] [PubMed] [Google Scholar]
- 6.Neurath AR. Strick N. Li YY. Anti-HIV-1 activity of anionic polymers: A comparative study of candidate microbicides. BMC Infect Dis. 2002;2:27. doi: 10.1186/1471-2334-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel S. Hazrati E. Cheshenko N, et al. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196(9):1394–1402. doi: 10.1086/522606. [DOI] [PubMed] [Google Scholar]
- 8.Keller MJ. Mesquita PM. Torres NM, et al. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: Implications for future microbicide clinical trials. PLoS One. 2010;5(1):e8781. doi: 10.1371/journal.pone.0008781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doncel GF. Chandra N. Fichorova RN. Preclinical assessment of the proinflammatory potential of microbicide candidates. J Acquir Immune Defic Syndr. 2004;37(Suppl 3):S174–180. [PubMed] [Google Scholar]
- 10.Fichorova RN. Tucker LD. Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184(4):418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 11.Mesquita PM. Cheshenko N. Wilson SS, et al. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: Model of microbicide safety. J Infect Dis. 2009;200(4):599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segarra TJ. Fakioglu E. Cheshenko N, et al. Bridging the gap between preclinical and clinical microbicide trials: Blind evaluation of candidate gels in murine models of efficacy and safety. PLoS One. 2011;6(11):e27675. doi: 10.1371/journal.pone.0027675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson SS. Cheshenko N. Fakioglu E, et al. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: Expansion of a murine model of microbicide safety. Antivir Ther. 2009;14(8):1113–1124. doi: 10.3851/IMP1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Network MTN. Microbicide Trials Network Statement on Decision to Discontinue Use of Tenofovir Gel in VOICE, a Major HIV Prevention Study in Women. 2012. http://www.mtnstopshiv.org/sites/default/files/attachments/MTNStatementNov17DSMB_final.pdf http://www.mtnstopshiv.org/sites/default/files/attachments/MTNStatementNov17DSMB_final.pdf [cited 2012 September 4th]
- 15.Dezzutti CS. Rohan LC. Wang L, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother. 2012;67(9):2139–2142. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesquita PM. Rastogi R. Segarra TJ, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012;67(7):1730–1738. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefanidou M. Herrera C. Armanasco N, et al. Saquinavir inhibits early events associated with establishment of HIV-1 infection: Potential role for protease inhibitors in prevention. Antimicrob Agents Chemother. 2012;56(8):4381–4390. doi: 10.1128/AAC.00399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano J. Variano B. Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25(5):483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 19.Woolfson AD. Malcolm RK. Morrow RJ, et al. Intravaginal ring delivery of the reverse transcriptase inhibitor TMC 120 as an HIV microbicide. Int J Pharm. 2006;325(1–2):82–89. doi: 10.1016/j.ijpharm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Singer R. Derby N. Rodriguez A, et al. The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J Virol. 2011;85(11):5504–5512. doi: 10.1128/JVI.02422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turville SG. Aravantinou M. Miller T, et al. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One. 2008;3(9):e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson DP. Coplan PM. Wainberg MA, et al. The paradoxical effects of using antiretroviral-based microbicides to control HIV epidemics. Proc Natl Acad Sci USA. 2008;105(28):9835–9840. doi: 10.1073/pnas.0711813105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson TJ. Srinivasan P. Albright TH, et al. Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. Antimicrob Agents Chemother. 2012;56(3):1291–1299. doi: 10.1128/AAC.05721-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turpin JA. Schito ML. Jenkins LM, et al. Topical microbicides: A promising approach for controlling the AIDS pandemic via retroviral zinc finger inhibitors. Adv Pharmacol. 2008;56:229–256. doi: 10.1016/S1054-3589(07)56008-4. [DOI] [PubMed] [Google Scholar]
- 25.Schito ML. Soloff AC. Slovitz D, et al. Preclinical evaluation of a zinc finger inhibitor targeting lentivirus nucleocapsid protein in SIV-infected monkeys. Curr HIV Res. 2006;4(3):379–386. doi: 10.2174/157016206777709492. [DOI] [PubMed] [Google Scholar]
- 26.Miller Jenkins LM. Ott DE. Hayashi R, et al. Small-molecule inactivation of HIV-1 NCp7 by repetitive intracellular acyl transfer. Nat Chem Biol. 2010;6(12):887–889. doi: 10.1038/nchembio.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeitlin L. Pauly M. Whaley KJ. Second-generation HIV microbicides: continued development of griffithsin. Proc Natl Acad Sci USA. 2009;106(15):6029–6030. doi: 10.1073/pnas.0902239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ham AS. Cost MR. Sassi AB, et al. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res. 2009;26(3):502–511. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welch BD. Francis JN. Redman JS, et al. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J Virol. 2010;84(21):11235–11244. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouschbacher M. Bomsel M. Verronese E, et al. Early events in HIV transmission through a human reconstructed vaginal mucosa. AIDS. 2008;22(11):1257–1266. doi: 10.1097/QAD.0b013e3282f736f4. [DOI] [PubMed] [Google Scholar]
- 31.Ganor Y. Zhou Z. Tudor D, et al. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol. 2010;3(5):506–522. doi: 10.1038/mi.2010.32. [DOI] [PubMed] [Google Scholar]
- 32.Ganor Y. Bomsel M. HIV-1 transmission in the male genital tract. Am J Reprod Immunol. 2011;65(3):284–291. doi: 10.1111/j.1600-0897.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- 33.Fischetti L. Barry SM. Hope TJ, et al. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS. 2009;23:319–328. doi: 10.1097/QAD.0b013e328321b778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hladik F. Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 35.Hope TJ. Microbicides. Delhi, India: 2008. Feb 25, 2008. Latest understanding of HIV sexual transmission. 2008. [Google Scholar]
- 36.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 37.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 38.Mesquita P. Torres N. Segarra T, et al. Intravaginal ring formulation of tenofovir disoproxil fumarate (TDF) facilitates rapid bioavailability of drug, protects cervical explants from HSV-2 infection. Protection from HIV: Targeted Intervention Strategies; Keystone Symposia. Mar 20–25;; British Columbia, Canada: 2011. [Google Scholar]
- 39.Mesquita P. Rastogi R. Johnson T, et al. Intravaginal ring delivery of the pyrimidinedione IQP-0528 results in protective concentrations in vaginal biopsies of pigtailed macaques, novel models to assess pharmacokinetics/pharmacodynamics. 19th Conference on Retroviruses and Opportunistic Infections; Mar 5–8;; Seattle, WA. 2012. [Google Scholar]
- 40.Sharkey DJ. Macpherson AM. Tremellen KP, et al. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol. 2012;189(2):1024–1035. doi: 10.4049/jimmunol.1200005. [DOI] [PubMed] [Google Scholar]
- 41.Sharkey DJ. Macpherson AM. Tremellen KP, et al. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13(7):491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 42.Sharkey DJ. Tremellen KP. Jasper MJ, et al. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 43.Kiser PF. Johnson TJ. Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012;14(1):62–77. [PubMed] [Google Scholar]
- 44.Naranbhai V. Abdool Karim SS. Altfeld M, et al. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis. 2012;206(7):993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vishwanathan SA. Guenthner PC. Lin CY, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57(4):261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 46.Madan RP. Carpenter C. Fiedler T, et al. Altered biomarkers of mucosal immunity and reduced vaginal lactobacillus concentrations in sexually active female adolescents. PLoS One. 2012;7(7):e40415. doi: 10.1371/journal.pone.0040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Low N. Chersich MF. Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: Individual participant data meta-analysis. PLoS Med. 2011;8(2):e1000416. doi: 10.1371/journal.pmed.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen CR. Lingappa JR. Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: A prospective cohort analysis among African couples. PLoS Med. 2012;9(6):e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell C. Hitti J. Paul K, et al. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retroviruses. 2011;27(1):35–39. doi: 10.1089/aid.2010.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shacklett BL. Anton PA. HIV infection and gut mucosal immune function: Updates on pathogenesis with implications for management and intervention. Curr Infect Dis Rep. 2010;12(1):19–27. doi: 10.1007/s11908-009-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patterson KB. Prince HA. Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: Implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re114. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbins BL. Srinivas RV. Kim C, et al. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxy-methylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42(3):612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao WY. Agbaria R. Driscoll JS, et al. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2',3'-dideoxynucleoside analogs in resting and activated human cells. J Biol Chem. 1994;269(17):12633–12638. [PubMed] [Google Scholar]
- 54.Garcia-Lerma JG. Aung W. Cong ME, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol. 2011;85(13):6610–6617. doi: 10.1128/JVI.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cihlar T. Ho ES. Lin DC, et al. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):641–648. doi: 10.1081/NCN-100002341. [DOI] [PubMed] [Google Scholar]