Abstract
As the AIDS epidemic continues with women being disproportionately affected, it is crucial to understand factors that predict the risk of heterosexual HIV-1 transmission. We investigated whether genital tract viral load (GTVL) in cervical-vaginal lavages (CVL) from HIV-1-positive women with moderately low CD4 T cell counts correlates with cytokine levels, antimicrobial concentrations, and intrinsic anti-HIV activity. CVL were collected from 19 HIV-1-positive women with moderately low CD4 T cell counts [mean 381 cells/mm3 (227–536 cells/mm3)]. None of the women was on antiretroviral therapy. The women were categorized into those with detectable GTVL or those with undetectable GTVL (detectable GTVL RNA levels > 400 copies/ml). Women were also categorized according to bacterial vaginosis (BV) status irrespective of GTVL. The TZM-bl assay was used to determine the presence of infectious virus and anti-HIV activity. Significantly higher levels of RANTES, Eotaxin, Fractalkine, IL-1α, IL-6, MCP-1, MIP1β, MIP1α, TNF-α, and GM-CSF were observed in women with detectable GTVL compared to women with undetectable GTVL. No significant differences were observed in the following cytokines and chemokines: G-CSF, IL-1RA, IL-8, and IP-10. GTVL did not correlate either with antimicrobials known to have anti-HIV activity or with the presence of infectious virus. BV status did not have a significant effect on anti-HIV activity. These findings further our understanding of the role of GTVL in determining the cytokine and chemokine milieu in the female reproductive tract.
Introduction
HIV is one of the leading cause of death and disease among women of reproductive age (15–49 years) worldwide.1 Genital tract shedding of HIV type 1 (HIV-1) in women increases the risk of heterosexual and mother-to-child transmission.2,3 Factors that increase genital tract HIV-1 shedding and the risk of HIV-1 transmission include genital coinfections,4–6 hormonal contraceptive use,7 pregnancy,4,8 and CD4 T cell count.9 Several studies have shown that plasma viral load (PVL) is an important predictor of HIV-1 RNA in genital tract secretions.5,10–13 However, a longitudinal study assessing genital tract HIV-1 RNA shedding in women on highly active antiretroviral therapy (HAART) indicated that over half the women at some time point had HIV-1 RNA present in their genital tract secretions although PVL was undetectable.14 Therefore, the risk of HIV-1 transmission may still exist in women with undetectable PVL and detectable genital tract viral load (GTVL). The actual percent of infectious HIV-1 in genital tract secretions from HIV-1-positive women is unknown and varies from one individual to the next. In a recent study, we reported that in 32 HIV-1-positive women, with a mean CD4 T cell count of 713 cells/mm3, only 10% had infectious virus in their cervical-vaginal secretions irrespective of PVL or GTVL.15 This finding may explain why over 99% of unprotected sexual exposures to HIV do not result in infection,16 and the transmission rate of HIV-1 per coital act between discordant heterosexual couples is relatively low.17–19
Female reproductive tract (FRT) cells produce and secrete a spectrum of cytokines, chemokines, and antimicrobials that inhibit the growth and/or infection by reproductive tract pathogens such as HIV-1, Neisseria gonorrheae, and Candida albicans.20 These antimicrobials include alpha/beta defensins, lactoferrin, secretory leukocyte protease inhibitor (SLPI), trappin-2/elafin, and MIP3α,20,21 all of which have been shown to inhibit HIV-1 infection of target cells through multiple mechanisms.17,22–24 Elevated trappin-2/elafin levels have also been found in genital tract secretions of a cohort of HIV-1-resistant Kenyan sex workers.25
The purpose of the current study was to determine whether the levels of various cytokines and antimicrobials in cervical-vaginal lavages (CVL) from HIV-1-positive women, with moderately low CD4 T cell counts, correlate with GTVL. As a part of these studies, we assessed whether GTVL was associated with infectious virus and anti-HIV activity in genital tract secretions and observed that CVL from HIV-1-positive women with detectable GTVL contained higher levels of specific cytokines compared to CVL with undetectable GTVL.
Materials and Methods
CVL collection from HIV+ women
CVL were collected from 19 HIV+ participants as part of an observational study on genital tract HIV shedding in women. The study was approved by the Miriam Hospital Institutional Review Board (Brown University, Providence, RI). All 19 HIV+ participants provided written informed consent for the collection of samples and subsequent analysis. The median age of the women was 35 years (range 21–50) and none was on antiretroviral (ARV) therapy. Enrollment criteria included a normal menstrual history and CD4 T cell counts above 200 cells/mm3 [mean 381 cells/mm3 (range 227–536 cells/mm3)]. Women were excluded if they were on hormonal contraceptives, had douched, used any vaginal products, or had sexual intercourse during the 48 h prior to CVL collection. Women were tested for genital tract infections including bacterial vaginosis (BV), Trichomonas vaginalis, Neisseria gonorrhea, Chlamydia trachomatis, and Candida albicans. BV was diagnosed based on the Amsel's criteria.26 CVL were collected by instilling 10 ml of normal saline into the vaginal cavity with the stream directed toward the external os of the cervix. The fluid was allowed to pool in the posterior fornix, and then aspirated. Secretions were centrifuged at 10,000×g for 5 min to separate out the cellular fraction and the supernatants were stored in aliquots in a −80°C freezer until use.
Genital and plasma HIV-1 load measurements
Nucleic acid sequence-based amplification (bioMérieux, Durham, NC) was used to measure HIV-1 RNA. All results are expressed as copies per milliliter with a lower limit of detection of 2.6 log10 (400) copies/ml for both plasma and CVL. Women were categorized into those with detectable GTVL (n=10) and those with undetectable GTVL (n=9). Semen in CVL fluid was assessed by the Abacus Diagnostic OneStep ABA card P30 test (Abacus Diagnostics, West Hills, CA).
Measurement of cytokines/chemokines and antimicrobial levels in CVL
Cytokines and chemokines measured in this study included Regulated upon Activation, Normal T-cell Expressed, and Secreted (RANTES), Eotaxin, Fractalkine, interleukin (IL)-1α, IL-6, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1 (MIP-1β), MIP-1α, tumor necrosis factor (TNF)-α, granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), IL-1 receptor antagonist (RA), IL-8, and interferon gamma-induced protein (IP)-10. Cytokines and chemokines were analyzed in CVL using multiplex cytokine bead technology (Luminex; Bio-Rad, Hercules, CA). Due to the limitation of CVL availability, five CVL with detectable GTVL and seven CVL with undetectable GTVL were measured for 14 cytokines and chemokines. Luminex assays were performed in a 96-well filtration plate (Millipore, Billerica, MA). To each well, 5000 beads coated with antibody to each cytokine or chemokine were mixed with either a standard, sample, spiked control or blank in a final volume of 100 μl and incubated for 30 min with continuous shaking at room temperature. After washing the beads three times, biotinylated antibodies were added for 30 min with shaking. Beads were again washed three times and streptavidin–phycoerythrin was added for 10 min. After further washes, the fluorescence intensity of the beads was measured using the Bio-Plex array reader, and Bio-Plex Manager software with five-parametric-curve fitting was used for data analysis. We also measured four antimicrobials with known anti-HIV activity using ELISA assays in 19 CVL. These antimicrobials were human β-defensin-2 (HBD2), secretory leukocyte protease inhibitor (SLPI), MIP-3α, and trappin-2/elafin. HBD2 was assayed with an ELISA test kit from PeproTech (Rocky Hill, NJ) according to the manufacturer's protocol. SLPI, MIP-3α and trappin-2/elafin were assayed with an ELISA quantikine kit or ELISA Duoset kit from R&D Systems (Minneapolis, MN) according to the manufacturer's protocol. HBD2, SLPI, MIP-3α, and trappin-2/elafin were quantified based on standard curves obtained using an ELISA reader (Dynex, Chantilly, VA).
HIV viral stocks
Laboratory-adapted viral strains HIV-1 IIIB (X4) and BaL (R5) stocks were propagated in PHA-stimulated human PBMCs and stored frozen at −80°C. These were kindly donated by Dr. P. Gupta (University of Pittsburgh, PA).
TZM-bl assay for HIV-1 infection: assessment of presence of infectious HIV-1 and intrinsic anti-HIV-1 activity
The TZM-bl indicator cell line is a HeLa cell derivative that expresses high levels of CD4, CCR5, and CXCR4.27 Cells contain HIV long terminal repeat (LTR)-driven β-galactosidase and firefly luciferase reporter cassettes that are activated by HIV infection and subsequent Tat protein expression. TZM-bl cells were routinely subcultured every 3 to 4 days by trypsinization and maintained in TZM-bl media consisting of phenol red-free DMEM (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% defined FBS (HyClone, Logan, UT), 2 mM l-glutamine (Invitrogen Life Technologies, Carlsbad, CA), and 50 μg/ml primocin (Invivogen, San Diego, CA). To measure infectious virus, CVL samples were diluted 1:4 in TZM-bl media prior to 100 μl being added to TZM-bl cells for 48 h at 37°C in 5% CO2. Luciferase activity was measured following the manufacturer's instructions. Briefly, following aspiration of supernatants, a beta-glo luciferase substrate (Promega, Madison, WI) solution (100 μl) was added to cells. The light intensity of each well was measured using a luminometer. Background luminescence was determined by analyzing uninfected TZM-bl cells. All infectivity assays were performed in quadruplicate. Media diluted CVL had neutral pH values (pH 7.0–7.2). Viability of TZM-bl cells upon treatment with CVL was quantified using the CellTiter Aqueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's instructions. Briefly, reagent was added directly to cell cultures and incubated for 30 min at 37°C followed by reading the plate in a plate reader at OD 490 nm.
CVL were diluted 1:4 and incubated with HIV-1 IIIB and BaL at a multiplicity of infection (MOI) of 1.0 for 1 h at 37°C prior to adding to TZM-bl indicator cells. Following incubation, media was aspirated from TZM-bl cells and the virus plus CVL mixture (100 μl) was added to the cells along with 100 μl of TZM-bl media. Positive controls included incubation of TZM-bl with virus alone. For negative controls we used cells treated with CVL alone and cells incubated in media alone. Luciferase activity was measured as described above. Uninfected cells were used to determine background luminescence and data were expressed in relative light units (RLU).
Statistical analysis
Data were analyzed using a two-tailed unpaired t test for all the comparisons between CVL with detectable GTVL and CVL with undetectable GTVL. Mean and standard error were calculated for all data sets. This was performed using GraphPad InStat version 3.0a (GraphPad Software, San Diego, CA). A p value of <0.05 was taken as indicative of statistical significance.
Results
Higher cytokine and chemokine levels in CVL with detectable GTVL compared to CVL with undetectable GTVL
Table 1 shows the patient demographics of HIV+ women indicating race, age, PVL, GTVL, CD4 T cell counts, and BV status. CD4 T cell count was not associated with detectable GTVL and there was no association between PVL and detectable GTVL (data not shown). To determine whether cytokine and chemokine levels in CVL are altered in women with detectable GTVL compared to women with undetectable GTVL, we compared levels of 14 different cytokines and chemokines. Owing to the limitation of CVL availability we assessed 12 out of the 19 CVL samples. As seen in Table 2, CVL from HIV+ women with detectable GTVL (n=5) had significantly higher levels of RANTES, Eotaxin, Fractalkine, IL-1α, IL-6, MCP-1, MIP-1β, MIP-1α, TNF-α, and GM-CSF compared to CVL with undetectable GTVL (n=7). Interestingly, we found no significant difference between the levels of G-CSF, IL-1RA, IL-8, and IP-10 in CVL with detectable GTVL compared to CVL with undetectable GTVL. This suggests that an increase in specific proinflammatory cytokines and chemokines is associated with HIV-1 shedding in the genital tract.
Table 1.
HIV-1 Patient Demographics Stratified According to Detectable or Undetectable Genital Track Viral Load
| Patient # | Race | Age | PVL | GTVL | CD4 count | BV Status |
|---|---|---|---|---|---|---|
| 1 | B | 41 | 3,500 | <400 | 353 | Neg |
| 2 | W | 42 | 250,000 | <400 | 428 | Neg |
| 3 | H | 21 | 4,100 | <400 | 448 | Pos |
| 4 | H | 43 | 3,800 | <400 | 470 | Pos |
| 5 | W | 32 | 34,000 | <400 | 510 | Pos |
| 6 | B | 50 | 8,900 | <400 | 393 | Pos |
| 7 | H | 34 | 3,300 | <400 | 371 | Pos |
| 8 | B | 42 | 1,800 | <400 | 490 | Pos |
| 9 | B | 47 | 2,200 | <400 | 432 | ND |
| 10 | W | 30 | 1,700 | 78,000 | 536 | Neg |
| 11 | H | 35 | 31,000 | 600 | 242 | Neg |
| 12 | A | 32 | 8,051 | 13,000 | 302 | Neg |
| 13 | B | 31 | 35,000 | 10,000 | 263 | Pos |
| 14 | W | 26 | 3,100 | 2,900 | 486 | Neg |
| 15 | W | 29 | 650,000 | 17,000 | 227 | Pos |
| 16 | B | 25 | 400 | 960 | 264 | Neg |
| 17 | B | 42 | 13,000 | 170,000 | 308 | Pos |
| 18 | B | 43 | 5,200 | 1,100 | 480 | Pos |
| 19 | H | 40 | 56,000 | 14,000 | 228 | Pos |
Patients self-identified themselves by race as Asian (A), Black (B), Hispanic (H) or White (W). The lower limit of viral load detection was 400 copies/ml. Indicated is the plasma viral RNA load (PVL), genital tract viral RNA load (GTVL), and CD4 counts (cells/mm3).
Table 2.
Analysis of Cytokines/Chemokines in Cervical-Vaginal Lavages with Detectable or Undetectable Genital Track Viral Load
| Cytokine/chemokine | Detectable GTVL N=5 pg/ml±SEM | Undetectable GTVL N=7pg/ml±SEM | p-value |
|---|---|---|---|
| RANTES | 101±37 | 23±3 | 0.02 |
| Eotaxin | 163±68 | 23±11 | 0.03 |
| Fractalkine | 1015±369 | 220±34 | 0.02 |
| IL-1α | 3421±1259 | 451±234 | 0.02 |
| IL-6 | 111±27 | 39±15 | 0.03 |
| MCP-1 | 511±193 | 116±45 | 0.04 |
| MIP-1β | 321±124 | 59±15 | 0.03 |
| MIP-1α | 151±61 | 6±3 | 0.02 |
| TNF-α | 66±27 | 4±1 | 0.02 |
| GM-CSF | 41±15 | 4±2 | 0.02 |
| G-CSF | 550±486 | 669±263 | 0.85 |
| IL-1Ra | 75,054±23,209 | 158,500±39,552 | 0.07 |
| IL-8 | 2,476±1,032 | 6,047±1,352 | 0.06 |
| IP-10 | 2,527±1,619 | 16,461±7,870 | 0.07 |
Significant increases in levels of several cytokines/chemokines were measured in CVL with detectable GTVL compared to those with undetectable GTVL. Cytokines/chemokines were measured as described in Materials and Methods. The p-values were calculated using a two-tailed unpaired t test and a p-value < 0.05 was considered significant.
CVL with both undetectable and detectable GTVL contain multiple antimicrobials
CVL contain more than 20 endogenous antimicrobials, some of which have known direct or indirect anti-HIV activity.17,22,28–30 To determine whether GTVL has an effect on antimicrobials with known anti-HIV activity, we measured concentrations of HBD2, SLPI, MIP-3α, and trappin-2/elafin in CVL with detectable and undetectable GTVL. For the purpose of this study we specifically examined HBD2, SLPI, MIP-3α, and trappin-2/elafin as a group of antimicrobials with known anti-HIV activity, although there are other chemokines such as RANTES, MIP-1β, and MIP-1α with known anti-HIV activity present in CVL (see Table 2).
Table 3 shows that CVL with both detectable and undetectable GTVL contained all the antimicrobials measured. In contrast to cytokines and chemokines measured in Table 2, there was no significant difference between levels of HBD2, SLPI, MIP-3α, and trappin-2/elafin in CVL with detectable GTVL compared to CVL with undetectable GTVL. When we stratified the women according to BV status irrespective of GTVL, the levels of HBD2, SLPI, and trappin-2/elafin in CVL from BV+ women tended to be lower than that seen in CVL from BV− women but were not statistically significant (Table 4). Due to a limitation in CVL samples we could not make comparisons of other cytokines or chemokines in CVL from BV+ women to CVL from BV− women.
Table 3.
Analysis of Antimicrobials in Cervical-Vaginal Lavages with Detectable or Undetectable Genital Track Viral Load
| Antimicrobial | Detectable GTVL N=10pg/ml±SEM | Undetectable GTVL N=9pg/ml±SEM |
|---|---|---|
| HBD2 | 206,481±44,286 | 213,758±81,381 |
| SLPI | 54,091±10,490 | 54,996±11,559 |
| MIP-3α | 204±94 | 230±188 |
| Trappin-2/elafin | 22,178±2,958 | 22,287±2,829 |
No significant differences were found in levels of antimicrobials in HIV+ women with detectable GTVL or undetectable GTVL. Levels of antimicrobials were measured as described In Materials and Methods.
Table 4.
Analysis of Antimicrobials in Bacterial Vaginosis (BV)-Negative and BV-Positive Cervical-Vaginal Lavages
| Antimicrobial | CVL from BV− women pg/ml±SEM | CVL from BV+ women pg/ml±SEM | p-value |
|---|---|---|---|
| HBD2 | 252,642±60,765 | 124,132±41,823 | 0.09 |
| SLPI | 71,340±11,515 | 40,544±8,992 | 0.05 |
| MIP-3α | 204±128 | 208.9±154 | 0.83 |
| Trappin-2/elafin | 25,388±3,027 | 20,019±2,781 | 0.22 |
Antimicrobial levels measured in CVL from BV-positive women are lower than in CVL from BV-negative women although not statistically significant. The p-values were calculated using a two-tailed unpaired t test and a p-value < 0.05 was considered significant.
Absence of infectious HIV-1 and intrinsic anti-HIV activity in CVL with detectable GTVL or undetectable GTVL
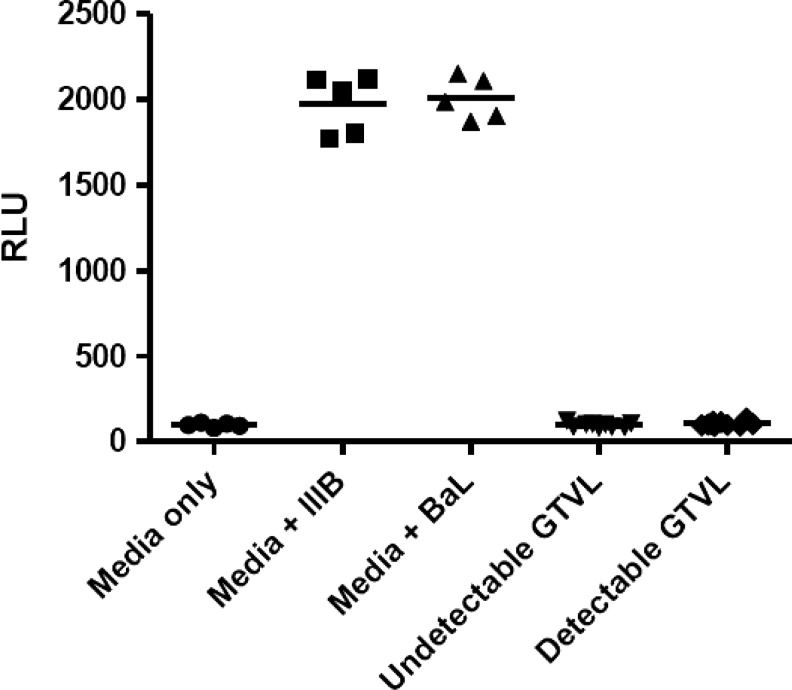
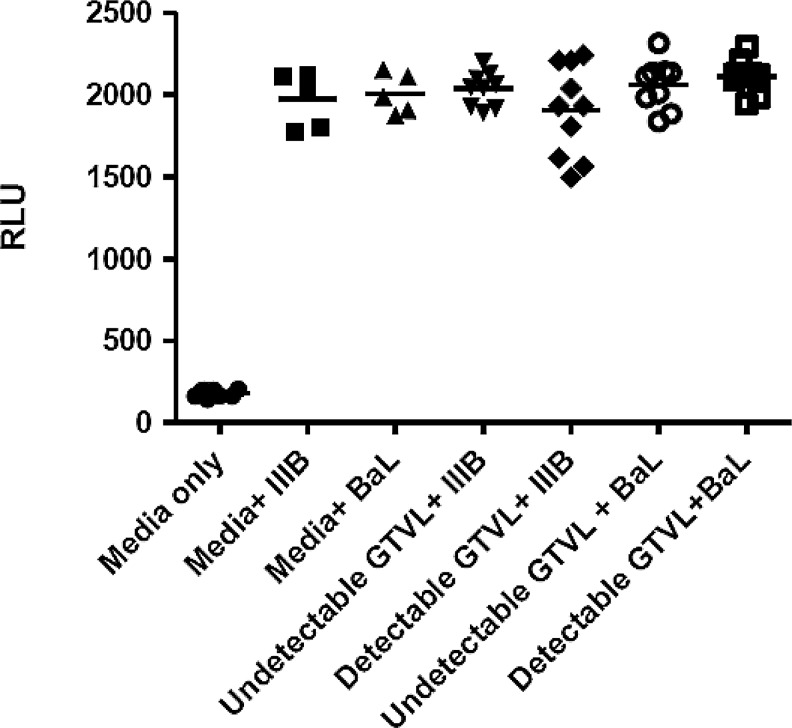
We did not detect any infectious HIV-1 virus in any of the 19 CVL samples analyzed (Fig. 1). Despite the presence of HIV-1 RNA in the genital tract, no infectious virus was found in this limited cohort of women. Previously, we observed intrinsic anti-HIV activity in CVL from relatively healthy HIV-positive women (CD4>500).15 To determine whether our cohort of HIV+ women with moderately low CD4 T cell counts also had anti-HIV activity we tested CVL against HIV IIIB (X4-tropic) and BaL (R5-tropic) using TZM-bl cells. CVL were incubated with either IIIB or BaL HIV-1 virus at an MOI of 1.0 for 1 h at 37°C prior to the measurement of HIV-1 infectivity of TZM-bl cells. Figure 2 shows that CVL, irrespective of whether they had detectable or undetectable GTVL, had no anti-HIV activity. This lack of anti-HIV activity in CVL was observed with both IIIB and BaL HIV-1 virus. CVL from our cohort of HIV+ women with moderately low CD4 T cell counts lacked intrinsic anti-HIV activity. Despite the presence of BV in some women, infectious virus was not recovered and anti-HIV activity was not found.31–35
FIG. 1.
Determination of the presence of infectious HIV-1 in 19 HIV+ women with detectable genital tract viral load (GTVL RNA levels > 400 copies/ml) and undetectable GTVL (GTVL RNA levels < 400 copies/ml). Cervical-vaginal lavages (CVL) were diluted 1:4 and added directly to TZM-bl cells. The assay was terminated 48 h postinfection and HIV-1 infection was quantified by measuring luciferase reporter gene activity using a luminometer and expressed as relative light units (RLU). Each data point in the graph represents one individual patient. In the negative control (media only) column and in the positive control [media+IIIB (X4-tropic) or BaL (R5-tropic)] each point represents replicate wells. There was no infectious HIV-1 in both detectable and undetectable GTVL groups.
FIG. 2.
Measurement of anti-HIV activity in HIV+ women with detectable GTVL (GTVL RNA levels > 400 copies/ml) and undetectable GTVL (GTVL RNA levels < 400 copies/ml). CVL were diluted 1:4 and incubated with HIV-1 IIIB (X4-tropic) and BaL (R5-tropic) at a multiplicity of infection (MOI) of 1.0 for 1 h at 37°C prior to infecting TZM-bl cells. Data points in each graph represent one individual patient. Media was set up as negative control, and replicate wells of virus (IIIB and BaL) were set up as positive controls.
Discussion
Our study of HIV+ women with moderately low CD4 T cell counts shows that GTVL correlated with an increase in specific cytokines and chemokines. We did not recover infectious virus and did not detect any anti-HIV activity in both CVL with detectable and undetectable GTVL. To delineate whether the absence of infectious HIV-1 virus or anti-HIV activity was associated with alterations in antimicrobial levels (HBD2, SLPI, MIP-3α, and trappin-2/elafin), we measured the antimicrobials in all 19 CVL samples. We observed that both CVL with detectable and undetectable GTVL contained all four antimicrobials with no significant differences seen between the groups.
Our study examined the differences in cytokine and chemokine levels between CVL with detectable and undetectable GTVL from HIV-1+ women with moderately low CD4 T cell counts. We found that CVL with detectable GTVL had significantly higher levels of RANTES, Eotaxin, Fractalkine (CX3CL1), IL-1α, IL-6, MCP-1, MIP-1β, MIP-1α, TNF-α, and GM-CSF. In a study carried out by Shebl at al. there was an association between Fractalkine (CX3CL1) and elevated HIV viral load in blood from male and female individuals.36 Lower CD4 T cell counts were also significantly associated with Fractalkine in the cohort Shebl at al. examined.36 These findings suggest that Fractalkine levels are elevated due to an increase in the frequency of T cells expressing CX3CR1, such as those observed by Combadiere et al. in HIV-1 infected patients.37 The chemokines, RANTES, MIP-1β, and MIP-1α are natural ligands for CCR5 and inhibit HIV-1 entry into target cells.24,38,39 Our studies with HIV+ women extend the findings of Kaul et al. who demonstrated an increase in genital RANTES levels and in the number of HIV susceptible target cells present in the cervical mucosa of HIV-exposed persistently seronegative women.40 In our study, RANTES, MIP-1β, and MIP-1α may be recruiting HIV target cells seen by Kaul et al. in the genital tract mucosa, which become infected with HIV-1 leading to an increase in GTVL.
Our study indicates that CVL with detectable GTVL had higher levels of the proinflammatory cytokines and chemokines—IL-1α, IL-6, MCP-1, TNF-α, and GM-CSF—than CVL with undetectable GTVL. Further studies are needed to determine if increases in proinflammatory cytokines and chemokines are due to HIV-1 infection or whether they contribute to elevated GTVL. These results are consistent with the findings that IL-1α, IL-6, and TNF-α increase HIV-1 expression.41–45 TNF-α increases HIV-1 proviral transcription and upregulates HIV replication through activation of the LTR promoter region.46 In contrast, we did not observe significant differences between the levels of G-CSF, IL-1RA, and IP-10 in CVL with detectable GTVL compared to CVL with undetectable GTVL. We found high levels of IL-8 in both CVL with detectable GTVL and undetectable GTVL; we did not observe a significant difference between these groups. In contrast to our finding, a recent study reported that IL-8 in CVL from HIV-1-positive women was associated with higher cervicovaginal HIV-1 RNA concentrations independent of genital coinfections.47 One explanation for the differences seen is that, unlike our study, which excluded women on antiretroviral therapy, 54% of the women were on antiretroviral therapy in the study reported.
Paradoxically, our studies indicate that CVL from HIV+ women contain high levels of HBD2, SLPI, MIP-3α, and trappin-2/elafin but lack intrinsic anti-HIV activity. Previously, we showed that CVL from HIV+ women with a mean CD4 T cell count of 713 cells/mm3 contain intrinsic anti-HIV activity and that anti-HIV activity in CVL correlated with MIP-3α and HBD2.15 An explanation for our findings in the present study is that our cohort consists of women with a lower mean CD4 T cell count than those analyzed in our earlier study. This may suggest that with disease progression, there is an overall reduction in anti-HIV activity possibly due to a compromised innate immune system in the FRT. Preliminary studies from our laboratory indicate that even lower CD4 T cell counts correlate with a complete loss of intrinsic anti-HIV activity (T. Lahey and M. Ghosh, unpublished observations). In these studies, loss of intrinsic anti-HIV was measured using the TZM-bl assay and then correlating anti-HIV activity with antimicrobial levels in CVL samples from different women stratified according to their CD4 T cell counts.
In our study, since antimicrobials were present in CVL, the absence of intrinsic anti-HIV activity may suggest that the antimicrobials measured might not be biologically active. As discussed elsewhere, others have found that CVL contain cathepsins, a family of matrix metalloproteases, which may compromise the antiprotease activity of SLPI and trappin-2/elafin.48,49 The mechanisms by which SLPI and trappin-2/elafin inhibit HIV-1 infection seem to be independent of their antiprotease properties but may be related to prevention of virus entry into target cells.49 However, disruption of the antiprotease activity of these antimicrobials may result in a compromised FRT mucosal immune response leading to an increase in susceptibility to HIV-1 infection. Kallikreins (KLK), a family of serine proteases, are also present in CVL and can activate or deactivate immune factors in the FRT.48,50 For example, KLK5 has been shown to regulate the antimicrobial activity of LL37 and defensins-1α.51,52
Whether HIV infection and disease progression lead to increases in protease activity in the FRT remains to be determined. What is clear is that analyses based on ELISA results do not accurately reflect biological activity of antimicrobials and as such may not reflect the level of immune protection present in the FRT. Although we did not categorize women according to their menstrual cycle stage, the finding that hormonal status influences trypsin-like activity in the FRT represents a further complexity in understanding the mechanisms through which the local FRT microenvironment regulates bioactivity of antimicrobials.48,50,53
BV and BV-associated microflora have been associated with increased HIV-1 RNA expression in the female genital tract.54–56 Due to a limitation in the number of CVL samples we were unable to determine whether there was a relationship between BV status, cytokine/chemokine levels, and GTVL. However, when HBD2, SLPI, MIP-3α, and trappin-2/elafin in CVL were measured, we observed that although they did not reach statistical significance, three out of four antimicrobials in CVL from BV+ women were lower than those in CVL from BV− women, suggesting that BV may increase susceptibility to HIV-1 infection. We did not detect the presence of infectious virus in any of the 19 CVL samples. The recovery of infectious virus from genital tract secretions is rare and there are only a few studies that have measured infectious virus in CVL.15,31 The percentage of infectious virus in CVL from HIV+ women may be independent of the GTVL but is an important factor in predicting heterosexual transmission of HIV-1. One of the limitations of our study was the small sample size of HIV+ women. In a study of African serodiscordant couples that included over 1500 HIV-1-seropositive women, Baeten et al. found an association between high concentrations of genital tract HIV-1 RNA and risk of HIV-1 transmission.2 Future studies on GTVL in HIV+ women should include different populations of HIV+ women. The race and genetics of HIV+ women in a population may be important factors to consider when evaluating concentrations of genital tract HIV-1 RNA and the risk of heterosexual transmission.
We found that alterations in specific cytokines and chemokines exist in CVL with detectable GTVL compared to CVL with undetectable GTVL indicating that GTVL is associated with local mediators that play a role in HIV-1 infection in women. As in previous studies, recovery of infectious virus was poor and in our study we did not recover infectious virus in CVL with detectable GTVL.
Acknowledgments
This work was supported by National Institutes of Health Grants AI51877 (awarded to Dr. Charles Wira) and AI40350 and AI066884 (awarded to Dr. Susan Cu-Uvin). The authors thank Zheng Shen, M.D. (Dartmouth Medical School), Deena Ratner, B.S. (University of Pittsburgh), and Irma Rodriguez (Brown University) for excellent technical assistance in the preparation of samples, cells, and virus stocks. The authors also thank Dr. Phalguni Gupta (University of Pittsburgh) for generous sharing of reagents and information.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.WHO, Women, Health. Today's evidence tomorrow's agenda. http://whqlibdoc.who.int/publications/2009/9789241563857_eng.pdf. 2009. http://whqlibdoc.who.int/publications/2009/9789241563857_eng.pdf
- 2.Baeten JM, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Translational Med. 2011;3(77):77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mofenson LM, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341(6):385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 4.Anderson BL, et al. Genital tract leukocytes and shedding of genital HIV type 1 RNA. Clin Infect Dis. 2008;47(9):1216–1221. doi: 10.1086/592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cu-Uvin S. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir Immune Defic Syndr. 2006;42(5):584–587. doi: 10.1097/01.qai.0000229997.52246.95. [DOI] [PubMed] [Google Scholar]
- 6.McClelland RS, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16(18):2425–2430. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 7.Heffron R, et al. Use of hormonal contraceptives, risk of HIV-1 transmission: A prospective cohort study. http://dx.doi.org/10.1016/S1473-3099(11)70247-X. Lancet Infect Dis. 2011(11) doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemetson DB, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269(22):2860–2864. [PubMed] [Google Scholar]
- 9.Kovacs A, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358(9293):1593–1601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 10.Vernazza PL, et al. Quantification of HIV in semen: Correlation with antiviral treatment and immune status. AIDS. 1997;11(8):987–993. [PubMed] [Google Scholar]
- 11.Speck CE, et al. Risk factors for HIV-1 shedding in semen. Am J Epidemiol. 1999;150:622–631. doi: 10.1093/oxfordjournals.aje.a010061. [DOI] [PubMed] [Google Scholar]
- 12.Mostad SB. Prevalence and correlates of HIV type 1 shedding in the female genital tract. AIDS Res Hum Retroviruses. 1998;14:S11–S15. [PubMed] [Google Scholar]
- 13.Kaul R, et al. The genital tract immune milieu: An important determinant of HIV susceptibility and secondary transmission. J Reproduct Immunol. 2008;77(1):32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Cu-Uvin S, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24(16):2489–2497. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh M, et al. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS ONE. 2010;5(6):e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royce RA, et al. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh M, et al. Trappin-2/Elafin: A novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2010;129(2):207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray RH, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 19.Wawer MJ, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 Infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 20.Wira CR, et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2011;4(3):335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey JV, et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1(4):317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh M, et al. CCL20/MIP3α is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reproduct Immunol. 2009;62(1):60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shine NR, et al. Secretory leukocyte protease inhibitor: Inhibition of human immunodeficiency virus-1 infection of monocytic THP-1 cells by a newly cloned protein. Bioorg Chem. 2002;30(4):249–263. doi: 10.1016/s0045-2068(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 24.Novak RM, et al. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin. Vaccine Immunol. 2007;14(9):1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal SM, et al. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS. 2009;23(13):1669–1677. doi: 10.1097/QAD.0b013e32832ea643. [DOI] [PubMed] [Google Scholar]
- 26.Amsel R, et al. Nonspecific vaginitis: Diagnostic criteria and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 27.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klotman ME. Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6(6):447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 29.Wira CR, et al. Innate immunity in the human female reproductive tract: Endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65(3):196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkataraman N, et al. cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol. 2005;175(11):7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 31.Cummins JE, et al. Detection of infectious human immunodeficiency virus type 1 in female genital secretions by a short-term culture method. J Clin Microbiol. 2003;41(9):4081–4088. doi: 10.1128/JCM.41.9.4081-4088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummins JE, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22(8):788–795. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs A, et al. HIV-1 RNA in plasma and genital tract secretions in women infected with HIV-1. J Acquir Immune Defic Syndr. 1999;22(2):124–131. doi: 10.1097/00126334-199910010-00003. [DOI] [PubMed] [Google Scholar]
- 34.Cohn JA, et al. HIV-inducing factor in cervicovaginal secretions is associated with bacterial vaginosis in HIV-1-infected women. J Acquir Immune Defic Syndr. 2005;39(3):340–346. doi: 10.1097/01.qai.0000146599.47925.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olinger GG, et al. Association of indicators of bacterial vaginosis with a female genital tract factor that induces expression of HIV-1. AIDS. 1999;13(14):1905–1912. doi: 10.1097/00002030-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 36.Shebl FM, et al. Increased levels of circulating cytokines with HIV-related immunosuppression. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0144. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Combadière B, et al. The chemokine receptor CX3CR1 controls homing and anti-viral potencies of CD8 effector-memory T lymphocytes in HIV-infected patients. AIDS. 2003;17(9):1279–1290. doi: 10.1097/00002030-200306130-00002. [DOI] [PubMed] [Google Scholar]
- 38.Capobianchi MR, et al. Inhibition of HIV type 1 BaL replication by MIP-1alpha, MIP-1beta, and RANTES in macrophages. AIDS Res Hum Retroviruses. 1998;14(3):233–240. doi: 10.1089/aid.1998.14.233. [DOI] [PubMed] [Google Scholar]
- 39.Cocchi F, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal SM, et al. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infect Dis. 2005;192(5):728–738. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 41.Asin SN, et al. HIV type 1 infection in women: Increased transcription of HIV type 1 in ectocervical tissue explants. J Infect Dis. 2009;200(6):965–972. doi: 10.1086/605412. [DOI] [PubMed] [Google Scholar]
- 42.Gregory T, et al. Positive association between HIV RNA and IL-6 in the genital tract of Rwandan women. AIDS Res Hum Retroviruses. 2008;24(7):973–976. doi: 10.1089/aid.2008.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poli G, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172(1):151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poli G. Fauci AS. Cytokine modulation of HIV expression. Sem Immunol. 1993;5(3):165–173. doi: 10.1006/smim.1993.1020. [DOI] [PubMed] [Google Scholar]
- 45.Zara F, et al. Markers of local immunity in cervico-vaginal secretions of HIV infected women: Implications for HIV shedding. Sex Transm Infect. 2004;80(2):108–112. doi: 10.1136/sti.2003.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osborn L. Kunkel S. Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell C, et al. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retroviruses. 2011;27(1):35–39. doi: 10.1089/aid.2010.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fahey JV, et al. New approaches to making the microenvironment of the female reproductive tract hostile to HIV. Am J Reprod Immunol. 2011;65(3):334–343. doi: 10.1111/j.1600-0897.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau T, et al. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie. 2008;90(2):284–295. doi: 10.1016/j.biochi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Shaw JL. Diamandis EP. A potential role for tissue kallikrein-related peptidases in human cervico-vaginal physiology. Biol Chem. 2008;389(6):681–688. doi: 10.1515/BC.2008.069. [DOI] [PubMed] [Google Scholar]
- 51.Shaw JLV, et al. Role of tissue kallikrein-related peptidases in cervical mucus remodeling and host defense. Biol Chem. 2008;389(12):1513–1522. doi: 10.1515/BC.2008.171. [DOI] [PubMed] [Google Scholar]
- 52.Yamasaki K, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20(12):2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 53.Keller MJ, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21(4):467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 54.Al-Harthi L, et al. Bacterial vaginosis-associated microflora isolated from the female genital tract activates HIV-1 expression. J Acquir Immune Defic Syndr. 1999;21(3):194–202. doi: 10.1097/00126334-199907010-00003. [DOI] [PubMed] [Google Scholar]
- 55.Cu-Uvin S, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33(6):894–896. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 56.Taha TE, et al. HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(1):52–59. doi: 10.1097/00042560-199901010-00008. [DOI] [PubMed] [Google Scholar]