Abstract
Background
There have been very few systematic reviews looking at maternal infections in the developing world, even though cutting maternal mortality by three quarters is United Nation’s Millennium Development Goal number five. This systematic review has two aims. The first is to present the prevalence of parasitic infections in the developing world over the last 30 years and the second is to evaluate the quality and distribution of research in this field.
Methods
A systematic review of Medline, EMBASE and Global Health databases was undertaken using pre-determined search criteria. Three levels of quality criteria for exclusion of inadequate studies identified 115 out of initial 8580 titles. The data were extracted for 5 domains: worldwide pathogen prevalence, year of study, study setting, sample size and diagnostic test for each pathogen.
Results
The initial search retrieved 8580 results. From these titles, 43 studies on malaria, 12 studies on helminths, 49 studies on Toxoplasma gondii, 7 studies on Chagas disease, 5 studies on Trichomonas, 1 leishmaniasis study and 1 study on trichinellosis were extracted for analysis. High prevalence of malaria was found in Gabon (up to 57%) India (55%), Cameroon (50%), Yemen (55%), Nigeria (up to 64%) and Ghana (54%). High prevalence of hookworm infections was found in Nepal at 78.8% and high values of Ascaris lumbricoides were found in Nepal, (56.2%), Kenya (52.3%) and Gabon (45.5%). High levels of Schistosoma mansoni were found in Zimbabwe (50%) and Tanzania (63.5%). The prevalence of active Toxoplasma gondii infection was found to be highest in India (27.7%).
Conclusion
This study highlights the large burden of maternal parasitic infections globally. It may serve as a useful starting point for health policy development and research prioritization in this area.
With 5 years to go until 2015 and the end of the period to achieve Millennium Development Goals (MDGs) targets, it is debatable how many of the MDGs will reach the aims defined in 2000. MDG 5 aims to: “Reduce by three quarters the maternal mortality ratio” (1). However, between 1990 and 2005, in Sub-Saharan Africa especially, little progress had been made and in some countries the figures have even increased (1). In 1990, the Democratic Republic of Congo had a maternal mortality ratio (MMR) of 870 per 100 000 and by 2005 it had increased to 1100 per 100 000 (1). In addition, in Tanzania, which is a more stable sub-Saharan African country, the MMR also increased from 770 per 100 000 in 1990 to 970 per 100 000 (1). At the recent review of the MDGs in 2010, the UN managed to secure US$ 40 billion (€ 30 billion) for women’s and children’s health alone (2).
Maternal deaths are due to many causes including haemorrhage, hypertensive disorders, abortion related complications, infections and sepsis (3). Maternal infections contribute to about 10%-20% of these deaths (3). It is therefore imperative that there is up to date research on these topics. So far, there have been no systematic reviews of the data.
This review aims to summarise the research that has been undertaken in the past 30 years. It will look at the global distribution of research and the year in which the research was done. It will also look at how this research was undertaken, where the research was done, how many subjects were included in papers and what microbiological diagnostic methods were used. Finally the paper will set out an up-to-date picture of the prevalence of parasitic infections in the developing world over the last 30 years.
METHODS
Literature search terms
Initial searches were conducted to identify suitable keywords and MeSH headings to use in the final search (Table 1). The search strategy was prepared with an input from a librarian. Searches were conducted in parallel by two reviewers (using OVID) in the following databases on 1 August 2010: Medline (1950 to August Week 4 2010); EMBASE (1980 to 2010 Week 30) and Global Health (1973 to August 2010).
Table 1.
Search terms used to identify published articles on the prevalence and aetiology of maternal infections in the developing world
| exp Infection/ |
| AND |
| exp Pregnancy/ OR exp Pregnancy Complications, Infectious/ |
| AND |
| exp Developing Countries OR africa/ or africa, northern/ or algeria/ or egypt/ or libya/ or morocco/ or tunisia/ or “africa south of the sahara”/ or africa, central/ or cameroon/ or central african republic/ or chad/ or congo/ or “democratic republic of the congo”/ or gabon/ or africa, eastern/ or burundi/ or djibouti/ or eritrea/ or ethiopia/ or kenya/ or rwanda/ or somalia/ or sudan/ or tanzania/ or uganda/ or africa, southern/ or angola/ or botswana/ or lesotho/ or malawi/ or mozambique/ or namibia/ or south africa/ or swaziland/ or zambia/ or zimbabwe/ or africa, western/ or benin/ or burkina faso/ or cape verde/ or cote d'ivoire/ or gambia/ or ghana/ or guinea/ or guinea-bissau/ or liberia/ or mali/ or mauritania/ or niger/ or nigeria/ or senegal/ or sierra leone/ or togo/ or caribbean region/ or west indies/ or “antigua and barbuda”/ or cuba/ or dominica/ or dominican republic/ or grenada/ or guadeloupe/ or haiti/ or jamaica/ or martinique/ or “saint kitts and nevis”/ or saint lucia/ or “saint vincent and the grenadines”/ or central america/ or belize/ or costa rica/ or el salvador/ or guatemala/ or honduras/ or nicaragua/ or panama/ or latin america/ or mexico/ or south america/ or argentina/ or bolivia/ or brazil/ or chile/ or colombia/ or ecuador/ or french guiana/ or guyana/ or paraguay/ or peru/ or suriname/ or uruguay/ or venezuela/ or asia/ or asia, central/ or kazakhstan/ or kyrgyzstan/ or tajikistan/ or turkmenistan/ or uzbekistan/ or asia, southeastern/ or borneo/ or brunei/ or cambodia/ or east timor/ or indonesia/ or laos/ or malaysia/ or mekong valley/ or myanmar/ or philippines/ or thailand/ or vietnam/ or asia, western/ or bangladesh/ or bhutan/ or india/ or sikkim/ or middle east/ or afghanistan/ or iran/ or iraq/ or jordan/ or lebanon/ or syria/ or turkey/ or yemen/ or nepal/ or pakistan/ or sri lanka/ or far east/ or china/ or tibet/ or “democratic people's republic of korea”/ or mongolia/ or taiwan/ or atlantic islands/ or azores/ or albania/ or lithuania/ or bosnia-herzegovina/ or bulgaria/ or byelarus/ or “macedonia (republic)”/ or moldova/ or montenegro/ or romania/ or russia/ or bashkiria/ or dagestan/ or moscow/ or siberia/ or serbia/ or ukraine/ or yugoslavia/ or armenia/ or azerbaijan/ or “georgia (republic)”/ or indian ocean islands/ or comoros/ or madagascar/ or mauritius/ or reunion/ or seychelles/ or fiji/ or papua new guinea/ or vanuatu/ or guam/ or palau/ or “independent state of samoa”/ or tonga/ |
Study inclusion and exclusion criteria
Studies were screened by title and then by abstract for relevance. Studies were deemed relevant if they provided information on the aetiology or epidemiology of parasitic infections in pregnant women in developing countries. These studies were then grouped according to pathogen studied, with some studies providing information on multiple pathogens. Studies providing information on the epidemiology of bacterial or viral infections in pregnant women were identified but not analyzed, as they were addressed in a separate review. Relevant English language papers were analyzed in this work, along with Chinese electronic databases, with the intention of translating and analyzing non-English papers, too. The inclusion criteria were:
Subjects: Pregnant women at any stage of pregnancy or labour, including the puerperium (up to 42 days after labour);
Study location: Low- and middle-income countries (as defined by the World Bank in 2010);
Study design and sampling methods: No restrictions applied;
Data collection: Only studies that provided evidence of parasitic infection using microbiological or serological test results were included;
Results: Papers were selected if they provided information on the burden of a particular pathogen (the prevalence of a particular infection in pregnant women in the community over time/incidence) and/or the aetiology of parasitical maternal infections (prevalence of a specific pathogen/infection).
Quality criteria
Papers were required to describe their samples and methods in detail, and provide microbiological or serological evidence of the aetiology of infection.
Data extraction
Information on pathogen studied, sample population (pregnant women studied during pregnancy or at labour) and size, study setting, duration and type, microbiological/serological test used and results were extracted from abstracts and full papers for analysis.
Data analysis
Epidemiology and aetiology of maternal parasitic infections were summarized according to the pathogen studied. Only pathogens with 5 or more studies reporting on its epidemiology and/or aetiology were analyzed. Median prevalence of each infection was calculated and trends in the prevalence of maternal infections were noted.
Selection of studies
The final search yielded 8580 relevant titles. Figure 1 outlines the results of the search process and application of inclusion and exclusion criteria, resulting in the final panel of studies from which data was extracted.
Figure 1.
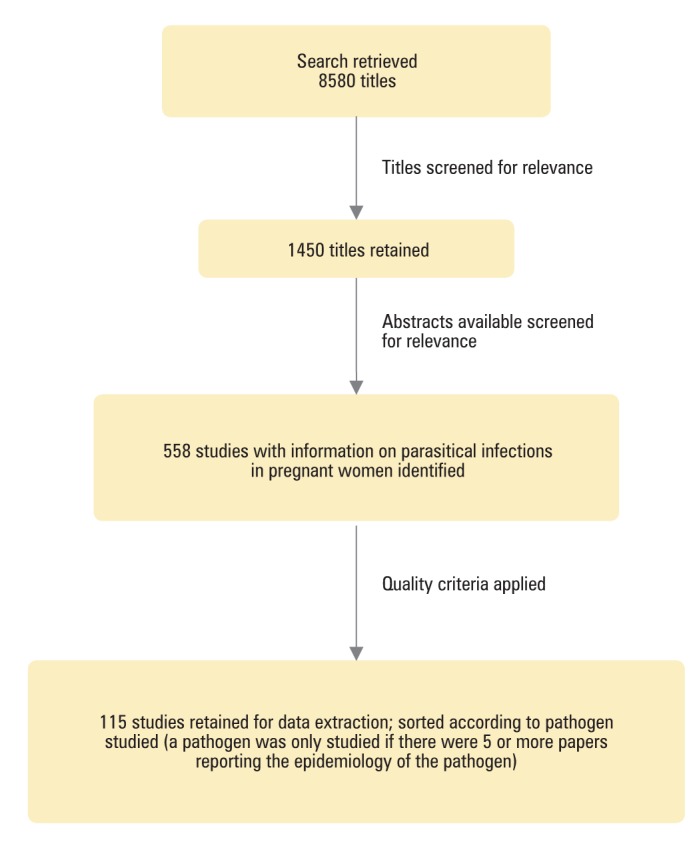
Summary of the literature search conducted.
Studies retained for data extraction (n=115) characterized the prevalence of 6 parasitic pathogens (malaria, helminths, Toxoplasma gondii, Chagas disease, trichinellosis and Trichomonas vaginalis) among pregnant women in developing countries, with 3 further reports providing secondary cross-sectional insights or reviews of the literature in this field, which were considered useful (4-118).
RESULTS
Prevalence of parasitical infections
Malaria. We identified 43 studies characterizing the prevalence of maternal malaria in 19 developing countries (Supplementary Table 1)(Supplementary Table 1). The features and results of these studies are summarised in Table 2 and Figures 2,3and4.
Table 2.
Distribution according to the size of population studied in 43 studies reporting maternal malaria prevalence
| Size of population | Number (%) |
|---|---|
| 0-500 |
28 (65%) |
| 501-1000 |
6 (14%) |
| 1001-1500 |
4 (9%) |
| 1501-2000 |
0 (0%) |
| 2001-2500 |
0 (0%) |
| 2501-3000 |
1 (2%) |
| 3001-3500 |
0 (0%) |
| 3501-4000 |
1 (2%) |
| 4001-4500 |
1 (2%) |
| 4501-5000 |
0 (0%) |
| >5000 | 2 (5%) |
Figure 2.
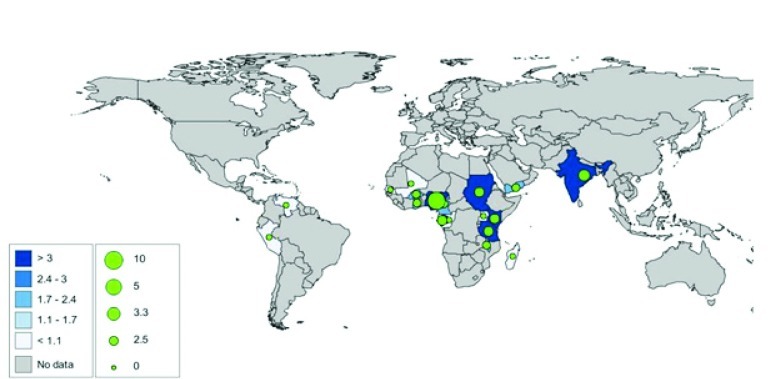
Geographical distribution of studies (n=43) reporting the prevalence of maternal malaria; “No data” in the legend refers to low- and middle-income countries only, as data from high-income countries were not the subject of this study.
Figure 3.
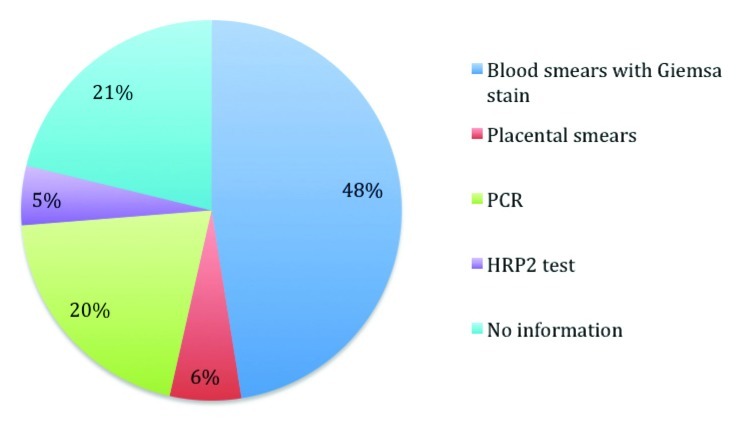
Techniques used to diagnose maternal malaria in the 43 studies identified. PCR – polymerase chain reaction, HRP2 test – histidine-rich protein 2.
Figure 4.
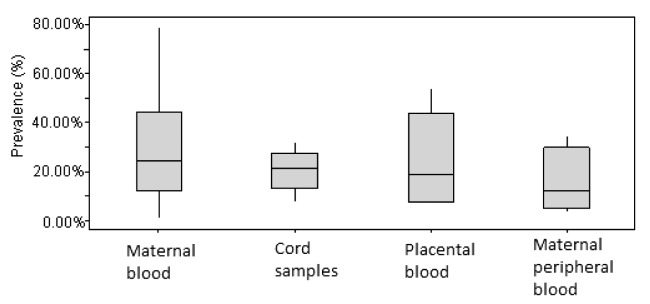
Box plot of malaria prevalence in different blood samples, reported by 43 relevant studies. The following number summaries are depicted in the boxplot: sample minimum, lower quartile, median, upper quartile, and sample maximum.
The majority of studies had small sample sizes (Table 2), between 0-500 subjects and most of them were conducted in antenatal clinics or hospitals (82.7%), suggesting either awareness towards the need for antenatal screening for maternal malaria infection, or merely that it is much easier to recruit study subjects in health care facilities. The remaining studies (8.9%) were community-based and the study setting was not specified in 8.4%.
Blood smears with Giemsa stains were by far the most regularly used diagnostic test, accounting for 47% of all tests used to detect Malaria (Figure 3). Very high prevalenceof Plasmodium vivax (78.69%) was found in Brazil (28). Nine studies in seven different countries reported prevalence of over 50%: Gabon (57% and 54%) India (55%), Cameroon (50%), Yemen (55%), Nigeria (57% and 64%) and Ghana (54%) (26,29,39,46,47,49,51). The mean prevalence of malaria, regardless of the blood site taken from, was between 20%-30%, suggesting a degree of consistency between tests from different blood sites (Figure 4).
Helminths. Fourteen studies characterizing the prevalence of maternal infections with helminths in 11 developing countries were identified (Supplementary Table 2)(Supplementary Table 2). The features and results of these studies are summarised in Table 3 and Figures 5,6 and 7.
Table 3.
Distribution according to size of population studied in 14 studies reporting maternal infection with helminth prevalence
| Size of population | Number (%) |
|---|---|
| 0-500 |
9 (69%) |
| 501-1000 |
0 (0%) |
| 1001-1500 |
1 (8%) |
| 1501-2000 |
0 (0%) |
| 2001-2500 |
1 (8%) |
| 2501-3000 |
2 (15%) |
| 3001-3500 |
0 (0%) |
| 3501-4000 |
0 (0%) |
| 4001-4500 |
0 (0%) |
| 4501-5000 |
0 (0%) |
| >5000 | 0 (0%) |
Figure 5.
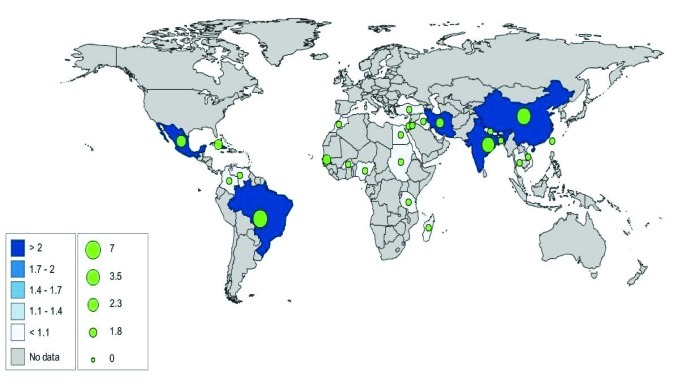
Geographical distribution of studies (n = 14) reporting the prevalence of maternal infection with helminths. “No data” in the legend refers to low- and middle-income countries only, as data from high-income countries were not the subject of this study.
Figure 6.
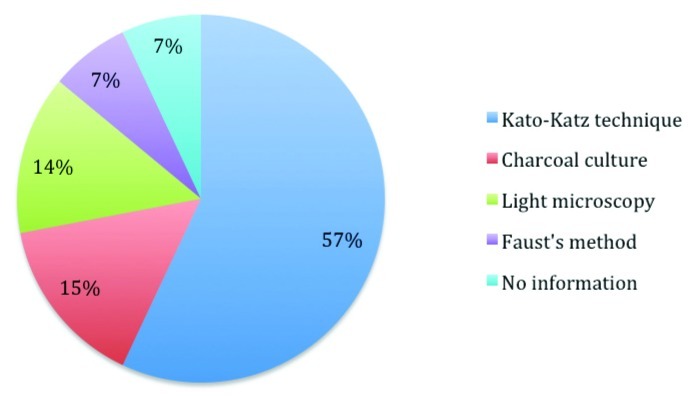
Techniques used to diagnose maternal infection with helminths in 14 studies identified.
Figure 7.
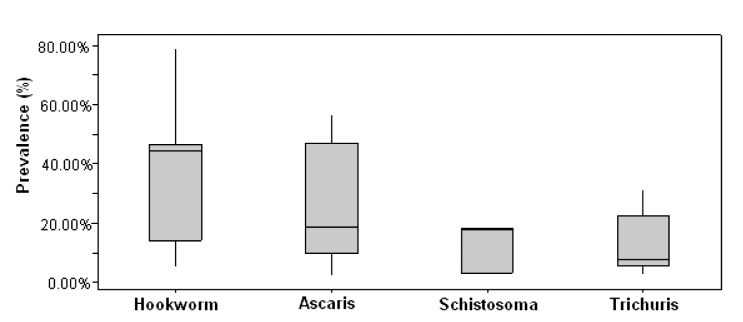
Box plot of the prevalence of maternal infections with helminths reported by the 14 relevant studies. The following number summaries are depicted in the boxplot: sample minimum, lower quartile, median, upper quartile, and sample maximum.
The majority of studies had small sample sizes (Table 3), between 0-500 subjects and most of them were conducted in antenatal clinics or hospitals (75%), suggesting either awareness towards the need for antenatal screening for maternal malaria infection, or merely that it is much easier to recruit study subjects in health care facilities. The remaining studies (18%) were community-based and the study setting was not specified in 9%.
Between the studies there was a generalised trend to use the Kato-Katz technique for examining stool samples (Figure 6), which is the gold standard for an epidemiological survey of helminth eggs (119). The prevalence of the different helminths varied across the studies. The most prevalent was hookworm, with a mean prevalence of 45%. The highest prevalence of hookworm infections was found in Nepal at 78.8% and high values were found in Uganda (44.5% and 45% in 2 studies), Tanzania (56.3%) and Kenya (39.5%) (12,16,18,19,24). For Ascaris lumbricoides relatively high prevalences were found in Nepal, (56.2%), Kenya (52.3%) and Gabon (45.5%) (11,18,19). Trichuris trichiura was least prevalent with a highest prevalence of 31% in Gabon and a mean value of 13% (11). High levels of Schistosoma mansoni were found in Zimbabwe (50%) and Tanzania (63.5%), but the prevalence in other countries was typically around 30% (10,24) (Figure 7).
Toxoplasma gondii. Forty-nine studies characterizing the prevalence of maternal infections with T. gondii in 26 developing countries were identified (Supplementary Table 3)(Supplementary Table 3). The features and results of these studies are summarised in Table 4 and Figures 8,9and 10.
Table 4.
Distribution according to size of population studied in 48 studies reporting maternal infection with Toxoplasma gondii prevalence
| Size of population | Number (%) |
|---|---|
| 0-500 |
29 (60%) |
| 501-1000 |
6 (13%) |
| 1001-1500 |
4 (8%) |
| 1501-2000 |
1 (2%) |
| 2001-2500 |
2 (4%) |
| 2501-3000 |
0 (0%) |
| 3001-3500 |
0 (0%) |
| 3501-4000 |
0 (0%) |
| 4001-4500 |
1 (2%) |
| 4501-5000 |
1 (2%) |
| >5000 | 4 (8%) |
Figure 8.
Geographical distribution of studies (n=49) reporting the prevalence of maternal infection with Toxoplasma gondii; “No data” in the legend refers to low- and middle-income countries only, as data from high-income countries were not the subject of this study.
Figure 9.
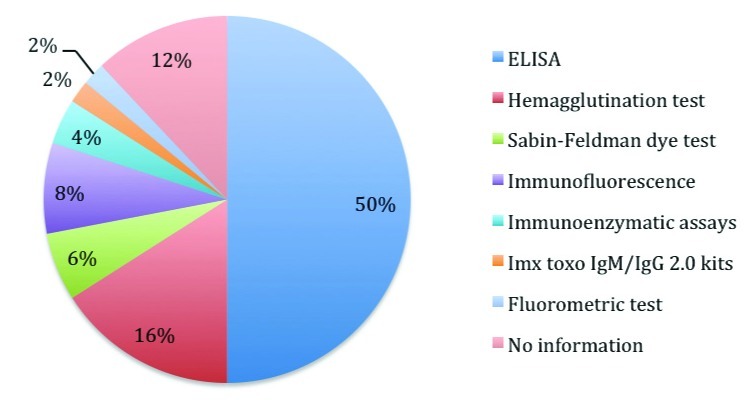
Techniques used to diagnose maternal infection with Toxoplasma gondii in 49 studies identified.
Figure 10.
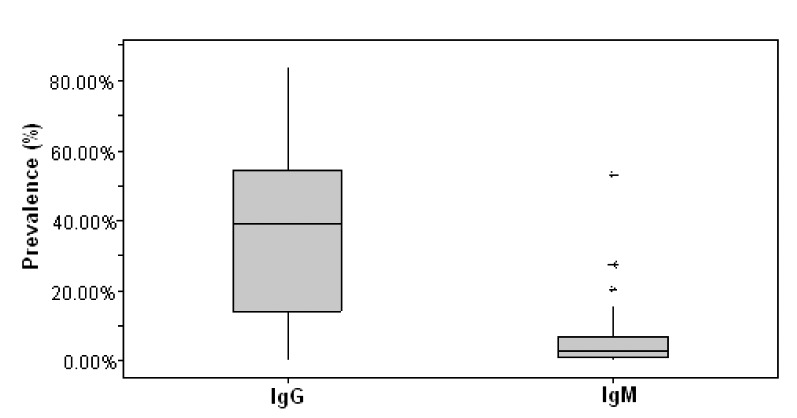
Box plot of maternal infections with Toxoplasma gondii prevalence reported by 48 relevant studies. The following number summaries are depicted in the boxplot: sample minimum, lower quartile, median, upper quartile, and sample maximum. Asterisks indicate outliers.
The majority of studies had small sample sizes (Table 4), between 0-500 subjects. Twenty five percent of the studies were conducted in antenatal clinics, hospitals, health care facilities or prenatal clinics. The remaining studies (2%) were community-based and the study setting was not specified in 75% of the studies.
The most commonly used test was ELISA, which is the gold standard for T. gondii analysis (Figure 9). The prevalence of both IgG and IgM was measured in 22 out of the 49 studies, useful for analysing current (IgM) and past (IgG) infections in pregnant mothers. The prevalence of active infection was low with a mean of 4% but there were high levels in India (27.7%), Mexico (20.7%) and Sudan (14.3%). The mean IgG (past infection) prevalence was 39%, highest prevalence in Brazil (75.1%) (Figure 10).
Chagas disease. Seven characterizing the prevalence of maternal infections with Chagas disease in 7 developing countries were identified (Supplementary Table 4)(Supplementary Table 4). The features and results of these studies are summarised in Table 5 and Figures 11,12 and 13.
Table 5.
Distribution according to size of population studied in 7 studies reporting maternal infection with Chagas disease prevalence
| Size of population | Number (%) |
|---|---|
| 0-500 |
1 (14%) |
| 501-1000 |
2 (29%) |
| 1001-1500 |
0 (0%) |
| 1501-2000 |
0 (0%) |
| 2001-2500 |
3 (43%) |
| 2501-3000 |
0 (0%) |
| 3001-3500 |
1 (14%) |
| 3501-4000 |
0 (0%) |
| 4001-4500 |
0 (0%) |
| 4501-5000 |
0 (0%) |
| >5000 | 0 (0%) |
Figure 11.
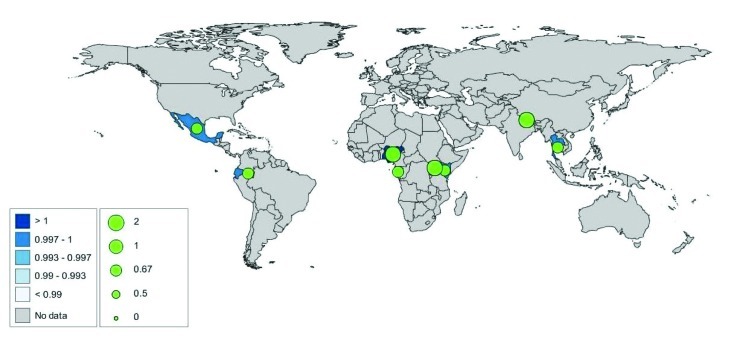
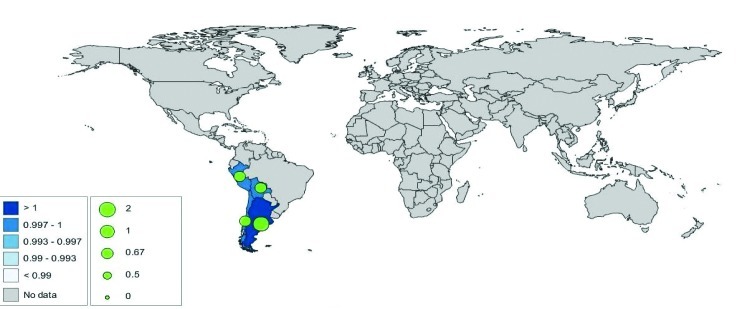
Geographical distribution of studies (n=7) reporting the prevalence of maternal infection with Chagas disease; “No data” in the legend refers to low- and middle-income countries only, as data from high-income countries were not the subject of this study.
Figure 12.
Techniques used to diagnose maternal infection with Toxoplasma gondii in the 49 studies identified.
Figure 13.
Box plot of maternal infections with Chagas disease prevalence reported by the 749 relevant studies. The following number summaries are depicted in the boxplot: sample minimum, lower quartile, median, upper quartile, and sample maximum. Asterisk outlines an outlier.
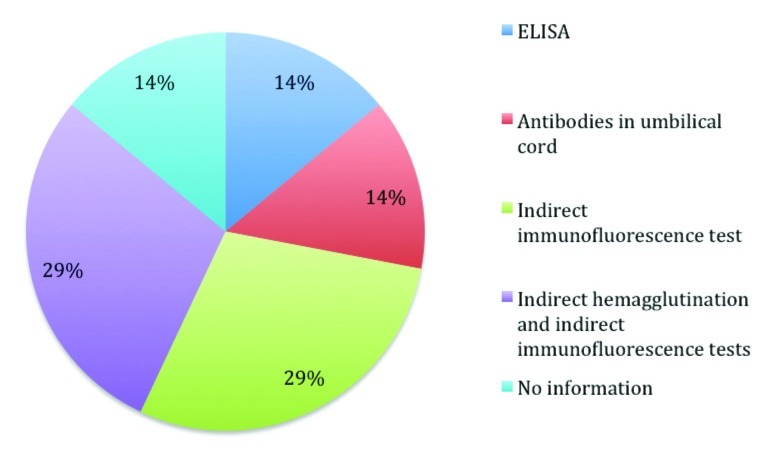
The majority of studies had small sample sizes (Table 5), between 0-500 subjects. Fourteen percent of the studies were conducted in hospitals, 29% in endemic and 29% in non-endemic areas. The remaining studies (14%) were conducted in parturient and the study setting was not specified in 14% of the studies.
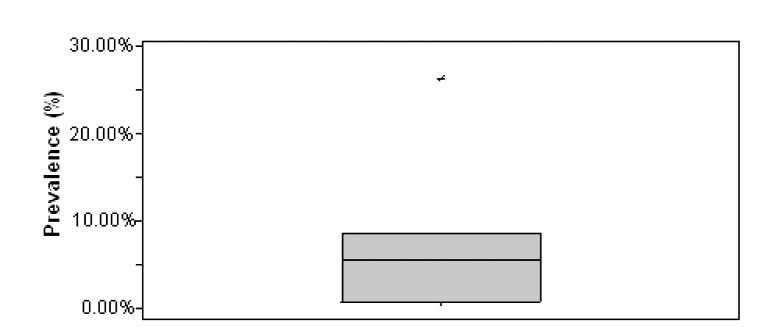
The most commonly used tests were indirect immunoflouresence and indirect haemogluttination tests (Figure 12). Chagas disease had a low mean prevalence of around 7.2% in pregnant women but a study in Bolivia (9) reported a prevalence of 26.3% (Figure 13).
Trichinellosis. There was only one study looking at trichinellosis (Supplementary Table 5)(Supplementary Table 5). Because of the scarcity of studies it is hard to make any inference to trends, so more research needs to be done in this area.
Trichomonas vaginalis. We found only four studies focused on trichomoniasis (Supplementary Table 6)(Supplementary Table 6). Because of the scarcity of studies it is hard to make any inference to trends and more research is needed.
DISCUSSION
Prevalence of parasitic infections
Our search of published literature relevant to the aetiology and epidemiology of maternal parasitic infections in the developing world provided detailed epidemiological information on 6 maternal infections. These 6 parasitical maternal infections were most extensively studied, suggesting that these infections have a high burden on pregnancy outcomes in the developing world. These infections also have potential adverse effects on neonates.
Malaria in its own right is a major contributing factor towards maternal deaths worldwide. It causes severe anaemia and can affect newborn‘s birth-weight and long-term survival (119). For this reason, it is recommended that research conducted in South and Central America, especially Brazil where the prevalence of P. vivax was so high (78.69%) to be expanded (28). Further research should also be concentrated on regions classified as high risk but with a current lack of data like Democratic Republic of Congo, Angola and Zambia, from where no evidence of research was found. Finally, prevention strategies should be encouraged in the maternal populations from the countries with evidence of very high malaria prevalence, namely Gabon, India, Cameroon, Yemen, Nigeria and Ghana (26,29,39,46,47,49,51).
Hookworm infections were found to be well studied, which is very promising as these parasites cause anaemia during pregnancy and have been noted to increase the maternal and child mortality rates (120). However more studies looking at Schistosoma would definitely be recommended as there were only 4 studies in pregnant women, despite WHO’s statement that Schistosoma is “second only to malaria in public health importance” and pregnant women are one of the important at risk groups (121). Specific countries of note for further investigation and preventative measures would be Nepal for hookworm (78.8%) and A. lumbricoides (56.2%) (19); Tanzania for hookworms (56.3%) and Schistosoma mansoni (63.5%) (24); Kenya for hookworms (39.5%) and A. lumbricoides (52.3%); the Gabon for A. lumbricoides (45.5%) and Trichuris trichuria (31%); and finally for S. mansoni (50%) (10,11,18).
Chagas disease is important because of its potential to cause mortality in all age-groups. It can be transmitted vertically so that the knowledge of maternal prevalence is important (122). Although this review has shown studies in South America, the disease is no longer confined to just South America, as with Latino American emigration there has been a movement of the disease across the world. Therefore global research in areas of high Latino American immigration would be recommended to assess the extent this emigration has had (123). More funding in Bolivia would be recommended to assess the nature of the 26.3% prevalence of maternal infection and its effects on maternal and neonatal health (9).
There is a need for more studies on Leishmania would also be recommended from the results. Although not much evidence can be taken from the single study from Brazil (124), pregnant woman tended to have more severe Leishmania than the non-pregnant infected subjects and there was a possible link with increased problems with pregnancy (124).
Strengths and limitations
To our knowledge, this is the first review that summarises the epidemiology of maternal parasitic infections in the developing world. The search strategy devised was sensitive and specific, which allowed for a comprehensive review of available literature on this topic. The information generated in this review can be used to guide public health policy and the allocation of resources within local governments and by the international community towards improving maternal health. Although the search did not exclude non-English papers, we did not search some of the databases where we would have expected a higher concentration of foreign language papers (e.g. LILACS). Although the databases used were very extensive, especially in the case of EMBASE and Medline there is a high chance that important papers could have been recovered from smaller, more specialist databases.
This study could be further improved by analysing non-English studies conducted in francophone parts of Africa (in French), South America (in Spanish) and in China (in Chinese), which could be accessed from appropriate databases. Reviewing non-English articles may assist in defining the epidemiology of pathogens for which we managed to identify few (<5) studies, as well as providing more robust data on the pathogens presented in this review. In addition, searching grey (unpublished) literature or contacting health officials and researchers in the field may also yield more country specific data on the subject, thus enabling more targeted and context-specific public health measures.
Recommendations and future work
This systematic review highlights the quantity of maternal parasitic infection research and, to a lesser extent, quality of research that has been achieved over the last 30 years. This paper would therefore be useful to decision makers, especially in light of the US$ 40 billion (€ 30 billion) pledged at the last UN’s MDG summit, to help them assess where best to implement resources for research. It could also be a useful tool to measure how good the data are for each individual parasite and in assessing what areas of the world have been neglected in terms of research. With a full picture where different maternal infections occur, it could be used as a tool to target research and could ultimately lead to big leap forward maternal infections knowledge, therefore helping in the fight towards cutting down maternal infections.
Conclusion
No mother should die during childbirth and every step should be taken to stop this happening. This review is a first step, in a long chain of events, trying to prevent maternal mortality. If research and knowledge is channelled into the right areas and decision makers have accurate knowledge regarding maternal infections then resources can be allocated to those areas that need it most.
Researchers have a responsibility to reflect on which part of the world current knowledge is stemming from and should look retrospectively to see how much research has been done in the past. By doing this, the public health community can positively expand research into areas of the world and into diseases where that information is lacking. And with this information, informed and important decision can be made about the factors that affect maternal mortality most and maybe, just maybe, Millennium Development Goal 5 can be achieved.
Acknowledgements
We thank Shiela Fiskin, librarian, for her advice on databases and refining of search terms and Edinburgh medical student Pascal.
Funding: Bill and Melinda Gates Foundation
Ethical approval: Not required
Authorship declaration: All authors designed and conducted the study and contributed to the writing of the paper.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare support from Bill and Melinda Gates Foundation for the submitted work. The authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Additional Material
References
- 1.United Nations. United Nations Millennium Development Goals. Available at: http://www.un.org/millenniumgoals/maternal.shtml Accessed: 2 August 2011.
- 2.United Nations. We can end poverty, Millennium Development Goals, 2015: UN summit, 20-22 September 2010, New York. Available at: http://www.un.org/en/mdg/summit2010/ Accessed: 4 August 2011.
- 3.Tebeu PM, Ngassa P, Kouam L, Major AL, Fomulu JN.Maternal mortality in Maroua Provincial Hospital, Cameroon (2003-2005). West Indian Med J 200756502–507.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 4.Araujo AB, Castagno VD, Gallina T, Aires Berne ME.Prevalence of Chagas disease among pregnant women in the southern region of Rio Grande Do Sul. Rev Soc Bras Med Trop 200942732–733.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 5.Arancibia AM, Sagua H, Neira I, Gonzalez J, Varela H. Chagas disease in northern Chile. Serological prevalence in pregnant women of the city of Antofagasta, 1991-1993. Bol Chil Parasitol. 1995;50:45–47. [PubMed] [Google Scholar]
- 6.Arcavi M, Orfus G, Griemberg G. Incidence of Chagas infection in pregnant women and newborn infants in a non-endemic area. Medicina (B Aires) 1993;53:217–222. [PubMed] [Google Scholar]
- 7.Mendoza Ticona CA, Cordova Benzaquen E, Ancca Juarez J, Saldana Diaz J, Torres Choque A, Velasquez Talavera R, et al. The prevalence of Chagas' disease in puerperal women and congenital transmission in an endemic area of Peru. Rev Panam Salud Publica. 2005;17:147–153. doi: 10.1590/S1020-49892005000300001. [DOI] [PubMed] [Google Scholar]
- 8.Sosa-Estani S, Gamboa-Leon MR, Del Cid-Lemus J, Althabe F, Alger J, Almendares O, et al. Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and Mexico. Am J Trop Med Hyg. 2008;79:755–759. [PubMed] [Google Scholar]
- 9.Torrico F, Castro M, Solano M, Rodriguez P, Torrico MC, Truyens C, et al. Effects of maternal infection with Trypanosoma cruzi in pregnancy development and in the newborn infant. Rev Soc Bras Med Trop. 2005;38(Suppl 2):73–76. [PubMed] [Google Scholar]
- 10.Patana M, Nyazema NZ, Ndamba J, Munatsi A, Tobaiwa O. Schistosomiasis and Hepatitis B infection in pregnancy: Implications for vaccination against Hepatitis B. Cent Afr J Med. 1995;41:288–292. [PubMed] [Google Scholar]
- 11.Adegnika AA, Agnandji ST, Chai SK, Ramharter M, Breitling L, Kendjo E, et al. Increased prevalence of intestinal Helminth infection during pregnancy in a sub-Saharan African community. Wien Klin Wochenschr. 2007;119:712–716. doi: 10.1007/s00508-007-0907-z. [DOI] [PubMed] [Google Scholar]
- 12.Muhangi L, Woodburn P, Omara M, Omoding N, Kizito D, Mpairwe H, et al. Associations between mild-to-moderate anaemia in pregnancy and Helminth, malaria and HIV infection in Entebbe, Uganda. Trans R Soc Trop Med Hyg. 2007;101:899–907. doi: 10.1016/j.trstmh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah BK, Baig LA.Association of anemia with parasitic infestation in pregnant Nepalese women: Results from a hospital-based study done in Eastern Nepal. JAMC 2005175–9.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 14.Ozumba UC, Ozumba NA, Anya S. Helminthiasis in pregnancy in Enugu, Nigeria. J Health Sci. 2005;51:291–293. doi: 10.1248/jhs.51.291. [DOI] [Google Scholar]
- 15.Rodriguez-Garcia R, Rodriguez-Guzman LM, Sanchez-Maldonado MI, Gomez-Delgado A, Rivera-Cedillo R. Prevalence and risk factors associated with intestinal parasitoses in pregnant women and their relation to the infant's birth weight. Ginecol Obstet Mex. 2002;70:338–343. [PubMed] [Google Scholar]
- 16.Ndibazza J, Muhangi L, Akishule D, Kiggundu M, Ameke C, Oweka J, et al. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: A randomized controlled trial. Clinical Infectious Diseases. 2010;50:531–540. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liabsuetrakul T, Chaikongkeit P, Korviwattanagarn S, Petrueng C, Chaiya S, Hanvattanakul C, et al. Epidemiology and the effect of treatment of soil-transmitted Helminthiasis in pregnant women in southern Thailand. Southeast Asian J Trop Med Public Health. 2009;40:211–222. [PubMed] [Google Scholar]
- 18.van Eijk AM, Lindblade KA, Odhiambo F, Peterson E, Rosen DH, Karanja D, et al. Geohelminth infections among pregnant women in rural western Kenya: A cross-sectional study. PLoS Negl Trop Dis. 2009;3:e370. doi: 10.1371/journal.pntd.0000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navitsky RC, Dreyfuss ML, Shrestha J, Khatry SK, Stoltzfus RJ. Albonico. Ancylostoma duodenale is responsible for hookworm infections among pregnant women in the rural plains of Nepal. J Parasitol. 1998;84:647–651. doi: 10.2307/3284746. [DOI] [PubMed] [Google Scholar]
- 20.Guerra EM, Vaz AJ, de Toledo LA, Ianoni SA, Quadros CM, Dias RM, et al. Helminth and protozoan intestinal infections in pregnant women in their first consultation at Health Centers of the state in the Butanta Subdistrict, Sao Paulo City. Rev Inst Med Trop Sao Paulo. 1991;33:303–308. doi: 10.1590/S0036-46651991000400010. [DOI] [PubMed] [Google Scholar]
- 21.Brooker S, Hotez PJ, Bundy DA. Hookworm-related anaemia among pregnant women: A systematic review. PLoS Negl Trop Dis. 2008;2:e291. doi: 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egwunyenga AO, Ajayi JA, Nmorsi OP, Duhlinska-Popova DD. Plasmodium/intestinal Helminth co-infections among pregnant Nigerian women. Mem Inst Oswaldo Cruz. 2001;96:1055–1059. doi: 10.1590/S0074-02762001000800005. [DOI] [PubMed] [Google Scholar]
- 23.Weigel MM, Calle A, Armijos RX, Vega IP, Bayas BV, Montenegro CE. The effect of chronic intestinal parasitic infection on maternal and perinatal outcome. Int J Gynaecol Obstet. 1996;52:9–17. doi: 10.1016/0020-7292(95)02442-5. [DOI] [PubMed] [Google Scholar]
- 24.Ajanga A, Lwambo NJS, Blair L, Nyandindi U, Fenwick A, Brooker S. Schistosoma mansoni in pregnancy and associations with anaemia in northwest Tanzania. Trans R Soc Trop Med Hyg. 2006;100:59–63. doi: 10.1016/j.trstmh.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Gomez E, Lopez E, Ache A. Malaria and pregnancy. San Isidro parish, municipality Sifontes, state of Bolivar, Venezuela, 2005-2006. Invest Clin. 2009;50:455–464. [PubMed] [Google Scholar]
- 26.Bouyou-Akotet MK, Nzenze-Afene S, Ngoungou EB, Kendjo E, Owono-Medang M, Lekana-Douki JB, et al. Burden of malaria during pregnancy at the time of IPTp/SP implementation in Gabon. Am J Trop Med Hyg. 2010;82:202–209. doi: 10.4269/ajtmh.2010.09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akanbi OM, Odaibo AB, Ademowo OG. The burden of malaria infection on pregnant women and birth weight of infants in south western Nigeria. East Afr J Public Health. 2009;6:63–68. doi: 10.4314/eajph.v6i1.45750. [DOI] [PubMed] [Google Scholar]
- 28.Chagas ECDS, Nascimento CTD, De Filho FSS, Botto-Menezes CH, Martinez-Espinosa FE. Impact of malaria during pregnancy in the Amazon region. Rev Panam Salud Publica. 2009;26:203–208. doi: 10.1590/S1020-49892009000900003. [DOI] [PubMed] [Google Scholar]
- 29.Aribodor DN, Nwaorgu OC, Eneanya CI, Okoli I, Pukkila-Worley R, Etaga HO. Association of low birth weight and placental malarial infection in Nigeria. J Infect Dev Ctries. 2009;3:620–623. doi: 10.3855/jidc.554. [DOI] [PubMed] [Google Scholar]
- 30.Adam I, Adamt GK, Mohmmed AA, Salih MM, Ibrahuim SA, Ryan CA. Placental malaria and lack of prenatal care in an area of unstable malaria transmission in eastern Sudan. J Parasitol. 2009;95:751–752. doi: 10.1645/GE-1912.1. [DOI] [PubMed] [Google Scholar]
- 31.Nwonwu EU, Ibekwe PC, Ugwu JI, Obarezi HC, Nwagbara OC. Prevalence of malaria parasitaemia and malaria related anaemia among pregnant women in Abakaliki, South East Nigeria. Niger J Clin Pract. 2009;12:182–186. [PubMed] [Google Scholar]
- 32.Clerk CA, Bruce J, Greenwood B, Chandramohan D. The epidemiology of malaria among pregnant women attending antenatal clinics in an area with intense and highly seasonal malaria transmission in northern Ghana. Trop Med Int Health. 2009;14:688–695. doi: 10.1111/j.1365-3156.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 33.Kabanywanyi AM, Macarthur JR, Stolk WA, Habbema JD, Mshinda H, Bloland PB, et al. Malaria in pregnant women in an area with sustained high coverage of insecticide-treated bed nets. Malar J. 2008;7:133. doi: 10.1186/1475-2875-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uneke CJ, Iyare FE, Oke P, Duhlinska DD. Assessment of malaria in pregnancy using rapid diagnostic tests and its association with HIV infection and hematologic parameters in South-Eastern Nigeria. Haematologica. 2008;93:143–144. doi: 10.3324/haematol.11695. [DOI] [PubMed] [Google Scholar]
- 35.Parekh FK, Hernandez JN, Krogstad DJ, Casapia WM, Branch OH.Prevalence and risk of Plasmodium falciparum and P. vivax malaria among pregnant women living in the hypoendemic communities of the Peruvian Amazon. Am J Trop Med Hyg 200777451–457.[REMOVED HYPERLINK FIELD] [PMC free article] [PubMed] [Google Scholar]
- 36.Coulibaly SO, Gies S, D'Alessandro U. Malaria burden among pregnant women living in the rural district of Boromo, Burkina Faso. Am J Trop Med Hyg. 2007;77(6) Suppl:56–60. [PubMed] [Google Scholar]
- 37.Tarimo SD.Appraisal on the prevalence of malaria and anaemia in pregnancy and factors influencing uptake of intermittent preventive therapy with sulfadoxine-pyrimethamine in Kibaha district, Tanzania. East Afr J Public Health 2007480–83.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 38.Lukuka KA, Fumie OS, Mulumbu MR, Lokombe BJ, Muyembe TJ.Malaria prevalence at delivery in four maternity hospitals of Kinshasa city, Democratic Republic of Congo. Bull Soc Pathol Exot 200699200–201.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 39.Idowu OA, Mafiana CF, Dapo S. Malaria among pregnant women in Abeokuta, Nigeria. Tanzan Health Res Bull. 2006;8:28–31. doi: 10.4314/thrb.v8i1.14267. [DOI] [PubMed] [Google Scholar]
- 40.Mockenhaupt FP, Bedu-Addo G, Von Gaertner C, Boye R, Fricke K, Hannibal I, et al. Detection and clinical manifestation of placental malaria in southern Ghana. Malar J. 2006;5:119. doi: 10.1186/1475-2875-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhotra I, Mungai P, Muchiri E, Kwiek JJ, Meshnick SR, King CL. Umbilical cord-blood infections with Plasmodium falciparum malaria are acquired antenatally in Kenya. J Infect Dis. 2006;194:176–183. doi: 10.1086/505150. [DOI] [PubMed] [Google Scholar]
- 42.Bassiouny HK, Al-Maktari MT.Malaria in late pregnancy in Al Hodeidah Governorate, Yemen. East Mediterr Health J 200511606–617.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 43.Adam I. A-Elbasit IE, Salih I, Elbashir MI. Submicroscopic Plasmodium falciparum infections during pregnancy, in an area of Sudan with a low intensity of malaria transmission. Ann Trop Med Parasitol. 2005;99:339–344. doi: 10.1179/136485905X36244. [DOI] [PubMed] [Google Scholar]
- 44.Mwapasa V, Rogerson SJ, Molyneux ME, Abrams ET, Kamwendo DD, Lema VM, et al. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS. 2004;18:1051–1059. doi: 10.1097/00002030-200404300-00014. [DOI] [PubMed] [Google Scholar]
- 45.Elghazali G, Adam I, Hamad A, El-Bashir MI.Plasmodium falciparum infection during pregnancy in an unstable transmission area in eastern Sudan. East Mediterr Health J 20039570–580.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 46.Bouyou-Akotet MK, Ionete-Collard DE, Mabika-Manfoumbi M, Kendjo E, Matsiegui PB, Mavoungou E, et al. Prevalence of Plasmodium falciparum infection in pregnant women in Gabon. Malar J. 2003;2:18. doi: 10.1186/1475-2875-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assabri AM, Muharram AA.Malaria in pregnancy in Hodiedah, Republic of Yemen. East Mediterr Health J 20028245–253.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 48.van Eijk AM, Lindblade KA, Odhiambo F, Peterson E, Rosen DH, Karanja D, et al. Geohelminth infections among pregnant women in rural western Kenya; a cross-sectional study. PLoS Negl Trop Dis. 2009;3:e370. doi: 10.1371/journal.pntd.0000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou A, Megnekou R, Leke R, Fogako J, Metenou S, Trock B, et al. Prevalence of Plasmodium falciparum infection in pregnant Cameroonian women. Am J Trop Med Hyg 200267566–570.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 50.Cot M, Deloron P. Med Trop (Mars) 2003;63:369–80. [Malaria during pregnancy: Consequences and interventional perspectives] [PubMed] [Google Scholar]
- 51.Singh N, Mehra RK, Srivastava N. Malaria during pregnancy and infancy, in an area of intense malaria transmission in central India. Ann Trop Med Parasitol. 2001;95:19–29. doi: 10.1080/00034980020035889. [DOI] [PubMed] [Google Scholar]
- 52.Tobian AAR, Mehlotra RK, Malhotra I, Wamachi A, Mungai P, Koech D, et al. Frequent umbilical cord-blood and maternal-blood infections with Plasmodium falciparum, P. malariae, and P. ovale in Kenya. J Infect Dis. 2000;182:558–563. doi: 10.1086/315729. [DOI] [PubMed] [Google Scholar]
- 53.Kasumba IN, Nalunkuma AJ, Mujuzi G, Kitaka FS, Byaruhanga R, Okong P, et al. Low birthweight associated with maternal anaemia and Plasmodium falciparum infection during pregnancy, in a peri-urban/urban area of low endemicity in Uganda. Ann Trop Med Parasitol. 2000;94:7–13. doi: 10.1080/00034980057563. [DOI] [PubMed] [Google Scholar]
- 54.Singh N, Saxena A, Chand SK, Valecha N, Sharma VP. Studies on malaria during pregnancy in a tribal area of central India (Madhya Pradesh). Southeast Asian J Trop Med Public Health. 1998;29:10–17. [PubMed] [Google Scholar]
- 55.Egwunyenga OA, Ajayi JA, Popova-Duhlinska DD, Nmorsi OP. Malaria infection of the cord and birthweights in Nigerians. Cent Afr J Med. 1996;42:265–268. [PubMed] [Google Scholar]
- 56.Steketee RW, Wirima JJ, Slutsker L, Breman JG, Heymann DL. Comparability of treatment groups and risk factors for parasitemia at the first antenatal clinic visit in a study of malaria treatment and prevention in pregnancy in rural Malawi. Am J Trop Med Hyg. 1996;55:17–23. doi: 10.4269/ajtmh.1996.55.17. [DOI] [PubMed] [Google Scholar]
- 57.Okonofua FE, Abejide OR. Prevalence of malaria parasitaemia in pregnancy in Nigerian women. J Obstet Gynaecol. 1996;16:311–315. doi: 10.3109/01443619609030034. [DOI] [Google Scholar]
- 58.Singh N, Shukla MM, Srivastava R, Sharma VP. Prevalence of malaria among pregnant and non-pregnant women of district Jabalpur, Madhya Pradesh. Indian J Malariol. 1995;32:6–13. [PubMed] [Google Scholar]
- 59.Gazin PP, Compaore MP, Hutin Y, Molez JF. Placental infections with Plasmodium in an endemic zone. Risk factors. Bull Soc Pathol Exot. 1994;87:97–100. [PubMed] [Google Scholar]
- 60.Ibhanesebhor SE, Okolo AA. Placental malaria and pregnancy outcome. Int J Gynaecol Obstet. 1992;37:247–252. doi: 10.1016/0020-7292(92)90324-C. [DOI] [PubMed] [Google Scholar]
- 61.Bako BG, Audu BM, Geidam AD, Kullima AA, Ashiru GM, Malah MB, et al. Prevalence, risk factors and effects of placental malaria in the UMTH, Maiduguri, North-Eastern, Nigeria: A cross-sectional study. J Obstet Gynaecol. 2009;29:307–310. doi: 10.1080/01443610902878783. [DOI] [PubMed] [Google Scholar]
- 62.Agomo CO, Oyibo WA, Anorlu RI, Agomo PU. Prevalence of malaria in pregnant women in Lagos, South-West Nigeria. Korean J Parasitol. 2009;47:179–183. doi: 10.3347/kjp.2009.47.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kayentao K, Mungai M, Parise M, Kodio M, Keita AS, Coulibaly D, et al. Assessing malaria burden during pregnancy in Mali. Acta Trop. 2007;102:106–112. doi: 10.1016/j.actatropica.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 64.N'Dao CT, N'Diaye JL, Gaye A, Hesran JY. Placental malaria and pregnancy outcome in a peri-urban area in Senegal. Rev Epidemiol Sante Publique. 2006;54:149–156. [PubMed] [Google Scholar]
- 65.Akum AE, Kuoh AJ, Minang JT, Achimbom BM, Ahmadou MJ, Troye-Blomberg M. The effect of maternal, umbilical cord and placental malaria parasitaemia on the birthweight of newborns from South-Western Cameroon. Acta Paediatr. 2005;94:917–923. doi: 10.1080/08035250510028605. [DOI] [PubMed] [Google Scholar]
- 66.Wanachiwanawin D, Sutthent R, Chokephaibulkit K, Mahakittikun V, Ongrotchanakun J, Monkong N. Toxoplasma gondii antibodies in HIV and non-HIV infected thai pregnant women. Asian Pac J Allergy Immunol. 2001;19:291–3. [PubMed] [Google Scholar]
- 67.Lopes FMR, Mitsuka-Bregano R, Goncalves DD, Freire RL, Karigyo CJT, Wedy GF, et al. Factors associated with seropositivity for anti-toxoplasma gondii antibodies in pregnant women of londrina, Parana, Brazil. Mem Inst Oswaldo Cruz. 2009;104:378–382. doi: 10.1590/S0074-02762009000200036. [DOI] [PubMed] [Google Scholar]
- 68.Varella IS, Canti ICT, Santos BR, Coppini AZ, Argondizzo LC, Tonin C, et al. Prevalence of acute toxoplasmosis infection among 41,112 pregnant women and the mother-to-child transmission rate in a public hospital in south Brazil. Mem Inst Oswaldo Cruz. 2009;104:383–388. doi: 10.1590/S0074-02762009000200037. [DOI] [PubMed] [Google Scholar]
- 69.Khurana S, Bagga R, Aggarwal A, Lyngdoh V, Shivapriya Diddi K, Malla N. Serological screening for antenatal toxoplasma infection in India. Indian J Med Microbiol. 2010;28:143–146. doi: 10.4103/0255-0857.62492. [DOI] [PubMed] [Google Scholar]
- 70.Vaz RS, Thomaz-Soccol V, Sumikawa E, Guimaraes ATB. Serological prevalence of Toxoplasma gondii antibodies in pregnant women from southern Brazil. Parasitol Res. 2010;106:661–665. doi: 10.1007/s00436-009-1716-2. [DOI] [PubMed] [Google Scholar]
- 71.Alvarado-Esquivel C, Torres-Castorena A, Liesenfeld O, Garca-Lpez CR, Estrada-Martinez S, Sifuentes-Alvarez A, et al. Seroepidemiology of Toxoplasma gondii infection in pregnant women in rural Durango, Mexico. J Parasitol. 2009;95:271–274. doi: 10.1645/GE-1829.1. [DOI] [PubMed] [Google Scholar]
- 72.Maggi P, Volpe A, Carito V, Schinaia N, Bino S, Basho M, et al. Surveillance of toxoplasmosis in pregnant women in Albania. New Microbiol. 2009;32:89–92. [PubMed] [Google Scholar]
- 73.Barbosa IR, de Carvalho Xavier Holanda CM, de Andrade-Neto VF. Toxoplasmosis screening and risk factors amongst pregnant females in natal, northeastern Brazil. Trans R Soc Trop Med Hyg. 2009;103:377–382. doi: 10.1016/j.trstmh.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 74.Liu Q, Wei F, Gao S, Jiang L, Lian H, Yuan B, et al. Toxoplasma gondii infection in pregnant women in China. Trans R Soc Trop Med Hyg. 2009;103:162–166. doi: 10.1016/j.trstmh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Ribeiro AC, Mutis MS, Fernandes O. Association of the presence of residual anti-toxoplasma gondii IgM in pregnant women and their respective family groups in Miracema, northwest Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2008;103:591–594. doi: 10.1590/S0074-02762008000600013. [DOI] [PubMed] [Google Scholar]
- 76.Rosso F, Les JT, Agudelo A, Villalobos C, Chaves JA, Tunubala GA, et al. Prevalence of infection with Toxoplasma gondii among pregnant women in Cali, Colombia, South America. Am J Trop Med Hyg. 2008;78:504–508. [PubMed] [Google Scholar]
- 77.Abdi J, Shojaee S, Mirzaee A, Keshavarz H. Seroprevalence of toxoplasmosis in pregnant women in Ilam province, Iran. IJP. 2008;3:34–37. [Google Scholar]
- 78.Lin YL, Liao YS, Liao LR, Chen FN, Kuo HM, He S. Seroprevalence and sources of toxoplasma infection among indigenous and immigrant pregnant women in Taiwan. Parasitol Res. 2008;103:67–74. doi: 10.1007/s00436-008-0928-1. [DOI] [PubMed] [Google Scholar]
- 79.Ndiaye D, Ndiaye A, Sene PD, Ndiaye JL, Faye B, Ndir O.Evaluation of serological tests of toxoplasmosis in pregnant women realized at the laboratory of parasitology and mycology of Le Dantec teaching hospital in 2002. Dakar Med. 20075258–61.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 80.El Mansouri B, Rhajaoui M, Sebti F, Amarir F, Laboudi M, Bchitou R, et al. Seroprevalence of toxoplasmosis in pregnant women in Rabat, Morocco. Bull Soc Pathol Exot. 2007;100:289–290. [PubMed] [Google Scholar]
- 81.Castilho-Pelloso MP, Falavigna DLM, Falavigna-Guilherme AL. Suspected acute toxoplasmosis in pregnant women. Rev Saude Publica. 2007;41:27–34. doi: 10.1590/S0034-89102007000100005. [DOI] [PubMed] [Google Scholar]
- 82.Razzak AH, Wais SA, Saeid AY. Toxoplasmosis: The innocent suspect of pregnancy wastage in Duhok, Iraq. East Mediterr Health J. 2005;11:625–32. [PubMed] [Google Scholar]
- 83.Spalding SM, Reis Annendoeira MR, Klein CH, Ribeiro LC. Serological screening and toxoplasmosis exposure factors among pregnant women in south of Brazil. Rev Soc Bras Med Trop. 2005;38:173–177. doi: 10.1590/S0037-86822005000200009. [DOI] [PubMed] [Google Scholar]
- 84.Ndir I, Gaye A, Faye B, Gaye O, Ndir O.Seroprevalence of toxoplasmosis among women having spontaneous abortion and pregnant women following in a center of health up-town in Dakar. Dakar Med. 2004495–9.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 85.Buchy P, Follezou JY, Lien TX, An TT, Tram LT, Tri DV, et al. Serological study of toxoplasmosis in Vietnam in a population of drug users (Ho Chi Minh city) and pregnant women (Nha Trang). Bull Soc Pathol Exot. 2003;96:46–47. [PubMed] [Google Scholar]
- 86.Elnahas A, Gerais AS, Elbashir MI, Eldien ES, Adam I.Toxoplasmosis in pregnant Sudanese women. Saudi Med J 200324868–870.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 87.Akoijam BS, Shashikant Singh S, Kapoor SK.Seroprevalence of toxoplasma infection among primigravid women attending antenatal clinic at a secondary level hospital in north India. J Indian Med Assoc 2002100591–592, 594-596, 602.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 88.Mahdi NK, Sharief M. Risk factors for acquiring toxoplasmosis in pregnancy. J Bahrain Med Soc. 2002;14:148–151. [Google Scholar]
- 89.Rai SK, Shibata H, Sumi K, Rai G, Rai N, Manandhar R, et al. Toxoplasma antibody prevalence in Nepalese pregnant women and women with bad obstetric history. Southeast Asian J Trop Med Public Health. 1998;29:739–743. [PubMed] [Google Scholar]
- 90.Chintana T, Sukthana Y, Bunyakai B, Lekkla A. Toxoplasma gondii antibody in pregnant women with and without HIV infection. Southeast Asian J Trop Med Public Health. 1998;29:383–386. [PubMed] [Google Scholar]
- 91.Ashrafunnessa S, Nazrul Islam M, Huq T. Seroprevalence of toxoplasma antibodies among the antenatal population in Bangladesh. J Obstet Gynaecol Res. 1998;24:115–119. doi: 10.1111/j.1447-0756.1998.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhang W, Zhao R, Qiu H.Toxoplasmosis infection in pregnant women in lanzhou. Zhonghua Fu Chan Ke Za Zhi 199732208–210.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 93.Onadeko MO, Joynson DH, Payne RA, Francis J. The prevalence of toxoplasma antibodies in pregnant Nigerian women and the occurrence of stillbirth and congenital malformation. Afr J Med Med Sci. 1996;25:331–334. [PubMed] [Google Scholar]
- 94.Zhang AM, Zhang T, Hao ZY. A seroepidemic survey on the infection of toxoplasma in pregnant women and its significance to better child-bearing. Chung-Hua Liu Hsing Ping Hsueh Tsa Chih. 1996;17:278–280. [PubMed] [Google Scholar]
- 95.Gonzalez-Morales T, Bacallo-Gallestey J, Garcia-Santana CA, Molina-Garcia JR. Prevalence of Toxoplasma gondii antibodies in a population of pregnant women in Cuba. Gac Med Mex. 1995;131:499–503. [PubMed] [Google Scholar]
- 96.de la Galvan Ramirez M.L., Soto Mancilla JL, Velasco Castrejon O, Perez Medina R. Incidence of anti-toxoplasma antibodies in women with high-risk pregnancy and habitual abortions. Rev Soc Bras Med Trop 199528333–337.[REMOVED HYPERLINK FIELD] [DOI] [PubMed] [Google Scholar]
- 97.Lelong B, Rahelimino B, Candolfi E, Ravelojaona BJ, Villard O, Rasamindrakotroka AJ, et al. Prevalence of toxoplasmosis in a population of pregnant women in Antananarivo (Madagascar). Bull Soc Pathol Exot 19958846–49.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 98.Sun RG, Liu ZL, Wang DC. The prevalence of toxoplasma infection among pregnant women and their newborn infants in Chengdu. Chung-Hua Liu Hsing Ping Hsueh Tsa Chih. 1995;16:98–100. [PubMed] [Google Scholar]
- 99.Martinez Sanchez R, Bacallao Gordo R, Alberti Amador E, Alfonso Berrio L.Prevalence of toxoplasmosis in pregnant women of the province of La Habana. Rev Inst Med Trop Sao Paulo 199436445–450.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 100.Bari A, Khan QA.Toxoplasmosis among pregnant women in northern parts of Pakistan. J Pak Med Assoc 199040288–289.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 101.Natu M, Joshi BN, Sali N. Toxoplasmosis prevalence in pregnancy with bad obstetric history. Indian J Med Sci. 1989;43:291–293. [PubMed] [Google Scholar]
- 102.Zhang GN.Epidemiological study on toxoplasma infection in human beings and animals in Shandong province. Chung-Hua Liu Hsing Ping Hsueh Tsa Chih. 19891030–33.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 103.Abdel-Hafez SK, Shbeeb I, Ismail NS, Abdel-Rahman F. Serodiagnosis of Toxoplasma gondii in habitually aborting women and other adults from north Jordan. Folia Parasitol. 1986;33:7–13. [PubMed] [Google Scholar]
- 104.Shanmugam J, Raveendranath M, Nair VR. Toxoplasmosis: Study of prevalence in infertile women and in healthy pregnant mothers from Kerala state. Indian J Med Microbiol. 1986;4:33–38. [Google Scholar]
- 105.Reis MM, Tessaro MM, d'Azevedo PA. Serologic profile of toxoplasmosis in pregnant women from a public hospital in Porto Alegre. Rev Bras Ginecol Obstet. 2006;28:158–64. [Google Scholar]
- 106.Harma M, Harma M, Gungen N, Demr N. Toxoplasmosis in pregnant women in Sanliurfa, Southeastern Anatolia city, Turkey. J Egypt Soc Parasitol. 2004;34:519–525. [PubMed] [Google Scholar]
- 107.Hou JH, Pu RZ, Liu JY, Li SP, Jiang CP, Wang Q. A study on IHA examination of toxoplasma infection in pregnant and postpartum women in Lanzhou district. Endemic Dis Bull. 1997;12:78–79. [Google Scholar]
- 108.Doehring E, Reiter Owona I, Bauer O, Kaisi M, Hlobil H, Quade G.et alToxoplasma gondii antibodies in pregnant women and their newborns in Dar Es Salaam, Tanzania. Am J Trop Med Hyg 199552546–548. [DOI] [PubMed] [Google Scholar]
- 109.Soto UR, Soto ST. Toxoplasmosis and pregnancy. Kasmera. 1993;21:1–36. [Google Scholar]
- 110.Franklin DM, Dror Z, Nishri Z. The prevalence and incidence of toxoplasma antibodies in pregnant women. Isr J Med Sci. 1993;29:285–286. [PubMed] [Google Scholar]
- 111.Ouermi D, Simpore J, Belem AM, Sanou DS, Karou DS, Ilboudo D, et al. Co-infection of toxoplasma gondii with HBV in HIV-infected and uninfected pregnant women in Burkina Faso. Pak J Biol Sci. 2009;12:1188–1193. doi: 10.3923/pjbs.2009.1188.1193. [DOI] [PubMed] [Google Scholar]
- 112.Hammouda NA, ElGebaly WM, Sadaka SM. Seroprevalence of toxoplasma and cytomegalovirus in complicated pregnancies. J Egypt Soc Parasitol. 1993;23:865–870. [PubMed] [Google Scholar]
- 113.Perazzi BE, Menghi CI, Coppolillo EF, Gatta C, Eliseth MC, de Torres RA, et al. Prevalence and comparison of diagnostic methods for Trichomonas vaginalis infection in pregnant women in Argentina. Korean J Parasitol. 2010;48:61–65. doi: 10.3347/kjp.2010.48.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stringer E, Read JS, Hoffman I, Valentine M, Aboud S, Goldenberg RL. Treatment of trichomoniasis in pregnancy in sub-Saharan Africa does not appear to be associated with low birth weight or preterm birth. S Afr Med J. 2010;100:58–64. [PMC free article] [PubMed] [Google Scholar]
- 115.Azargoon A, Darvishzadeh S.Association of bacterial vaginosis, trichomonas vaginalis, and vaginal acidity with outcome of pregnancy. Arch Iran Med 20069213–217.[REMOVED HYPERLINK FIELD] [PubMed] [Google Scholar]
- 116.Ogbonna CI, Ogbonna IB, Ogbonna AA, Anosike JC. Studies on the incidence of trichomonas vaginalis amongst pregnant women in Jos area of Plateau state, Nigeria. Angew Parasitol. 1991;32:198–204. [PubMed] [Google Scholar]
- 117.Assefa A, Asrat D, Woldeamanuel Y, G/Hiwot Y, Abdella A, Melesse T. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa specialized hospital Addis Ababa, Ethiopia. Ethiop Med J. 2008;46:227–235. [PubMed] [Google Scholar]
- 118.Taybouavone T, Hai TN, Odermatt P, Keoluangkhot V, Delanos-Gregoire N, Dupouy-Camet J, et al. Trichinellosis during pregnancy: A case control study in the Lao peoples' Democratic Republic. Vet Parasitol. 2009;159:332–336. doi: 10.1016/j.vetpar.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 119.Glinz D, Silue KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, et al. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis. 2010;4:e754. doi: 10.1371/journal.pntd.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.World Health Organization, Initiative for Vaccine Research (IVR). Parasitic diseases – Malaria. Available at: http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index4.html Accessed: 8 October 2011.
- 121.World Health Organization, Initiative for Vaccine Research (IVR). Parasitic diseases – Hookworm disease. Available at: http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index2.html Accessed: 8 October 2011.
- 122.World Health Organization, Initiative for Vaccine Research (IVR). Parasitic diseases - Schistosomiasis. Available at: http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index5.html Accessed: 8 October 2011.
- 123.World Health Organization. Chagas disease (American trypanosomiasis). Available at: http://www.who.int/mediacentre/factsheets/fs340/en/ Accessed: 8 October 20111.
- 124.Morgan DJ, Guimaraes LH, Machado PR, D'Oliveira A, Jr, Almeida RP, Lago EL, et al. Cutaneous leishmaniasis during pregnancy: Exuberant lesions and potential fetal complications. Clin Infect Dis. 2007;45:478–482. doi: 10.1086/520017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.