Abstract
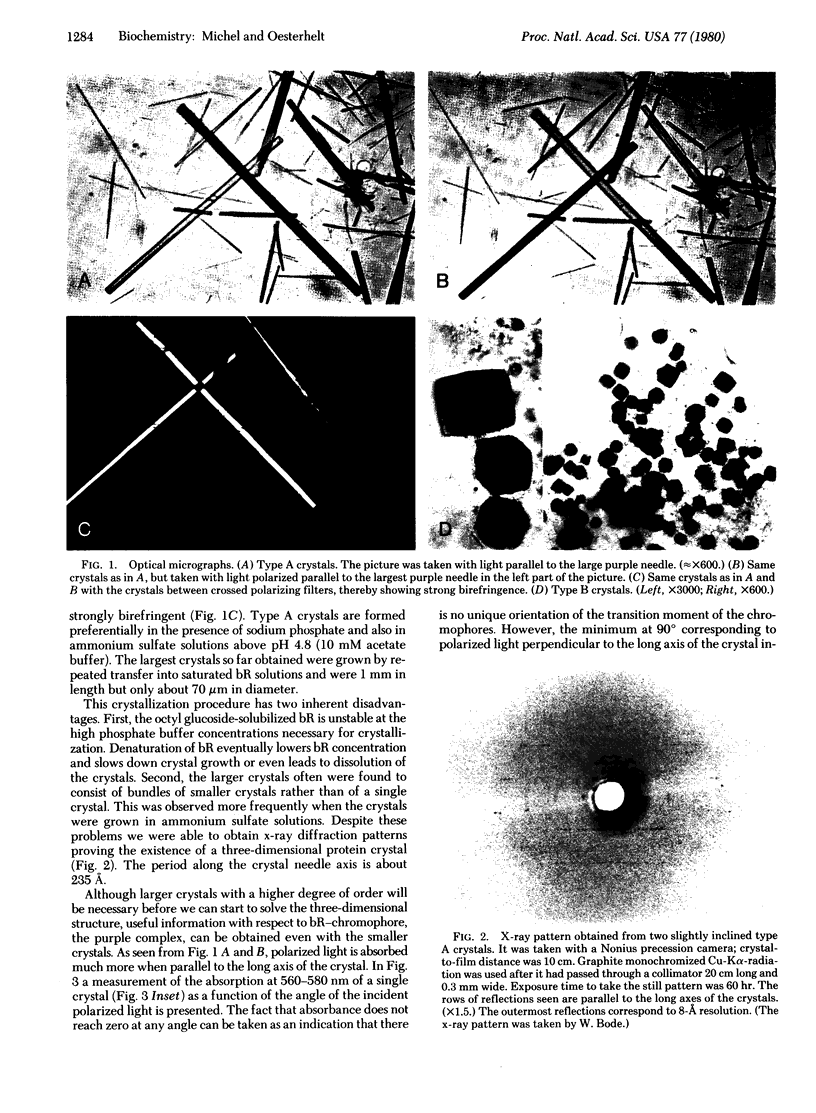
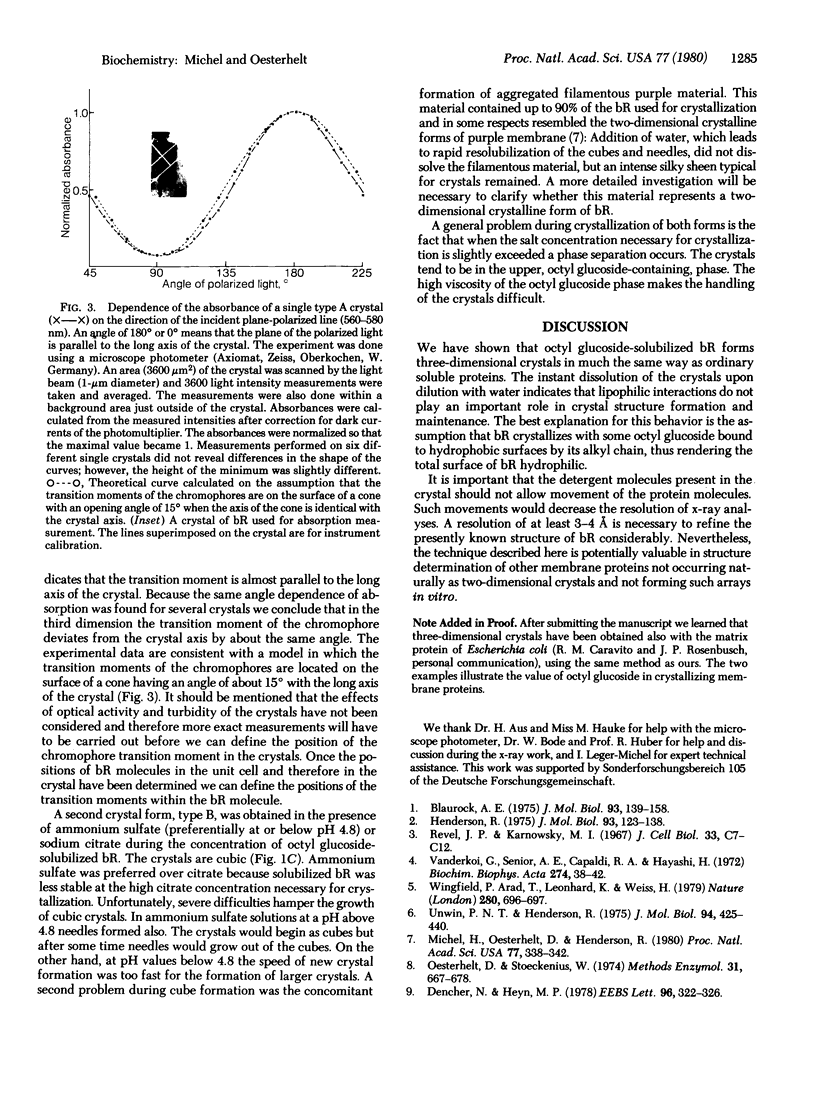
The intrinsic membrane protein bacteriorhodopsin has been crystallized by salt precipitation after solubilization by octyl glucoside. Two different crystal forms were obtained, depending on the nature of the salt used and the pH. Needles formed in the presence of sodium phosphate and in ammonium sulfate solutions above pH 4.8. Cubes appeared in sodium citrate solutions or ammonium sulfate. Unlike the cubic crystals, the birefringent needles showed strong linear dichroism, which allowed determination of the orientation of the chromophore's transition moment. The procedure described here may be of general use in crystallographic studies of membrane proteins.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Formation and properties of bacteriorhodopsin monomers in the non-ionic detergents octyl-beta-D-glucoside and Triton X-100. FEBS Lett. 1978 Dec 15;96(2):322–326. doi: 10.1016/0014-5793(78)80427-x. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D., Henderson R. Orthorhombic two-dimensional crystal form of purple membrane. Proc Natl Acad Sci U S A. 1980 Jan;77(1):338–342. doi: 10.1073/pnas.77.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Vanderkooi G., Senior A. E., Capaldi R. A., Hayashi H. Biological membrane structure. 3. The lattice structure of membranous cytochrome oxidase. Biochim Biophys Acta. 1972 Jul 3;274(1):38–48. doi: 10.1016/0005-2736(72)90278-7. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Arad T., Leonard K., Weiss H. Membrane crystals of ubiquinone:cytochrome c reductase from Neurospora mitochondria. Nature. 1979 Aug 23;280(5724):696–697. doi: 10.1038/280696a0. [DOI] [PubMed] [Google Scholar]