Abstract
Sexual transmission accounts for the majority of new HIV infections worldwide with sexually exposed cervicovaginal and colorectal mucosae being primary sites of infection. Two recent Phase 1 rectal microbicide trials included, as an ancillary endpoint, suppression of ex vivo HIV infection of in vivo microbicide-exposed rectal mucosal tissue biopsies. Both trials demonstrated significant suppression of biopsy infectibility in drug-exposed versus placebo-exposed tissue. This potential early biomarker of efficacy has raised the feasibility of utilizing “snap-frozen” tissue samples, acquired at multiple trial sites to be shipped for central processing, providing a mechanism to correlate tissue drug concentrations with a functional index of HIV prevention. While previous reports have indicated acceptable comparability of fresh versus freeze-thawed cervicovaginal tissue samples, no similar evaluations with colorectal tissue biopsies have been done. In this study, rectal biopsies from healthy, HIV-seronegative participants were assessed for structural integrity (histology), viability (MTT assays), and tissue infectibility to compare results from fresh versus combinations of freeze/thaw protocols. Results indicated that while all protocols showed equivalent viability with fresh samples (MTT), histology documented poor preservation of tissue integrity following freezing. Infectibility results from freeze-thawed colorectal tissue were markedly lower (usually<25% of fresh samples) and varied greatly and unpredictably. Centralized colorectal tissue infectibility assays using biopsies from remote trial sites cannot currently be supported under these protocols.
The colorectal explant model is emerging as a potentially valuable tool in microbicide development to assess product impact on ex vivo HIV infectivity.1–3 Recent inclusion of this assay in two Phase 1 rectal microbicide trials1,4 indicates this assay might be an important, albeit exploratory, endpoint of ex vivo efficacy in Phase 1 human clinical trials. Both of these recent rectal microbicide trials demonstrated statistically significant suppression of ex vivo infection using tissue biopsies from colorectal tissue exposed to UC781 or tenofovir gels in vivo despite the known assay variability at baseline.1,5 These results suggest that these ex vivo assays might function as a mechanism to assist in the selection of products that should be advanced to later stage development.
To support utilization of this assay in larger and/or multisite clinical trials, an important consideration is whether freshly acquired colorectal samples can be frozen and shipped to a central facility where viral challenge/tissue infectibility studies could be conducted on frozen/thawed tissues as has been shown with cervicovaginal samples.6,7 Given that colorectal tissue is more physiologically active, contains highly activated immune cells, has a single columnar epithelial layer, and is more prone to rapid apoptosis than other tissues,8,9 it was felt necessary to specifically evaluate whether comparable infectibility readouts would occur using fresh versus frozen/thawed rectal biopsies.
To evaluate whether rapid (“snap”) freezing of fresh, endoscopically acquired human colorectal tissue biopsies with subsequent thawing before explant set-up would detrimentally impact ex vivo infectibility assay readouts, three commonly utilized freeze/thaw protocols were compared (using conventional as well as specialized methods, such as for oocyte freeze/thaw).10 Histology (for architectural maintenance) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide] quantification (for sample viability) were also evaluated. Outcomes of each protocol were compared to freshly acquired/processed samples from the same subjects. Numbers of subjects recruited for this pilot study were too small for formal statistical analyses, but trends are indicated.
Based on published protocols for cryopreservation6,10,11 as well as input from collaborators, three freeze/thaw protocols were compared. The first protocol was a simple snap freeze/rapid thaw method that produced poor results. The second protocol was derived from the first by adding a cold freezing medium and utilizing a more gradual thawing step; this yielded better but still unsatisfactory results. The third protocol was adapted from the second protocol but utilized a Mr. Frosty (Nalgene) freezing apparatus to standardize followed by the same, slower thawing method used in Protocol 2.
• Protocol 1 (P1): Freezing Process #1 (snap frozen in liquid nitrogen) and Thawing Process #1 (rapid thawing at 37°C).
• Protocol 2 (P2): Freezing Process #2 [tissue collection on ice prior to the addition of freezing medium (7% DMSO/FBS)] and Thawing Process #2 (thawing by swirling at 37°C with gradual exposure to incremental volumes of culture medium).
• Protocol 3 (P3): Freezing Process #3 (tissue collection in cold culture medium (RPMI/Hepes/antibiotic-antimycotic) prior to the addition of freezing medium then brought to −80C° using Mr. Frosty (Nalgene cat # 5100-0001 at a cooling rate of −1C°/min) and Thawing Process #2 (thawing by swirling at 37°C with gradual exposure to incremental volumes of culture medium).
Rectosigmoid biopsies were acquired from 14 healthy, confirmed HIV-1-seronegative volunteers (11 men; 3 women; ages ranged from 34 to 65; mean age was 50) via flexible sigmoidoscopy, 30 cm from the anal verge as previously reported.5 The study was approved by the UCLA Office of Human Research Protection Program Institutional Review Board (UCLA IRB #11-000602) and all subjects provided written informed consent. Each of the 14 participants provided 27 colorectal tissue biopsies each to enable direct comparisons between the freshly acquired/processed and the freeze/thaw protocols. It should be noted that this was an assay evaluation assessed iteratively: the results for the first protocol were evaluated before advancing to try the second protocol, etc. Due to the limit on biopsy number, the need for replicates, and the sequential reviewing of data to eliminate nonproductive freeze/thaw protocols, not all participants' samples went through each endpoint assessment. Numbers of subjects' samples used in assessment of each freeze/thaw protocol are identified in Fig. 1.
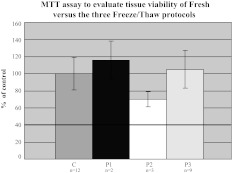
FIG. 1.
Comparative viability by MTT: Each of the three freeze/thaw protocols (P1: N=2; P2: N=3; P3: N=9) is compared to the freshly assayed controls (N=12). Histograms (±SD) are shown as percentage of fresh controls. A cut-off threshold of 40% defined nonviable samples (solid line).
Nine biopsies from each participant were utilized for the “freshly acquired” endpoints (tissue biopsy infection, MTT assay, and histology). The remaining 18 biopsies were frozen/thawed according to the three freeze protocols defined above. Following 1–2 weeks of freezing time, biopsy samples were thawed (according to assigned protocol) and evaluated by MTT, histology, and ex vivo infectibility with each subject's sample results compared to those from that subject's freshly processed samples. Conditions with inherent variability such as different laboratory staff, FBS lot numbers, DMSO storage, freezer temperatures, assay processing room air flow, media and reagents, hours of procedure, and sterility conditions were standardized to try and minimize variability other than whether the samples were fresh or frozen.
Samples were assessed for tissue viability using the overnight MTT assay (Millipore MTT Chemicon Kit #CT02) on two separate biopsies (read at 24 h). Results were read on a Bio-Rad Benchmark Plus spectrophotometer and then averaged. Viability was defined as ≥40% of that seen with fresh colorectal biopsies. MTT-defined viability readouts did not differ between freshly processed colorectal biopsies versus those from the three different freeze/thaw protocols (Fig. 1).
Histopathological scoring of tissue injury was conducted on batched samples in a blinded fashion using oriented, formalin-fixed, paraffin-embedded, hematoxylin-eosin-stained biopsy sections (4 μm thick; three tissue sections per prepared slide) of both fresh and frozen/thawed tissue. Scoring was done using a validated, qualitative scale of chronic active injury/inflammation previously adapted for use in exploratory and Phase 1 rectal microbicidal studies.1,12 A single “grade of injury” (range: 1=intact, attached epithelium with retained mucin and lamina propria/glandular array, to 6=lost epithelia with minimal cellular remnants) was assigned based on review of two slides per subject (100×) (Table 1 and Fig. 2).
Table 1.
Histopathology Grading Scale
| Grade | Histological description |
|---|---|
| 1 | Intact epithelium. Maintained lamina propria. Mucin visible within glands. Nuclei stained blue. |
| 2 | Detached epithelium. Maintained lamina propria. Mucin visible within glands. Nuclei stained blue. |
| 3 | Detached epithelium. Disaggregated lamina propria. Mucin visible within glands. Nuclei stained blue. |
| 4 | Mostly detached/lost epithelium. Disaggregated lamina propria. No mucin visible within glands. Karyorrhectic nuclei>30%. |
| 5 | Mostly detached/lost epithelium. Disaggregated lamina propria. No mucin visible within glands. Karyorrhectic nuclei>75%. |
| 6 | Lost epithelium. Minimal cellular remnants. Mostly structural elements remaining. |
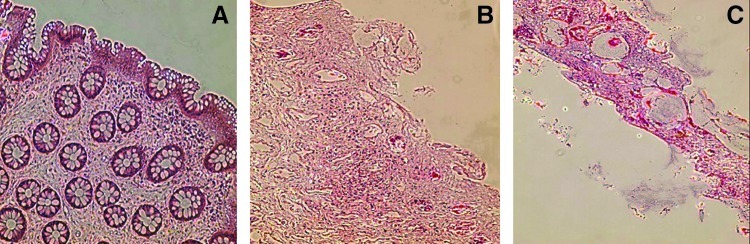
FIG. 2.
Photomicrograph representations of tissue sections showing (A) no damage, (B) moderate damage, and (C) severe damage at time of ex vivo infection. This figure shows three of the six grades of injury used. Samples were sectioned and paraffin-embedded tissues stained with hematoxylin and eosin at a final magnification of 100×.
Table 2 demonstrates that all histology samples from the freshly acquired control group (n=12) were scored as “normal” (Grade 1–2), consistent with our previously published results on healthy, seronegative tissues.1,12,13 In contrast, the majority of all samples undergoing any form of freeze/thaw were either moderately or severely damaged histologically (representative photomicrographs are shown in Fig. 2). It is interesting and important to note that all samples demonstrating moderate/severe histopathological tissue disruption were still reported as “within normal range” using the MTT assay.
Table 2.
Histopathological Scoring
| |
|
Histology grades |
||
|---|---|---|---|---|
| Protocol | n | Grade 1–2 no damage | Grade 3–4 moderate | Grade 5–6 severe |
| Fresh | 12 | 100% | 0% | 0% |
| P1a | 0 | ND | ND | ND |
| P2 | 3 | 0% | 67% | 33% |
| P3 | 9 | 45% | 22% | 33% |
ND, histology not done as no tissue infectibility was detected.
The same batch of HIV-1BaL viral stock14 (produced in PM1 cells) (NIH AIDS Research and Reagent Program) were used in all biopsy infection experiments at two titers (104 and 102 TCID50; determined using PBMCs)15 as previously reported.1,3 Briefly, biopsy tissues in triplicate (fresh or thawed) were incubated with both titers of virus for 2 h, washed thoroughly, and then explant cultures were set-up on gel-foam rafts in individual wells of a 24-well plate (Costar #3524, Corning, Inc., Corning, NY). Culture supernatants were collected every 3 days over a 14-day period for ELISA quantification of p24 (pg/ml) (AIDS & Cancer Virus Program, NCI, Bethesda, MD), frozen, and stored at −80°C for batched processing. Results are reported as cumulative p24 at day 14 with infectible defined as >300 pg p24/ml.
Under same-day/fresh conditions, all participants' samples (100%) were infectible using the higher HIVBaL viral titer of 104 TCID50 (median p24: 5579 pg/ml; range: 2,931–25,318 pg/ml); 93% of subject's samples were infectible using the 102 TCID50 viral titer (median p-24: 2669 pg/ml; range: <300–2,651 pg/ml) (Table 3). These results, including the wide intersubject variability in p24 production, are consistent with previous reports using fresh colorectal biopsies for ex vivo infections.1,4
Table 3.
Ex Vivo Infection of Colorectal Biopsies Is Reduced with All Freeze/Thaw Protocols
| |
|
Percentage of subjects with infectible biopsies |
|
|---|---|---|---|
| Protocol | n | 104 (TCID50) | 102 (TCID50) |
| Fresh | 13a | 100% | 93% |
| P1 | 2 | 50% | 0% |
| P2 | 3 | 67% | 0% |
| P3 | 9 | 67% | 44% |
One subject's fresh biopsies were contaminated at 104 (TCID50); the biopsies from this subject's same day samples did establish infection at 103, indicating nonresistance.
In contrast, all freeze/thaw protocols showed inconsistent but always decreased ex vivo infectibility (Table 3). With Protocol 1 (N=2), one of the two subjects' samples was infectible with the 104 titer (390 pg/ml p24) although this is barely above our threshold level for a positive p24 result. Neither subject's thawed frozen biopsies were infectible with the 102 titer. Protocol 2 (N=3) yielded 67% subject's samples infection with the 104 titer (median: 471 pg/ml, which was 8.4% of the fresh controls' median; range: <300–2,733 pg/ml); no tissue infection was seen with the lower, 102 titer. Protocol 3 (N=9) also demonstrated infectibility of 67% of participant's samples with the higher titer of virus (median: 1,226 pg/ml) but at a level that was 21.9% of the fresh controls' median and only 44% infection using the lower titer (median: 210 pg/ml; 7.8% of the fresh controls' median) (Table 3).
In comparison with immediate infection of freshly acquired colorectal biopsies, using the same subject's concurrently acquired biopsies, no combination of freeze/thaw protocols was identified that yielded reproducible results with the 104 TCID50 titer of HIV-1BaL. Infectibility was even less reproducable with the 102 TCID50 titer, a titer that is thought to more closely approximate the viral load in human ejaculate or rectal/vaginal fluids from untreated HIV-positive individuals.16
In a previous report, Gupta et al.6 demonstrated the viability of utilizing frozen cervical explants as a model to study the use of microbicides against HIV. Their results show maintained infection reproducibility, which may relate to the cervix being a more fibrous, less vascular and fragile structure, their use of surgically acquired versus freshly acquired biopsies, and larger explant samples. In other work7 it was shown that cervical tissues can be shipped on wet ice overnight and that viability of these tissues is sufficient to support HIV replication. This brief report demonstrates that various freeze/thaw protocols similar to those used with resected cervicovaginal samples are inadequate when applied to colon biopsies in these ex vivo infectibility assays. Explanations for this difference likely include a combination of the following compartment differences: (1) colorectal biopsy architecture is markedly different in terms of epithelial thickness, vascularity, surface area, endogenous microflora diversity/mucosal interaction, and extent of physiological inflammation in health1,5,12; (2) cervical or cervicovaginal samples (whether freshly acquired or derived from surgical resections) are more structurally intact, fibrous, and resilient to initial fragmentation and hypoxic effects; (3) as we and others have shown in this explant model, colonic biopsies demonstrate significantly altered epithelial integrity, mucin/goblet cell depletion, and architectural disarray by 24 h at 37°C3; and (4) given the known “within subject” variability when using freshly acquired biopsies that are immediately infected ex vivo,1 that “within-subject” variability is further increased when the same subject's samples are kept overnight for infectibility assays ex vivo the following day (data not shown).
While the recruited numbers of subjects were too small to provide statistical evidence, the trends indicate that none of the three freeze/thaw protocols reliably generated reproducible infectivity data in a believable range to merit inclusion in multisite clinical trials. Future protocols may improve these pitfalls and merit revisiting this issue. The documented high false-positive rates of the MTT assay as relates to both indices of histological injury and ex vivo infectibility support popular views that MTT as an index of tissue viability remains coarse and not terribly helpful. On the other hand, histology scoring did correlate with the ability of the tissue to sustain HIV replication (infectibility data); perhaps this is because preferential HIV target cells survive longer than other cell types incorporated in the histology score. Regardless, the discordance between MTT viability and histological assessment of tissue integrity as relates to explant infectibility raises questions about the robustness of the MTT assay in documenting tissue viability for explant infection studies. For more centralized assessment of ex vivo infectibility assays of colorectal biopsies, current freeze/thaw protocols are inadequate. Efforts to advance this will need to at least meet the level of baseline variability already seen with freshly acquired, immediately assayed tissue biopsies.
Acknowledgments
This study was funded under the NIAID/DAIDS/Prevention Sciences Integrated Preclinical-Clinical Program (IPCP) U19 grant to the Microbicide Development Program (MDP) (#AI606014). This study design benefited from input from CONRAD, the UCLA CFAR Mucosal Immunology Core and Biostatistics Core (#AI28697). In addition, supplemental funds were provided by the Hansen Family Foundation, Macy's Foundation, and the Oppenheimer Brothers Foundation. As always, we thank the willing volunteers who participated by donating samples for this pilot study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Anton PA. Saunders T. Elliott J, et al. First Phase 1 Double-Blind, Placebo-Controlled, Randomized Rectal Microbicide Trial Using UC781 Gel with a Novel Index of Ex Vivo Efficacy. PLoS One. 2011;6(9):e23243. doi: 10.1371/journal.pone.0023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrera C. Cranage M. McGowan I. Anton P. Shattock RJ. Colorectal microbicide design: Triple combinations of reverse transcriptase inhibitors are optimal against HIV-1 in tissue explants. AIDS. 2011;25(16):1971–1979. doi: 10.1097/QAD.0b013e32834b3629. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher PS. Elliott J. Grivel JC, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS. 2006;20(9):1237–1245. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 4.Anton P. Cranston R. Carballo-Dieguez A. Kashuba A. Khanukhova E. Elliot J, et al. RMP-02/MTN-006: A Phase 1, placebo controlled trial of rectally applied 1% vaginal tenofovir gel with comparison to oral tenofovir disoproxil fumarate. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. Feb;2011 ; Abstract 34LB. [Google Scholar]
- 5.Anton PA. Elliott J. Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14(12):1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 6.Gupta P. Ratner D. Patterson BK, et al. Use of frozen-thawed cervical tissues in the organ culture system to measure anti-HIV activities of candidate microbicides. AIDS Res Hum Retroviruses. 2006;22(5):419–424. doi: 10.1089/aid.2006.22.419. [DOI] [PubMed] [Google Scholar]
- 7.Lackman-Smith C. Osterling C. Luckenbaugh K, et al. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob Agents Chemother. 2008;52(5):1768–1781. doi: 10.1128/AAC.01328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanahan F. Intestinal lymphoepithelial communication. Adv Exp Med Biol. 1999;473:1–9. doi: 10.1007/978-1-4615-4143-1_1. [DOI] [PubMed] [Google Scholar]
- 9.Griffith TS. Ferguson TA. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997;18(5):240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- 10.Isachenko V. Isachenko E. Reinsberg J, et al. Cryopreservation of human ovarian tissue: Comparison of rapid and conventional freezing. Cryobiology. 2007;55(3):261–268. doi: 10.1016/j.cryobiol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Keros V. Hultenby K. Borgstrom B, et al. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22(5):1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 12.McGowan I. Elliott J. Cortina G, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056) J Acquir Immune Defic Syndr. 2007;46(4):417–425. doi: 10.1097/QAI.0b013e318156ef16. [DOI] [PubMed] [Google Scholar]
- 13.Anton PA. Ibarrondo FJ. Boscardin WJ, et al. Differential immunogenicity of vaccinia and HIV-1 components of a human recombinant vaccine in mucosal and blood compartments. Vaccine. 2008;26(35):4617–4623. doi: 10.1016/j.vaccine.2008.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson-Harman N. Lackman-Smith C. Fletcher PS, et al. Multi-site comparison of anti-HIV microbicide activity in explant assays using a novel endpoint analysis. J Clin Microbiol. 2009;47(11):3530–3539. doi: 10.1128/JCM.00673-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed LJ. Muench H. A simple method of estimating fifty per cent end-points. Am J Hygiene. 1938;27:493–497. [Google Scholar]
- 16.Chan DJ. McNally L. Batterham M. Smith DE. Relationship between HIV-RNA load in blood and semen in antiretroviral-naive and experienced men and effect of asymptomatic sexually transmissible infections. Curr HIV Res. 2008;6(2):138–142. doi: 10.2174/157016208783885074. [DOI] [PubMed] [Google Scholar]