ABSTRACT
Introduction: BK polyomavirus (BKV) as a member of polyomavirus family is prevalent in the human population. BKV persists in renal tissue after asymptomatic infection in childhood. The reactivation of BKV in renal transplant recipients sometimes can lead to BKV associated nephropathy. BKV isolates are classified into four serologically distinct subtypes. Present study was carried out to investigate the distribution pattern of BKV subtypes in Iranian Turkish renal transplant recipients.
Materials and Methods: Urine samples from 12 kidney transplant recipients infected with BKV were analyzed by RFLP-PCR technique for classification of subtypes.
Results: Our analysis showed that all samples were infected with BKV type I. BK virus types II, III, and IV were not detected in our patients.
Conclusions: Based on the results of the present study, BKV subtype I was the most frequently detected subtype in renal transplant recipients. To our knowledge, the present study provides the first data regarding distribution of BKV subtypes in Iranian renal transplant recipients.
Keywords: BKV, renal transplant, recipients, urine
INTRODUCTION
BKV as human polyomavirus has been isolated from urine after renal transplantation in 1971 (1). A large body of studies indicated that there is high prevalence of antibodies to BKV, in early childhood ranging from 60% to 100% (2-8). For the first time, individuals infected with BKV are in early childhood without prominent symptoms (8). BKV may found in a wide range of organs and tissues (9-11) especially renal tissues (12). Transmission of BKV occurs via body fluids (13). In immunocompromised patients, the condition is prepared for reactivation of BKV as well as BKV related disease (14). BKV has been associated to late-onset hemorrhagic cystitis in recipients of bone marrow transplants (15,16), interstitial nephritis, vasculopathy, and allograft dysfunction in the allograft kidney recipient (17-19), central nervous system in AIDS and transplant patients (20), nephritic-nephrotic syndrome (21), ureteric stenosis (22), encephalitis (23), interstitial desquamative pneumonitis (24).The gene organization of BKV genome was described previously (25-31), coding sequences of BKV genome that correspond to t-Ag,T-Ag, Agno, VP1, VP2, and VP3 proteins production (30, 31). Several variants of BKV have been identified based on a variable sequence of the protein VP1. Jin et al. reported that amino acid coding alteration within residues 61 to 83 of the VP1 gene product leads to typing of BKV in to four groups I, II, III, and IV (32). However the clinical importance of each group is poorly understood. The aim of present study was to identify distribution pattern of BKV subtypes in Iranian Turkish renal transplant recipients by PCR-restriction enzyme assay. ❑
MATERIALS AND METHODS
A total of 120 urine samples were obtained from the kidney transplant patients form the division of Nephrology and Renal Transplantation of Urmia University of Medical Sciences (Urmia, Iran). Subtyping was carried out using 12 patients' urine samples positive for BK viral DNA out of 120 cases screened for BK virus viruria. This study was approved by the Ethics committee of Urmia University of Medical Sciences. Informed written consent was taken from all patients before obtaining of urine samples. One ml of urine was centrifuged for 2 minutes. The sediment of samples washed in 1 ml PBS, and followed by spin for 2 min. The pellets were resuspent in 100 µl of distilled water and heated at 95 ° C for 5 min. 10 µL of supernatant was added to a 50 µl of reaction mixture containing Taq polymerase buffer, 1.25 u Taq polymerase, 200 µm of each dNTP, 50 pmol of each primer. The oligonucleotides VP1-327-1 5'-CAA GTG CCA AAA CTA CTA AT-3' and VP1-327-2r 5'-TGC ATG AAG GTT AAG CAT GC-3' were used for PCR amplification of VP1 segment. These primers amplify only BKV and not JC virus (34). PCR reaction was carried out as follow: 2 min of denaturation at 94 ° C, followed by 35 cycles consisting 1 min at 91 ° C, 1 min at 55 ° C and 1 min at 72 ° C, consequently a final extension cycle of 1 min at 55 ° C and 4 min at 72 ° C (33). The expected amplicon was 327 bp long which covers the type-specific region. PCR product was evaluated via electrophoresis on a 3% agarose gel. PCR products were digested by 1-2 µl of Alu I restriction enzyme in a total volume of 30 µl at 37 ° C for 2 h. Digested PCR products were separated on a 3% agarose gel and bands visualized by ethidium bromide. Type I and II BKV isolates were digested by Alu I producing fragments migrating at 186 and 141 bp while type III or IV isolates were not digested by Alu I (33). PCR products that digested by AluI consequently were cut with Xmn I. Type II BKV isolates produces two fragments of 244 and 83 bp in size, whereas type I remained uncut by Xmn I. PCR products defined as BKV type III or IV were digested with Ava II, and type III isolates produces product sizes of 237 and 90 bp, while type IV remained undigested. ❑
RESULTS
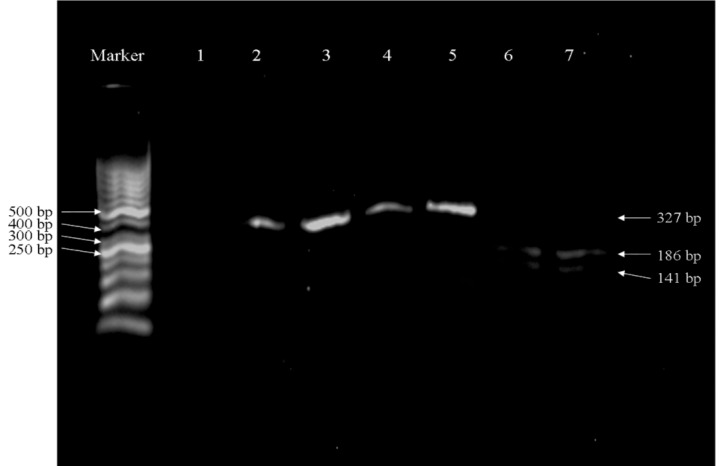
BKV DNA was analyzed by RFLP-PCR. Sub typing of BKV in renal transplant recipients showed that all samples were infected by BKV type I. BKV subtypes II, III, and IV were not found in our patients. Mixed infections were not detected in recipients. The restriction patterns obtained from some patients positive for BKV viruria are presented in Fig 1. ❑
Figure 1. Analysis of BKV DNA types by RFLP-PCR. 327 bp products of BKV DNA from four representative samples were produced by PCR and digested with Alu I Restriction enzyme. Then PCR products that digested by Alu I consequently were digested by Xmn I. Marker: 50 bp; lane1: negative control, lane2: un-cut PCR product; lanes 3, 4, 5: cut PCR products (remind un-cut after digestion with Xmn I); and lanes 6, 7: cut PCR products with Alu I producing two fragments of 186 and 141 bp.
DISCUSSION
BKV isolates are classified into four subtypes (I–IV) using either serological reactivity or genotyping methods. The distribution of BKV subtypes within the human population was previously studied in England, Tanzania, the United States, Japan and Germany (34-38). Nevertheless, no information is available about the distribution pattern of BKV subtypes in Iran. In this study, first data on the prevalence of BKV subtypes obtained from renal transplant recipients of Iranian Turkish patients are presented. Similar to other reports (34-38), subtype I was found to be predominant. A recent study (38) on sixty specimens obtained from German renal and bone marrow transplant recipients detected subtype I in 90.9% of the cases. Takasaka et al (37) found that subtype I was the most prevalent subtype (70-80%) in Japanese renal and bone marrow transplant recipients. Likewise, Jin et al. (35) reported that subtype I was the major subtype in English bone marrow transplant recipients. They detected BKV subtype III only in samples obtained from pregnant women and HIV-infected patients. In kidney transplant recipients (34,36,37), subtype IV was detected at lower rates and subtypes II and III were not or rarely detected. Subtype I is widespread throughout the world, whereas subtype IV is prevalent in East Asia, excluding Japan and Europe but rare in Africa (39). The data presented in this study are consistence with those results.
Based on phylogenetic analysis, Takasaka et al (37) proposed three subgroups (Ia, Ib and Ic) within subtype I and reported that most of subtype I sequences in Japanese patients belonged to subgroup Ic. The other study from Germany showed that most of sequences were demonstrated to cluster within subgroups Ic (38). The geographic distribution pattern of subtype IV BKV is in contrast with the hypothesis that BKV co-evolved with humans, since subtype IV rarely finds in Africa. It has proposed that subtype IV of BKV which is prevalent in modern humans is derived from a virus that infected ancestral Asians (40). Several studies conducted in order to explain how the geographical distribution of BKV subtypes and subgroups has developed (40-42). Zhong et al (41) analyzed sequences obtained from two American, European and Asian populations and found that subtype I was the highest through all groups, but subtype IV was variable. Distribution pattern of subtype I subgroups was also varied between Euro-American and Asian populations. By comparison of subtype and subgroups, authors suggested that BKV has co-migrated with human populations. In the present study, we were not able to analyze distribution pattern of subgroups within subtype I since no phylogenetic analysis was carried out due to cost limits.
The major limitation of this study is related to its small sample size. This limitation impairs the generalizability of the current findings and may fail to adequately detect abundance of less common subtypes in our study. Despite the restriction of present study to a limited number of 12 patients from Iranian Turkish recipients, the data indicate that BKV subtype I is predominant in North West of Iran. Additional studies with larger sample sizes are required to determine the frequency of other possible subtypes. ❑
CONCLUSIONS
To our knowledge, this is the first report regarding distribution of BKV subtypes in Iranian Turkish renal transplant recipients. Based on the results of present study, BKV subtype I was the most frequently detected subtype in renal transplant recipients. These results suggest lower distribution of subtypes II, III and IV in our recipients. Further studies are needed to provide a better understanding of the epidemiology of BKV subtypes in different provinces of Iran. ❑
ACKNOWLEDGEMENTS
This study was supported by a grant from the Urmia University of Medical Sciences. We would like to thank all staff and clinicians in Nephrology and Renal Transplantation Research Center of Urmia University of Medical Sciences for their contribution in this project.
References
- 1.Gardner SD, Field AM, Coleman DV, et al. New human papovavirus (B.K.) isolated from urine after renal transplantation. . Lancet. 1971;1(7712):1253–7. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 2.Pang XL, Doucette K, LeBlanc B, et al. Monitoring of polyomavirus BK virus viruria and viremia in renal allograft recipients by use of a quantitative real-time PCR assay: one-year prospective study. J Clin Microbiol. 2007;45:3568–73. doi: 10.1128/JCM.00655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–46. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 4.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–23. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 5.Bechert CJ, Schnadig VJ, Payne DA, et al. Monitoring of BK viral load in renal allograft recipients by real-time PCR assays. Am J Clin Pathol. 2010;133:242–50. doi: 10.1309/AJCP63VDFCKCRUUL. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–23. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 7.Pollara CP, Corbellini S, Chiappini S, et al. Quantitative viral load measurement for BKV infection in renal transplant recipients as a predictive tool for BKVAN. New Microbiol. 2011;34:165–71. [PubMed] [Google Scholar]
- 8.Cannon RM, Ouseph R, Jones CM, et al. BK viral disease in renal transplantation. Curr Opin Organ Transplant. 2011;16:576–9. doi: 10.1097/MOT.0b013e32834cd666. [DOI] [PubMed] [Google Scholar]
- 9.Krumbholz A, Bininda-Emonds OR, Wutzler P, et al. Phylogenetics, evolution, and medical importance of polyomaviruses. Infect Genet Evol. 2009;9:784–99. doi: 10.1016/j.meegid.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 10.van der Meijden E, Janssens RW, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:e1001024–e1001024. doi: 10.1371/journal.ppat.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison CJ, Meinke G, Kwun HJ, et al. Asymmetric assembly of Merkel cell polyomavirus large T-antigen origin binding domains at the viral origin. J Mol Biol. 2011;409:529–42. doi: 10.1016/j.jmb.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariharan S. BK virus nephritis after renal transplantation. . Kidney Int. 2006;69:655–62. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 13.Pietropaolo V, Di Taranto C, Degener AM, et al. Transplacental transmission of human polyomavirus BK. J Med Virol. 1998;56:372–6. doi: 10.1002/(sici)1096-9071(199812)56:4<372::aid-jmv14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Bendiksen S, Rekvig OP, Van Ghelue M, et al. VP1 DNA sequences of JC and BK viruses detected in urine of systemic lupus erythematosus patients reveal no differences from strains expressed in normal individuals. J Gen Virol. 2000;81:2625–33. doi: 10.1099/0022-1317-81-11-2625. [DOI] [PubMed] [Google Scholar]
- 15.Reploeg MD, Storch GA, Clifford DB. BK virus: a clinical review. Clin Infect Dis. 2001;33:191–202. doi: 10.1086/321813. [DOI] [PubMed] [Google Scholar]
- 16.Silva Lde P, Patah PA, Saliba RM, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–90. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus–associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103–9. doi: 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 18.Nickeleit V, Klimkait T, Binet IF, et al. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med. 2000;342:1309–15. doi: 10.1056/NEJM200005043421802. [DOI] [PubMed] [Google Scholar]
- 19.Petrogiannis-Haliotis T, Sakoulas G, Kirby J, et al. BK-related polyomavirus vasculopathy in a renal-transplant recipient. N Engl J Med. 2001;345:1250–5. doi: 10.1056/NEJMoa010319. [DOI] [PubMed] [Google Scholar]
- 20.Lopes da Silva R. Polyoma BK virus: an emerging opportunistic infectious agent of the human central nervous system. Braz J Infect Dis. 2011;15:276–84. doi: 10.1016/s1413-8670(11)70189-1. [DOI] [PubMed] [Google Scholar]
- 21.Derakhshan N, Derakhshan D, Torabinejad S, et al. Nephritic-nephrotic syndrome as a presentation of BK virus infection. Saudi J Kidney Dis Transpl. 2011;22:123–5. [PubMed] [Google Scholar]
- 22.Braun WE. BK polyomavirus: a newly recognized threat to transplanted kidneys. Cleve Clin J Med. 2003;70:1056,1059–62. doi: 10.3949/ccjm.70.12.1056. [DOI] [PubMed] [Google Scholar]
- 23.Voltz R, Jager G, Seelos K, et al. BK virus encephalitis in an immunocompetent patient. Arch Neurol. 1996;53:101–3. doi: 10.1001/archneur.1996.00550010121025. [DOI] [PubMed] [Google Scholar]
- 24.Sandler ES, Aquino VM, Goss-Shohet E, et al. BK papova virus pneumonia following hematopoietic stem cell transplantation. Bone Marrow Transplant. 1997;20:163–5. doi: 10.1038/sj.bmt.1700849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krumbholz A, Bininda-Emonds OR, Wutzler P, et al. Evolution of four BK virus subtypes. Infect Genet Evol. 2008;8:632–43. doi: 10.1016/j.meegid.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Fioriti D, Videtta M, Mischitelli M, et al. The human polyomavirus BK: Potential role in cancer. J Cell Physiol. 2005;204:402–6. doi: 10.1002/jcp.20300. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto C, Hara K, Taguchi F, et al. Growth efficiency of naturally occurring BK virus variants in vivo and in vitro. J Virol. 1989;63:3195–9. doi: 10.1128/jvi.63.7.3195-3199.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubinstein R, Harley EH. BK virus DNA cloned directly from human urine confirms an archetypal structure for the transcriptional control region. Virus Genes. 1989;2:157–65. doi: 10.1007/BF00315259. [DOI] [PubMed] [Google Scholar]
- 29.Jin L, Gibson PE. Genomic Function and Variation of Human Polyomavirus BK (BKV). Rev Med Virol. 1996;6:201–214. doi: 10.1002/(SICI)1099-1654(199612)6:4<201::AID-RMV177>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Gosert R, Rinaldo CH, Funk GA, et al. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J Exp Med. 2008;205:841–52. doi: 10.1084/jem.20072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seif I, Khoury G, Dhar R. The genome of human papovavirus BKV. Cell. 1979;18:963–77. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- 32.Jin L, Gibson PE, Knowles WA, et al. BK virus antigenic variants: sequence analysis within the capsid VP1 epitope. J Med Virol. 1993;39:50–6. doi: 10.1002/jmv.1890390110. [DOI] [PubMed] [Google Scholar]
- 33.Jin L. Rapid genomic typing of BK virus directly from clinical specimens. Mol Cell Probes. 1993;7:331–4. doi: 10.1006/mcpr.1993.1047. [DOI] [PubMed] [Google Scholar]
- 34.Jin L, Gibson PE, Booth JC, et al. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol. 1993;41:11–7. doi: 10.1002/jmv.1890410104. [DOI] [PubMed] [Google Scholar]
- 35.Jin L, Pietropaolo V, Booth JC, et al. Prevalence and distribution of BK virus subtypes in healthy people and immunocompromised patients detected by PCR-restriction enzyme analysis. Clin Diagn Virol. 1995;3:285–95. doi: 10.1016/s0928-0197(94)00044-1. [DOI] [PubMed] [Google Scholar]
- 36.Baksh FK, Finkelstein SD, Swalsky PA, et al. Molecular genotyping of BK and JC viruses in human polyomavirus-associated interstitial nephritis after renal transplantation. Am J Kidney Dis. 2001;38:354–65. doi: 10.1053/ajkd.2001.26101. [DOI] [PubMed] [Google Scholar]
- 37.Takasaka T, Goya N, Tokumoto T, et al. Subtypes of BK virus prevalent in Japan and variation in their transcriptional control region. J Gen Virol. 2004;85:2821–7. doi: 10.1099/vir.0.80363-0. [DOI] [PubMed] [Google Scholar]
- 38.Krumbholz A, Zell R, Egerer R, et al. Prevalence of BK virus subtype I in Germany. J Med Virol. 2006;78:1588–98. doi: 10.1002/jmv.20743. [DOI] [PubMed] [Google Scholar]
- 39.Chen Q, Zheng HY, Zhong S, et al. Subtype IV of the BK polyomavirus is prevalent in East Asia. Arch Virol. 2006;151:2419–29. doi: 10.1007/s00705-006-0814-z. [DOI] [PubMed] [Google Scholar]
- 40.Nishimoto Y, Zheng HY, Zhong S, et al. An Asian origin for subtype IV BK virus based on phylogenetic analysis. J Mol Evol. 2007;65:103–11. doi: 10.1007/s00239-006-0269-6. [DOI] [PubMed] [Google Scholar]
- 41.Zhong S, Randhawa PS, Ikegaya H, et al. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J Gen Virol. 2009;90:144–52. doi: 10.1099/vir.0.83611-0. [DOI] [PubMed] [Google Scholar]
- 42.Ikegaya H, Saukko PJ, Tertti R, et al. Identification of a genomic subgroup of BK polyomavirus spread in European populations. J Gen Virol. 2006;87:3201–8. doi: 10.1099/vir.0.82266-0. [DOI] [PubMed] [Google Scholar]