ABSTRACT
Background: Clinical presentation in microscopic colitis (MC) is similar in many cases to that of diarrhea-predominent irritable bowel syndrome (IBS-D). The proper differential diagnosis requires total colonoscopy with multiple biopsies from normal-appearing mucosa and a detailed histopathological exam. Specific treatment may improve symptomatology.
Aim: To evaluate the prevalence of MC in patients with an initial diagnosis of IBS-D, to analyse demographic and clinical features of MC patients and to assess the efficacy of specific treatment.
Material and methods: Our retrospective study analyzed patients diagnosed with microscopic colitis in clinic during a three-year period. Diagnosis was established on histological exams of the samples obtained during colonoscopy in patients previously thought to have IBS-D. We evaluated clinical manifestations, time lapsed from their onset to definitive diagnosis, the association of MC with autoimmune diseases or with prior medication and the efficacy of treatment with budesonide or mesalazine.
Results: From 247 patients considered to have IBS-D, 15 patients (6.07%) had actually MC (13 lymphocytic colitis and 2 collagenous colitis). MC was associated with nonsteroidal antiinflammatory drugs (3 patients), Lansoprazole (2 patients) and autoimmune diseases (6 patients). Watery, non-bloody diarrhea was present in all patients with MC. Other frequent complaints were nocturnal diarrhea (11 patients), abdominal pain (8 patients), abdominal bloating and flatulence (8 patients) and slight weight loss (6 patients). The diagnostic samples were obtained from the right colon in 6 cases and from rectosigmoid or transverse colon in 9 patients. Treatment was initial symptomatic in all patients, but there were 5 patients that required mesalazine and/or Budesonide, with favourable outcome.
Conclusions: All the patients thought to have diarrhea-irritable bowel syndrome should be evaluated for microscopic colitis. Symptomatology is almost superimposable, but a few distinct features can be noticed. The proper and early diagnosis and the specific treatment may lead to significant clinical improvement in some difficult cases of the so-called "irritable bowel syndrome".
Keywords: irritable bowel syndrome, diarrhea-predominent irritable bowel syndrome, microscopic colitis, collagenous colitis, lymphocytic colitis, intraepithelial lymphocytes, thickened collagen band, budesonide, mesalazine
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders with a reported prevalence between 7 and 15% in the USA and between 2.5 and 22% in Europe. Its prevalence is greater in middle-age women and a decrease in reported frequency among older subject was noticed (1-4). This disorder is associated with a significantly impaired health-related quality of life (compared with healthy controls and patients with GERD, diabetes and end-stage renal disease), reduced work productivity and high health care costs (1,3,5,6).
Properly diagnosing IBS can be challenging and uncertain for many reasons: the absence of a clinical, biological or endoscopical marker of IBS, the difficulty to quantify objectively the symptoms reported by patients, the variations in symptoms between different persons and even in the same subject, the presence of many organic conditions that can mimic IBS (7-9).
One gastrointestinal disorder frequently misdiagnosed as irritable bowel syndrome is microscopic colitis (MC). Its incidence varies between 0.2 and 5.5/100.000 inhabitants/year and its prevalence between 10 and 16/100.000 inhabitants/year (10,11). MC is regarded as a common cause of chronic watery, non-bloody diarrhea, accounting for approximately 10% of patients presenting with this symptom (reaching 15-20% in elderly patients) (12-14).
The diagnosis of microscopic colitis is dependent on well-defined histological criteria in association with chronic watery, non-bloody diarrhea and normal macroscopic findings at endoscopy (15). Because of difficulties in histopathological assessment the diagnosis of MC may be missed in one third of cases in the primary examination (14).
Microscopic colitis may be associated with other autoimmune diseases or with administration of some drugs weeks or months before onset of the symptoms. A detailed medical history associated with multiple biopsies from normal intestinal mucosa taken throughout the colon is essential for a proper diagnosis (16-19).
Thus, differentiating patients with diarrhea-IBS from those with microscopic colitis can be challenging. Our study is therefore aimed to estimate the prevalence of microscopic colitis in patients diagnosed with IBS according to Rome III criteria and to evaluate epidemiological features, clinical manifestations and outcomes after proper treatment. ❑
MATERIALS AND METHODS
Our study was designed as a retrospective analysis of patients diagnosed with diarrhea-IBS (based on Rome III criteria) who underwent total colonoscopy and who were diagnosed with microscopic colitis in the Gastroenterology and Hepatology Centre, Fundeni Clinical Institute, during a three-year period (April 2007 – April 2010). Multiple biopsies from normal colonic mucosa were taken during colonoscopy and the diagnosis of microscopic colitis was established on histopathological features.
Other investigations performed in this subgroup of patients with diarrhea-IBS were upper endoscopy with jejune biopsy, microbiological and parasites stool examination, fecal calprotectin test and screening abdominal ultrasound. Those patients with organic colonic diseases detected by colonoscopy were excluded from our analysis.
The biopsy samples have been analyzed in detail by two experienced pathologists using special stains, including those for collagen. Diagnostic histopathological features for collagenous colitis were: subepithelial collagenous band thickness more than 10 µm, increased number of inflammatory cells in lamina propria, increased intraepithelial lymphocytes and possible surface epithelial damage. In lymphocytic colitis diagnostic criteria were: intraepithelial lymphocytes increased to more than 20 per 100 epithelial cells and inflammatory cells increased in lamina propria, associated with normal collagen band. Also in lymphocytic colitis injuries of superficial epithelium were identified, with flattening of epithelial cells and areas of detachment or absence of the epithelium.
In the group of patients diagnosed with microscopic colitis we took notice of the demographic features (age, sex), the smoking status, the subtype of MC (collagenous colitis or lymphocytic colitis), the presence of other autoimmune diseases and the prior usage of nonsteroidal antiinflammatory drugs (NSAIDs) or proton pump inhibitors. We tried to identify the most characteristic symptoms of the disease (diarrhea, nocturnal diarrhea, nausea, abdominal pain, abdominal bloating, flatulence, urgency, anal incontinence, weight loss) and to evaluate the severity of clinical manifestations.
We also reviewed the result of the fecal calprotectin test, the presence/absence of some minimal colonic mucosal changes (erythema, edema, small ulcers) and the part of the colon where from the diagnostic samples were obtained during colonoscopy. We were also interested in determining the period of time until the proper diagnosis and we evaluated the outcome after specific treatment when this was possible.
The study protocol was approved by the Fundeni Clinical Institute Ethics Committee. Due to the small number of patients included in our analysis statistical data are not relevant, but we hereby report one of the largest case series of microscopic colitis diagnosed in our country. ❑
RESULTS
During a three-year period, in our clinic, 314 patients diagnosed with diarrhea-IBS underwent total colonoscopy. By colonoscopy there were identified various colonic disorders such as inflammatory bowel diseases, colorectal cancer, colonic diverticula with mild diverticulitis or unspecified chronic inflammation of the colonic mucosa in 67 patients.
In 247 patients no significant macroscopic lesions were detected during colonoscopy and the prior diagnosis of diarrhea-IBS was confirmed. Multiple biopsies from normal mucosa were obtained and upper digestive endoscopy with jejune biopsy was performed in all these 247 patients. After a detailed histopathological examination of the samples, the diagnosis of microscopic colitis was established in 15 cases (6.07%): 13 cases of lymphocytic colitis (5.27%) and 2 cases of collagenous colitis (0.8%). 232 patients (93.93%) had normal histological examination and these were redefined as the IBS-D group (Figure 1).
Figure 1. Diagnostic steps from IBS-D to microscopic colitis.
The mean age of patients diagnosed with microscopic colitis was 45.93 ± 16.10 years (range 25-76) and the majority of patients were women (female/male = 2/1). Among the 15 patients with MC 7 patients were smokers. The duration of symptoms prior to histological diagnosis of microscopic colitis ranged between 3 and 94 months, with a mean of 28 months.
6 patients (40%) were diagnosed with concomitant autoimmune diseases: 3 patients with celiac disease, 2 patients with diabetes mellitus type I and 1 patient with rheumatoid arthritis. No case of autoimmune thyroiditis was detected among the patients with MC in our study. As concerns the history of drug consumption before the onset of MC symptoms we noticed NSAIDs usage for osteoarticular pains in 3 cases (20%) and proton pump inhibitors (Lansoprazole) usage for gastroesophageal reflux disease in 2 cases (13.33%). Fecal test for calprotectin was positive in 11 patients (73.33%). Evaluation of the patients with lymphocytic and collagenous colitis is summarized in Table 1.
Table 1.
Features of the patients with lymphocytic and collagenous colitis
| Lymphocytic colitis (n = 13) | Collagenous colitis (n = 2) | |
|---|---|---|
| Mean age (years) | 42.69 | 67 |
| Sex (female/male) | 8/5 | 2/0 |
| Smokers | 6 | 1 |
| Duration of symptoms (months), (mean, range) | 22 (3-94) | 34 (18-50) |
| Celiac disease | 2 | 1 |
| Diabetes mellitus type I | 2 | 0 |
| Rheumatoid arthritis | 1 | 0 |
| NSAIDs | 2 | 1 |
| PPI (Lansoprazole) | 2 | 0 |
| Fecal calprotectin test + | 9 | 2 |
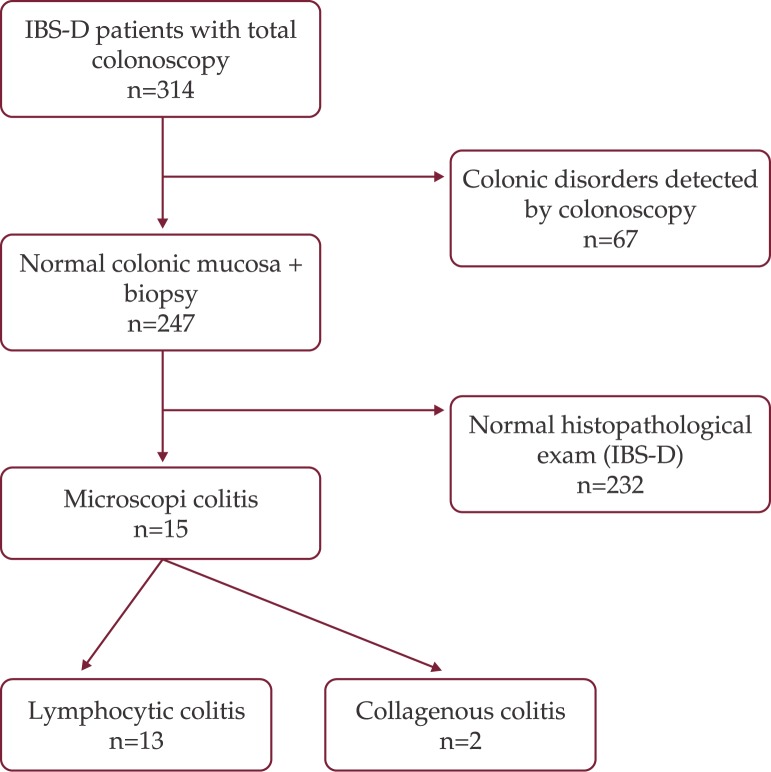
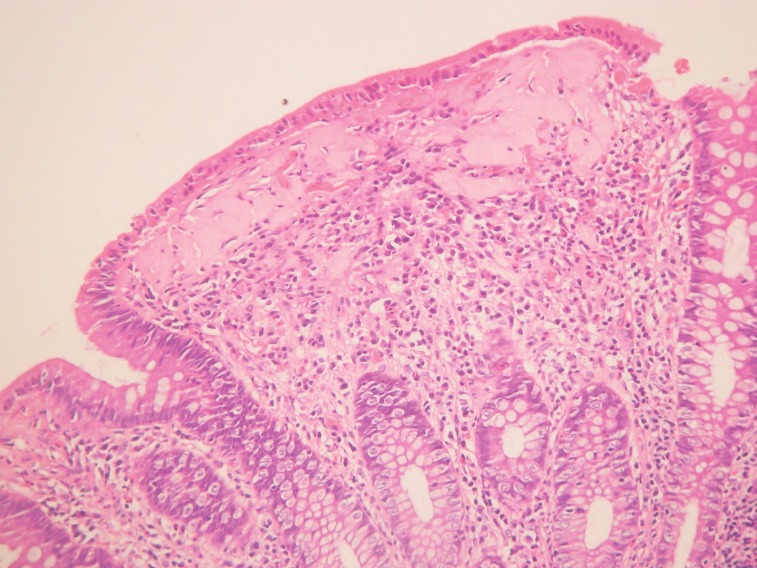
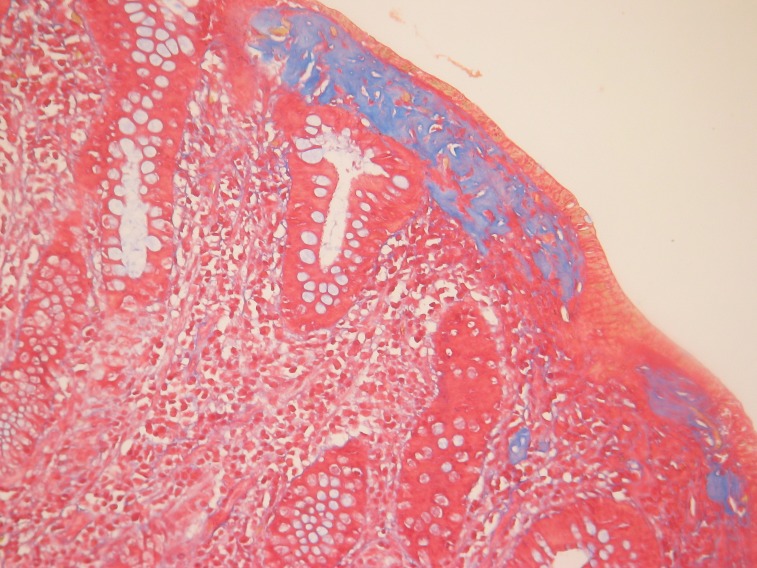
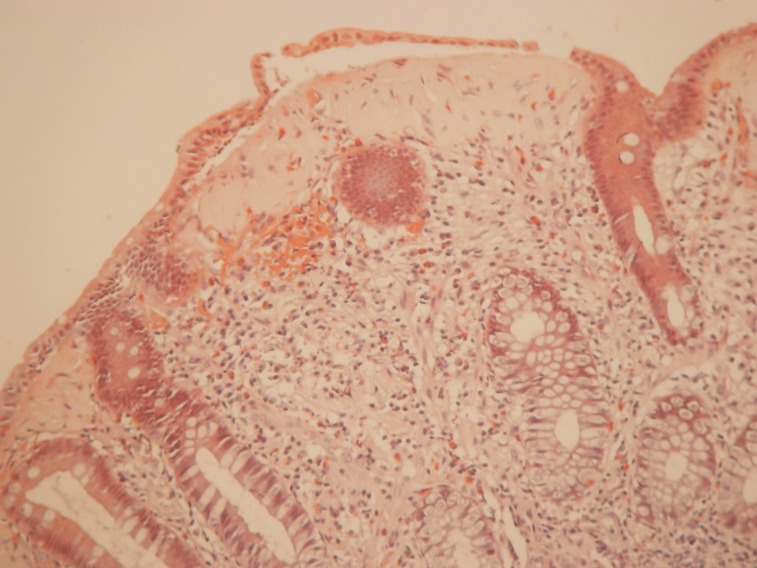
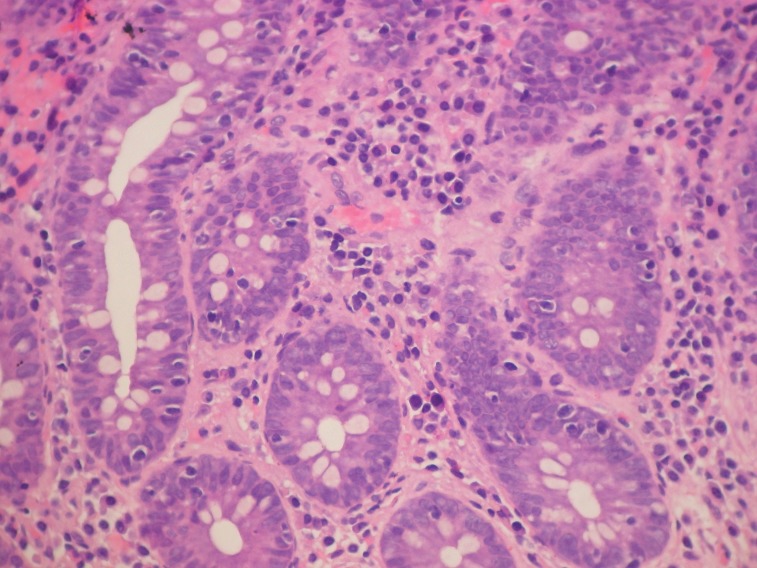
Pathology examination of the two cases of collagenous colitis showed mild to moderate chronic inflammation in the lamina propria and a thick collagen band, changes detected by H&E and Masson's trichrome staining (Figure 2 and 3). Surface epithelial damage with parts of detached epithelium was also detected by van Gieson staining in one case (Figure 4). In the lymphocytic colitis group the chronic inflammation in the lamina propria was moderate and 5 out of 13 cases showed surface epithelial damage. Intraepithelial lymphocytes were increased to more than 20 per 100 epithelial cells in all 13 cases; they were identified and counted with H&E staining (Figure 5).
Figure 2. Collagenous colitis. Thickened collagen band; H&E staining.
Figure 3. Collagenous colitis. Thickened collagen band; Masson≈s trichrome staining.
Figure 4. Collagenous colitis. Thickened collagen band and area of partially detached epithelium; van Gieson staining.
Figure 5. Lymphocytic colitis. Chronic inflammation in the lamina propria and increased intraepithelial lymphocytes; H&E staining.
In 12 patients with MC the colonoscopic examination was absolutely normal and in 3 patients minimal macroscopic changes such as erythema, edema or small and superficial ulcers of mucosa were seen. The diagnostic samples for microscopic colitis were obtained from the right colon (ascending colon or cecum) in 6 cases and from the rectosigmoid or transverse colon in 9 patients. A capsule endoscopy examination was performed in 3 patients, but findings were normal.
Diarrhea was the main symptom in all patients diagnosed with microscopic colitis. They had non-bloody, watery or loose stools. The mean stool frequency per day was 5 (range 3-12) and many patients (11 patients, 73.33%) had also nocturnal stools. Other common symptoms reported by patients were abdominal pain (8 patients, 53.33%), abdominal bloating and flatulence (8 patients, 53.33%) and slight weight loss (6 patients, 40%).
Correlating the type of the disease with some features of MC and IBS-D, we found that nocturnal diarrhea, slight weight loss and positive fecal calprotectin were predominant in the group of patients with MC (p <0.0001, p = 0.0005, p <0.0001), while abdominal pain and abdominal bloating were more frequent in patients with IBS-D (p = 0.0017, p = 0.022). Comparative data between MC and IBS-D patients are shown in Table 2.
Table 2.
Comparison between MC patients and IBS-D patients
| MC (n = 15) | IBS-D (n = 232) | p | |
|---|---|---|---|
| nocturnal diarrhea | 11 (73.33%) | 23 (9.91%) | p<0.0001 |
| abdominal pain | 8 (53.33%) | 204 (87.93%) | p=0.0017 |
| abdominal bloating ± flatulence | 8 (53.33%) | 186 (80.17%) | p=0.022 |
| slight weight loss | 6 (40%) | 15 (6.46%) | p=0.0005 |
| fecal calprotectin test + | 11 (73.33%) | 7 (3.01%) | p<0.0001 |
First line treatment in all 15 patients with MC was symptomatic medication (antidiarrheals and spasmolytic agents), associated, when necessary, with the avoidance of the potential colitis-inducing drugs. Despite this symptomatic therapy 5 patients had more complaints and they were further treated with 5-ASA agents – mesalazine (4 patients) and corticosteroids – budesonide (1 patient). 3 patients treated with mesalazine achieved complete remission, but 1 patient required further treatment with budesonide. In 4 patients symptomatic treatment proved its efficacy and there was not necessary another therapeutic approach. Unfortunately one patient died of other causes not related to gastrointestinal pathology and 5 patients were lost from follow-up. ❑
DISCUSSION
Diarrhea-predominant IBS is a relatively widespread gastrointestinal disorder, but because of the lack of patognomonic clinical features and of specific diagnostic methods it may be easily misdiagnosed. After successfully excluding the most frequent causes of chronic diarrhea (inflammatory bowel diseases, colorectal cancer, celiac disease, infectious or endocrine diarrhea, etc.) a number of rarely identified diseases can still remain undiagnosed under the assumption of an irritable bowel syndrome.
Thus, microscopic colitis represents a cause of chronic watery non-bloody diarrhea whose incidence seems to be rising in the last years. This fact may be explained by an increase in the awareness of physicians, as well as by a greater accuracy of diagnostic methods. The importance of recognizing this condition is crucial because chronic diarrhea is a debilitating illness and a proper treatment may help a lot of patients to return to normal life.
A study from Sweden on 1018 patients identified microscopic colitis in 9.5% of the patients who underwent colonoscopy for chronic watery, non-bloody diarrhea: 5% were diagnosed with collagenous colitis and 4.5% with lymphocytic colitis. The percentage increased to almost 20% if only patients older than 70 years referred for colonoscopy were considered (14). Another study on 30 patients previously diagnosed with IBS reported an incidence of lymphocytic colitis of 23.5% (20), while other authors found among IBS patients with diarrhea an incidence for collagenic colitis of 7.2% and for lymphocytic colitis of only 2.2% (21). Although the reported data about the incidence and the prevalence of microscopic colitis in IBS patients differ by large amounts, they emphasize the importance of performing complete colonoscopy and taking multiple biopsies from area of normal appearing mucosa in all patients diagnosed with diarrhea-predominant IBS.
In our analysis the frequency of microscopic colitis among IBS-D patients was around 6%: 5.27% for lymphocytic colitis and 0.8% for collagenous colitis. Due to the small number of patients diagnosed with microscopic colitis on histopathological criteria (n = 15), statistical interpretation was not significant, but demographic and clinical characteristics of our patients resemble the data reported by other studies.
The mean age of microscopic colitis patients in our group was 45.93 years, little less than in other cohorts, although there were papers reporting a mean age of about 40 years (21,22). The female/male ratio of 2/1 confirmed the female predominance characteristic for this disease. Although smoking was associated with collagenic colitis in a few studies (23), we found no obvious connection in our analysis: among the 7 smoking patients one was found to have collagenous colitis and the other 6 had lymphocytic colitis.
Microscopic colitis is known to have a fluctuating course, with periods of clinical activity and periods free of symptoms. The definitory symptom in MC patients is watery, non-bloody diarrhea, which can be continuous or intermittent. Indeed, all our patients diagnosed with MC in our hospital were referred for colonoscopy due to the presence of watery or loose stools. The presence of nocturnal diarrhea is usually a distinguishing feature that helps differentiating other diseases from IBS (24). The frequency of nocturnal stools in MC was reported between 30% and 37% in some studies (21,22). In our group of MC patient's nocturnal diarrhea was noticed in 73.33% of cases and this emphasizes the importance of this symptom in differentiating MC from IBS. Other frequent complaints were abdominal pain, abdominal bloating and/or flatulence and slight weight loss, all similar to the clinical presentation seen in other studies (21,22,25,26).
Duration of symptoms before diagnosis (mean duration = 28 months) was similar to those reported by other studies (22,26-28). It is important to emphasize that a large number of patients use to be referred to several centers before the diagnosis of microscopic colitis is made and that the delay is also related to previous colonoscopies in which a normal mucosa was found, but biopsies were not performed.
A correlation between MC and the use of specific drugs was reported, especially NSAIDs (up to 34%), proton pump inhibitors (Lansoprazole) and ticlopidine (21,25,29). Less frequently a connection between ranitidine, carbamazepine, sertraline, flutamide, carbose or simvastatin and microscopic colitis was described (10,15,30). In our analysis we found that 3 patients were treated with NSAIDs and 2 with Lansoprazole before the onset of diarrhea and this determined us to consider these drugs as causative agents for microscopic colitis. For this reason we think that a detailed medical history that includes the history of previous drug intake is essential for a proper diagnosis.
Autoimmune diseases are associated with microscopic colitis in up to 50% of cases, the most frequent being rheumatoid arthritis, celiac disease, thyroiditis and type I diabetes mellitus. Less frequent associations were found with pernicious anemia, CREST syndrome, scleroderma, Sjogren's syndrome, primary billiary cirrhosis (21,25,26). We identified in our group 6 cases of association between MC and autoimmune diseases (3 celiac disease, 2 diabetes mellitus, 1 rheumatoid arthritis) that represents 40%, data that are comparable to those from other studies.
It is known that lymphocytic colitis and collagenous colitis assume a patchy distribution and that topographic gradient of intraepithelial lymphocytes decreases from the right colon to the rectum (10,15,31). Therefore, representative biopsies should be taken from each part of the colon and submitted in separate containers. Indeed, in our study we observed that the diagnostic samples for microscopic colitis were obtained from the right colon in 6 cases and from the rectosigmoid or transverse colon in 9 patients. We can conclude that biopsies obtained only from the rectum are not enough for a proper diagnosis of microscopic colitis.
As concerns the treatment, budesonide appears to be the optimal drug choice nowadays. However, we had a gradual therapeutic approach in MC patients: withdrawal of possible colitis-inducing medication, symptomatic treatment (antidiarrheals, spasmolytic agents), followed by mesalazine and/or budesonide. This specific treatment (mesalazine and/or budesonide) was necessary in 5 patients with MC (33.33%) and proved good results. ❑
CONCLUSIONS
In conclusion, nocturnal diarrhea, slight weight loss and fecal calprotectin can be helpful in distinguishing microscopic colitis from diarrhea-predominant IBS, but a conclusive diagnosis of this disease can be attained only based on well-defined histological criteria. We therefore suggest that all patients previously thought to have IBS-D should undergo a total colonoscopy with multiple level biopsies from normal-appearing mucosa. Obtaining adequate biopsy specimens and an accurate histopathological examination are crucial for an early diagnosis of microscopic colitis. Specific treatment with budesonide or mesalazine should be applied whenever it is possible because it may lead to significant clinical improvement in those difficult to treat cases of "irritable bowel syndrome".
References
- 1.AGA technical review in irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 2.Khan S, Chang Li. Diagnostic and management of IBS. Nat Rev Gastroenterol Hepatol. 2010;7:565–81. doi: 10.1038/nrgastro.2010.137. [DOI] [PubMed] [Google Scholar]
- 3.Jha RK, Zou Y, Li J, et al. Irritable bowel syndrome (IBS) at a glance. BJMP. 2010;5:342–8. [Google Scholar]
- 4.WGO Global Guideline – IBS: a global perspective 2009 [Google Scholar]
- 5.El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a sistematic review. Aliment Pharmacol Ther. 2002;16:1171–856. doi: 10.1046/j.1365-2036.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- 6.Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–60. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 7.Gilkin RJ. The spectrum of irritable bowel syndrome: a clinical review. Clinical Therapeutics. 2005;27:1696–1709. doi: 10.1016/j.clinthera.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel BMR, Farid M, Esrailian E, et al. Is irritable bowel syndrome a diagnosis of exclusion?: a survey of primary care providers, gastroenterologists and IBS experts. Am J Gastroenterol. 2010;105:848–58. doi: 10.1038/ajg.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel B. Do physicians follow evidence-based guidelines in the diagnostic work-up of IBS? Nat Clin Pract Gastroenterol Hepatol. 2007;4:296–7. doi: 10.1038/ncpgasthep0820. [DOI] [PubMed] [Google Scholar]
- 10.Mulder CJJ, Harkema IM, Meijer JWR, et al. Microscopic colitis. Rom J Gastroenterology. 2004;13:113–7. [PubMed] [Google Scholar]
- 11.Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology. 2011;140:1155–65. doi: 10.1053/j.gastro.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese C, Gionchinetti P, Liguori G, et al. Clinical course of microscopic colitis in a single-center cohort study. JCC. 2011;5:218–21. doi: 10.1016/j.crohns.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Tysk C, Bohr J, Nyhlin N, et al. Diagnosis and management of microscopic colitis. World J Gastroenterol. 2008;14:7280–8. doi: 10.3748/wjg.14.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993-1998. Gut. 2004;53:346–50. doi: 10.1136/gut.2003.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed N, Marais M, Beyuidenhout J. Microscopic colitis as a missed cause of chronic diarrhea. World J Gastroenterol. 2011;17:1996–2002. doi: 10.3748/wjg.v17.i15.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen OH, Vainer B, Schaffalitzky de Muckadell OB. Microscopic colitis: a missed diagnosis? Lancet. 2004;364:2055–7. doi: 10.1016/S0140-6736(04)17518-1. [DOI] [PubMed] [Google Scholar]
- 17.Nyhlin N, Bohr J, Eriksson S, et al. Systematic review: microscopic colitis. Aliment Pharmacol Ther. 2006;23:1525–34. doi: 10.1111/j.1365-2036.2006.02913.x. [DOI] [PubMed] [Google Scholar]
- 18.Barto A, Heller S. Microscopic colitis: identical clinical presentations of subtypes create diagnostic challenges. Postgrad Med. 2002;112:69–75. doi: 10.3810/pgm.2002.11.1346. [DOI] [PubMed] [Google Scholar]
- 19.Tagkalidis P, Bhatal P, Gibson P. Microscopic colitis. J Gastroenterol Hepatol . 2002;17:236–48. doi: 10.1046/j.1440-1746.2002.02640.x. [DOI] [PubMed] [Google Scholar]
- 20.Tuncer C, Cindoruk M, Dursun A, et al. Prevalence of microscopic colitis in patients with symptoms suggesting irritable bowel syndrome. Acta Gastroenterol Belg. 2003;66:133–6. [PubMed] [Google Scholar]
- 21.Tavakkoli H, Esmaeili FS, Emami MH, et al. Is microscopic colitis a missed diagnosis in diarrhea-predominant irritable bowel syndrome? J of Research in Medical Sciences. 2008;13:202–6. [Google Scholar]
- 22.Calabrese C, Gionchetti P, Liguori G, et al. Clinical course of microscopic colitis in a single-center cohort study. JCC. 2011;5:218–21. doi: 10.1016/j.crohns.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Baert F, Wouters K, D'Haens G, et al. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut. 1999;45:375–81. doi: 10.1136/gut.45.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagkalidis P, Bhathal P, Gibson P. Microscopic colitis. J Gastroenterol Hepatol. 2002;17:236–48. doi: 10.1046/j.1440-1746.2002.02640.x. [DOI] [PubMed] [Google Scholar]
- 25.Chande N, Driman DK, Reynolds RP. Collagenous colitis and lymphocytic colitis: patient characteristics and clinical presentation. Scand J Gastroenterol. 2005;40:343–7. doi: 10.1080/00365520510011623. [DOI] [PubMed] [Google Scholar]
- 26.Barta Z, Mekkel G, Csipo I, et al. Microscopic colitis: A retrospective study of clinical presentation in 53 patients. World J Gastroenterol. 2005;11:1351–5. doi: 10.3748/wjg.v11.i9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chande N, MacDonald JK, McDonald JW. Interventions for treating microscopic colitis: a Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Review Group systematic review of randomized trials. Am J Gastroenterol. 2009;104:235–41. doi: 10.1038/ajg.2008.16. [DOI] [PubMed] [Google Scholar]
- 28.Bonderup OK, Folkersen BH, Gjersoe P, et al. Collagenous colitis: a long term follow-up study. Eur J Gastroenterol Hepatol. 1999;11:493–5. [PubMed] [Google Scholar]
- 29.Capurso G, Marignani M, Attilia F, et al. Lansoprazole-induced microscopic colitis: an increasing problem? Results of a prospective case-series and systematic review of the literature. Digestive and Liver Disease. 2011;43:380–5. doi: 10.1016/j.dld.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Carmack SW, Lash RH, Gulizia JM, et al. Lymphocytic disorders of thr gastrointstinal tract: a review for practicing pathologist. Adv Anat Pathol. 2009;16:290–306. doi: 10.1097/PAP.0b013e3181b5073a. [DOI] [PubMed] [Google Scholar]
- 31.Kirby JA, Bone M, Robertson H, et al. The number of intraepithelial T cells decreases from ascending colon to rectum. J Clin Pathol. 2003;56:158–158. doi: 10.1136/jcp.56.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]