Abstract
Isoprenylation is a posttranslational modification that is believed to be necessary, but not sufficient, for the efficient association of numerous eukaryotic cell proteins with membranes. Additional modifications have been shown to be required for proper intracellular targeting and function of certain isoprenylated proteins in mammalian and yeast cells. Although protein isoprenylation has been demonstrated in plants, postisoprenylation processing of plant proteins has not been described. Here we demonstrate that cultured tobacco (Nicotiana tabacum cv Bright Yellow-2) cells contain farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities with apparent Michaelis constants of 73 and 21 μm for N-acetyl-S-trans,trans-farnesyl-l-cysteine and N-acetyl-S-all-trans-geranylgeranyl-l-cysteine, respectively. Furthermore, competition analysis indicates that the same enzyme is responsible for both activities. These results suggest that α-carboxyl methylation is a step in the maturation of isoprenylated proteins in plants.
Isoprenoid protein modifications are necessary for the interaction of certain proteins with membranes and/or other proteins (Hancock et al., 1989, 1991; Glomset et al., 1990; Hwang and Lai, 1993; Kuroda et al., 1993; Marshall, 1993; Beranger et al., 1994; Kisselev et al., 1994; Porfiri et al., 1994). These modifications involve the formation of a thioether bond between a 15-carbon farnesyl or a 20-carbon geranylgeranyl moiety and a Cys residue at or near the carboxyl terminus of a protein. Only proteins bearing a recognition sequence at the carboxyl terminus for one of three isoprenyl:protein transferases are isoprenylated (for review, see Clarke, 1992; Randall and Crowell, 1997). These sequences are: CXXX, which is recognized by farnesyl:protein transferase; CXXL, which is recognized by geranylgeranyl:protein transferase type I; and XXCC, CCXX, XCXC, or XCCX, which is recognized by geranylgeranyl:protein transferase type II or Rab geranylgeranyl:protein transferase. In the above sequences, “C” represents Cys, “X” represents one of several possible amino acids, and “L” represents Leu.
Recent studies in mammalian and yeast systems suggest that protein isoprenylation is not sufficient for high-affinity protein-membrane or protein-protein interactions (Hancock et al., 1991; Volker et al., 1991b; Sapperstein et al., 1994; Marom et al., 1995). Most isoprenylated proteins (with the exception of certain Rab proteins bearing an XXCC carboxyl terminus; Wei et al., 1992) undergo further posttranslational modifications, including proteolytic removal of amino acids downstream of the isoprenylated Cys residue, α-carboxyl methylation of the prenylcysteine residue, and, in a few cases, fatty acid acylation of upstream Cys residues (Hancock et al., 1989; Clarke, 1992; Randall and Crowell, 1997). Proteolysis and α-carboxyl methylation of fungal mating pheromones (Marcus et al., 1991; Sapperstein et al., 1994; Boyartchuk et al., 1997), Ras proteins (Clarke et al., 1988; Gutierrez et al., 1989; Hancock et al., 1989; Fujiyama et al., 1991; Boyartchuk et al., 1997), Ras-related small G-proteins (Kawata et al., 1990; Huzoor-Akbar et al., 1991), heterotrimeric G-protein γ-subunits (Yamane et al., 1990; Fukada, 1995; Parish et al., 1995), nuclear lamin B (Vorburger et al., 1989), and retinal cGMP phosphodiesterase subunits (Ong et al., 1989) are dependent on previous protein isoprenylation.
A single methyltransferase catalyzes the S-adenosyl Met-dependent α-carboxyl methylation of farnesylated and geranylgeranylated proteins in mammalian and yeast cells, but no such activity has been detected in prokaryotes (Ota and Clarke, 1989; Hrycyna and Clarke, 1990; Stephenson and Clarke, 1990, 1992; Hrycyna et al., 1991; Pérez-Sala et al., 1991, 1992; Volker et al., 1991a; Pillinger et al., 1994; Sapperstein et al., 1994; Philips and Pillinger, 1995). This conclusion is based on the results of competition experiments using farnesylated and geranylgeranylated substrates and on analyses of yeast mutants (Volker et al., 1991a; Pérez-Sala et al., 1992). No protein determinants other than a carboxyl-terminal prenylcysteine residue appear to be required for recognition by the methyltransferase, because the enzyme very efficiently methylates prenylated Cys analogs, including AFC and AGGC, but not AGC (Tan et al., 1991; Shi and Rando, 1992; Ma et al., 1995).
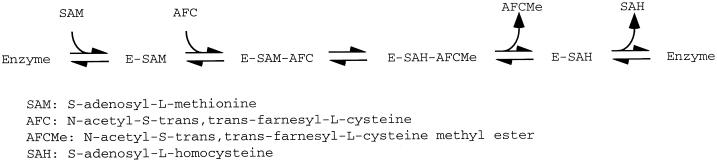
Prenylcysteine carboxyl methyltransferases are membrane-bound enzymes found in association with virtually all intracellular membranes, particularly plasma membrane in neutrophils (Pillinger et al., 1994) and the nuclear/ER membrane fraction in brain, liver, and heart (Stephenson and Clarke, 1990, 1992; Volker et al., 1991b, 1995). In general, they are phospholipid-dependent, detergent-sensitive enzymes that use an ordered Bi Bi reaction mechanism (Shi and Rando, 1992), as shown in Figure 1. In Saccharomyces cerevisiae, prenylcysteine α-carboxyl methyltransferase is encoded by the STE14 gene (Hrycyna and Clarke, 1990; Hrycyna et al., 1991; Sapperstein et al., 1994). The functionally homologous gene from Schizosaccharomyces pombe is called MAM4 (Imai et al., 1997). Yeast cells defective in the STE14 gene completely lack detectable prenylcysteine α-carboxyl methyltransferase activity and are viable but sterile, demonstrating that carboxyl methylation of mating pheromones is essential for mating. The STE14 gene encodes a polypeptide of 239 amino acids that is predicted to contain multiple membrane-spanning domains (Sapperstein et al., 1994).
Figure 1.
Reaction mechanism of prenylcysteine α-carboxyl methyltransferase (Shi and Rando, 1992).
Carboxyl methylation is essential for the membrane association and function of certain isoprenylated signal-transducing proteins. For example, proteolysis and carboxyl methylation have been shown to be required for efficient membrane binding of p21ras (Hancock et al., 1991; Marom et al., 1995). Furthermore, recent data on the effects of competitive inhibitors of prenylcysteine α-carboxyl methyltransferase (e.g. AFC), which have been shown to block Ras-dependent and G-protein-dependent signaling processes in a number of systems, support the notion that methylation is required for protein function. For example, prenylcysteine α-carboxyl methyltransferase inhibitors block Glc-induced insulin secretion in pancreatic islet cells (Li et al., 1996), Ras-dependent cell growth in Ha-Ras-transformed cells (Marom et al., 1995), chemoattractant-induced superoxide production in human neutrophils (Philips et al., 1993), chemotaxis in mouse peritoneal macrophages (Volker et al., 1991b), and agonist-mediated aggregation of human platelets (Huzoor-Akbar et al., 1993). Responses to downstream activators that bypass G-protein-dependent signal transduction (e.g. phorbol esters) are not affected by these inhibitors (Huzoor-Akbar et al., 1993; Philips et al., 1993), suggesting that G-protein function is impaired in the absence of prenylcysteine α-carboxyl methyltransferase activity. In some cases, methylation may be required for protein function, but not for membrane association. γ-Subunit carboxyl methylation was recently reported to be required for G-protein function, but not for βγ membrane association (Rosenberg et al., 1998).
Unlike isoprenylation, carboxyl methylation of prenylcysteine residues is a reversible modification and is therefore subject to regulation. Consistent with this hypothesis, a methyl ester hydrolase has been described in mammalian rod outer-segment membranes that specifically catalyzes the demethylation of carboxyl-methylated prenylcysteine residues (Pérez-Sala et al., 1991; Tan and Rando, 1992). Furthermore, receptor agonists and nonhydrolyzable analogs of GTP have been found to increase the carboxyl methylation of Ras-related small G-proteins without affecting prenylcysteine α-carboxyl methyltransferase activity (Huzoor-Akbar et al., 1991, 1993; Philips et al., 1993; Pillinger et al., 1994; Volker et al., 1995). This observation suggests that the GTP-bound state of these proteins may be more susceptible to carboxyl methylation. One possible explanation for this phenomenon is the GTP-dependent release of Ras-related small G-proteins from their respective GDP-dissociation inhibitors and subsequent translocation to intracellular membranes, where they would be expected to become readily available to the prenylcysteine α-carboxyl methyltransferase (Volker et al., 1995). Prenylcysteine α-carboxyl methyltransferase activity has been shown to be higher in certain tumor types than in normal cells, suggesting that tumorigenesis is associated with disregulation of this enzyme (Klein et al., 1994). These findings point to the possibility of regulated carboxyl methylation of prenylated proteins.
Protein isoprenylation has recently been described in plants (Randall et al., 1993; Swiezewska et al., 1993; Morehead et al., 1995; Randall and Crowell, 1997) and shown to be involved in cell-cycle control (Qian et al., 1996) and phytohormone signal transduction (Cutler et al., 1996). Additional studies have focused on the characterization of prenylated proteins in plants (Zhu et al., 1993; Biermann et al., 1994; Lin et al., 1996; Trainin et al., 1996) or plant prenyl:protein transferases (Randall et al., 1993; Yang et al., 1993; Loraine et al., 1996; Parmryd et al., 1996; Schmitt et al., 1996; Yalovsky et al., 1996, 1997). However, postisoprenylation processing of plant proteins has not been reported. The present study was undertaken to determine whether plants contain farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities and, if so, to determine whether the same enzyme catalyzes the methylation of these two prenylcysteine residues.
MATERIALS AND METHODS
Tobacco Tissue Culture
Suspension cultures of tobacco BY-2 cells (derived from Nicotiana tabacum cv Bright Yellow-2 callus) were used for all experiments (Nagata et al., 1992). Cultures were grown in Murashige-Skoog medium (Murashige and Skoog, 1962) containing 0.9 μm 2,4-D at 26°C ± 1°C in continuous fluorescent light. Cultures were propagated by transferring 3 mL of a 14-d-old culture into 30 mL of fresh medium.
Preparation of Tobacco Cell Membranes
To prepare tobacco cell membranes, cultures (usually 4-d-old cultures) were harvested by vacuum filtration and resuspended in 2× homogenization buffer (50 mm Hepes/Tris, pH 7.4, 500 mm mannitol, 6 mm EGTA, 5 mm DTT, 0.1 mg/mL aprotinin, 0.01 mg/mL leupeptin, and 1 mm PMSF) at 1 mL g−1 fresh weight at 4°C. Cells were ground in a mortar at 4°C and the resulting extract was passed through four layers of cheesecloth into 50-mL centrifuge bottles. Unbroken cells and large organelles were sedimented by centrifugation at 10,000g for 10 min at 4°C, after which membranes were sedimented from the extract by centrifugation at 100,000g for 1 h at 4°C. The membranes were resuspended in 1 volume of 2.5 mm Hepes, pH 7.4, 250 mm mannitol, 1 mm DTT, and stored in 100-μL aliquots at −80°C in the presence of 15% (w/v) glycerol.
In Vitro Carboxyl Methyltransferase Assays
Farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase assays were first performed as described previously (Hrycyna and Clarke, 1990) in the presence of up to 100 μg of tobacco membrane protein and 100 mm Hepes, pH 7.0 (50 μL total volume), except that S-adenosyl-l-[3H-methyl]Met (Amersham) was used as a methyl donor (24 μm, 2.5 Ci/mmol, 60 μCi/mL) and 200 μm AFC or 200 μm AGGC were used as methyl acceptors (200 μm AGC was used as a negative control). Stock solutions of AFC, AGGC, and AGC (BioMol, Plymouth Meeting, PA) were prepared in DMSO at a concentration of 10 mm. Reactions were incubated at 30°C for 1 h and then terminated by the addition of 50 μL of 1 m NaOH, 1% (w/v) SDS. Terminated reactions were immediately mixed and 50 μL was spotted onto a piece of pleated filter paper that was wedged in the neck of a counting vial above 3 mL of BioSafe II scintillation cocktail. Vials were capped and volatile radioactivity (i.e. [3H]methanol generated by base hydrolysis of methyl esters) was trapped for 2 h at room temperature and counted.
The assay described above generated a high background in the presence of tobacco membrane proteins. Consequently, the product detection portion of the assay was modified. Reactions were terminated by the addition of 50 μL of 90% (v/v) methylene chloride, 9.75% (v/v) methanol, and 0.25% (v/v) acetic acid, mixed extensively, centrifuged for 1 min in a microcentrifuge, and 10 μL of the organic phase was spotted onto a plastic-backed silica gel plate (Polygram Sil G, Aldrich). Plates were developed in 90% methylene chloride, 9.75% methanol, and 0.25% acetic acid, sprayed with En3hance fluorographic reagent (NEN/DuPont), and fluorography was performed for 3 d at −80°C using X-Omat AR film (Kodak). After alignment with the corresponding fluorogram, reaction products were cut out of the silica gel plate and quantitated by liquid scintillation.
Reaction products were identified by HPLC comigration with chemically synthesized standards (AFC and AGGC methyl esters) and by other criteria described in Discussion. HPLC conditions were as follows: A Microsorb-MV C18 column was first equilibrated in a 50% solvent A:50% solvent B mixture. Solvent A was 0.1% (v/v) trifluoroacetic acid in water; solvent B was 90% (v/v) acetonitrile, 9.9% (v/v) water, and 0.1% (v/v) trifluoroacetic acid. Samples dissolved in 50% acetonitrile:50% water were injected at a flow rate of 1 mL min−1; after 5 min a 50-min linear gradient was begun starting with a 50% solvent A:50% solvent B mixture and ending with 100% solvent B. Eluted products were detected by measuring A214 (chemical standards) or by liquid scintillation of 1-mL fractions (enzymatic products).
Synthesis of AFC and AGGC Methyl Esters
The methyl esters of AFC and AGGC were synthesized by reaction with (trimethylsilyl)diazomethane (Tan et al., 1991; Tan and Rando, 1992). (Trimethylsilyl)diazomethane (2 m in hexane, 5 equivalents) at 0°C was added to a solution of acid (5 μmol of AFC or 2 μmol of AGGC) in anhydrous tetrahydrofuran. The reaction mixture was stirred at 0°C to 5°C for 1 h, then directly passed through a small silica gel column. Elution with hexane yielded the methyl esters (78% AFC methyl ester and 72% AGGC methyl ester), which were analyzed by TLC, GC, 1H-NMR spectroscopy, and high-resolution MS as described below.
AFC Methyl Ester
RF (5% methanol/CH2Cl2) 0.37; GC (DB-5, 30 m, 300°C) 6.74 min; 1H-NMR spectroscopy (CDCl3, 300 mHz) 6.23 (d, J = 7.3 Hz, 1 H); 5.20 (t, J = 8.1 Hz, 1 H); 5.1 (t, J = 5.1 Hz, 2 H); 4.8 (m, 1 H); 3.77, 3.32 (2 s, 3 H), 3.16 (m, 2 H), 2.99 (dd, J = 13.9 Hz, J = 8.8 Hz, 1 H), 2.88 (dd, J = 13.2 Hz, J = 5.1 Hz, 1 H), 2.05 (s, 3 H), 2.10 to 1.95 (m, 8 H), 1.68 (s, 3 H), 1.66 (s, 3 H), 1.57 (s, 6 H); high-resolution MS (chemical ionization) 378.23508 (378.23395 calculated for C21H35SO3N).
AGGC Methyl Ester
RF (5% methanol/CH2Cl2) 0.40; 1H-NMR spectroscopy (CDCl3, 300 mHz) 6.22 (d, J = 7.3 Hz, 1 H), 5.20 (t, J = 7.3 Hz), 5.10 (m, 3 H), 4.81 (dt, J = 8.1 Hz, J = 5.9 Hz, 1 H), 3.77, 3.36 (2 s, 3 H), 3.16 (m, 2 H), 2.97 (dd, J = 13.9 Hz, J = 5.1 Hz, 1 H), 2.88 (dd, J = 13.2 Hz, J = 5.1 Hz, 1 H), 2.10 to 1.97 (m, 12 H), 2.05 (s, 3 H), 1.68 (s, 3 H), 1.66 (s, 3 H), 1.60 (s, 6 H), 1.57 (s, 3 H).
RESULTS
Prenylcysteine α-Carboxyl Methyltransferase Activity in Plants
To determine whether plants process isoprenylated Cys residues by carboxyl-terminal methylation, membrane preparations from tobacco BY-2 cells were assayed for the presence of farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities. The assay utilized S-adenosyl-l-[3H-methyl]Met as a methyl donor and AFC (200 μm) or AGGC (200 μm) as methyl acceptors in the presence of tobacco membranes. AGC (200 μm) was used as a control to ensure that product formation was dependent on the presence of a biologically relevant prenylcysteine substrate.
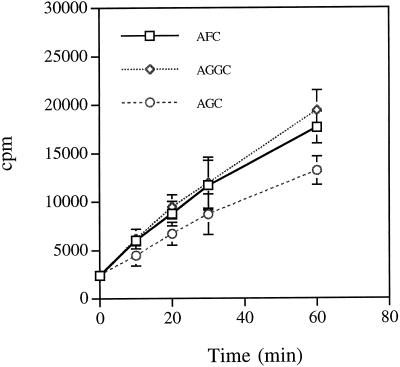
The formation of base-labile radioactivity was assayed as described previously (Hrycyna and Clarke, 1990). This assay is based on the release of volatile [3H]methanol from methyl ester products in the presence of 1 m NaOH. However, as shown in Figure 2, high background levels of base-labile radioactivity were detected by this method using tobacco membranes in the presence or absence of AGC. To alleviate this problem, a product-purification step was incorporated into the assay by extracting hydrophobic products into 90% methylene chloride:10% methanol under acidic conditions and resolving the organic-soluble material by silica gel TLC using acidic 90% methylene chloride:10% methanol as a mobile phase.
Figure 2.
Farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase assays on isolated membranes from cultured tobacco BY-2 cells. Assays were performed essentially as described by Hrycyna and Clarke (1990). Production of base-labile radioactivity was measured as a function of time in the presence of tobacco membranes, S-adenosyl-l-[3H-methyl]Met, and 200 μm AFC, AGGC, or AGC. The background in the absence of exogenous methyl acceptor was identical to that detected in the presence of 200 μm AGC (data not shown).
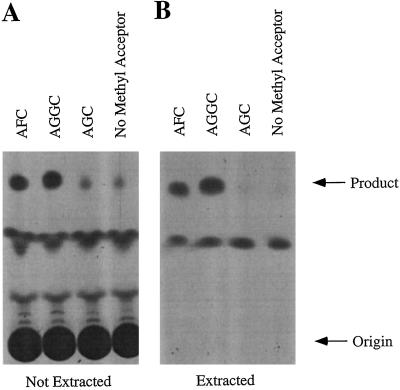
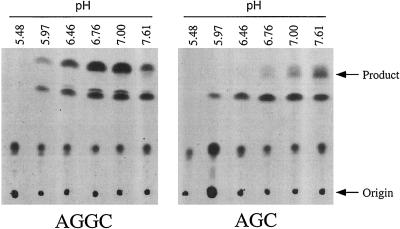
As shown in Figure 3, this modified assay provided clear evidence of methyltransferase activities in tobacco membranes that use AFC or AGGC but not AGC as methyl acceptors (no difference in product formation was observed in the presence or absence of AGC, suggesting that AGC is not a substrate of membrane-associated methyltransferases from tobacco). As expected, the radioactive materials labeled “Product” in Figure 3 were base labile (data not shown), suggesting that they represent the methyl esters of AFC or AGGC. Several other radioactive materials shown in Figure 3A were also base labile, indicating the presence of methyl esters, but none of these appeared to be dependent on the presence of a biologically relevant prenylcysteine substrate (i.e. these products formed in the presence or absence of AGC). The tobacco prenylcysteine α-carboxyl methyltransferase activity had a pH optimum near 7.0 (Fig. 4).
Figure 3.
Modified assay for tobacco farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase. Assays were performed in the presence of tobacco membranes, S-adenosyl-l-[3H-methyl]Met, and 200 μm AFC, AGGC, AGC, or no exogenous methyl acceptor. Assay mixtures were then resolved by silica gel TLC either before (A) or after (B) extraction into 90% methylene chloride, 9.75% methanol, and 0.25% acetic acid.
Figure 4.
pH optimum for tobacco prenylcysteine α-carboxyl methyltransferase. Assays were performed in the presence of 200 μm AGGC or AGC using the following buffers at 100 mm: sodium acetate at pH 5.48, sodium acetate at pH 5.97, sodium acetate at pH 6.46, Hepes at pH 6.76, Hepes at pH 7.00, and Hepes at pH 7.61.
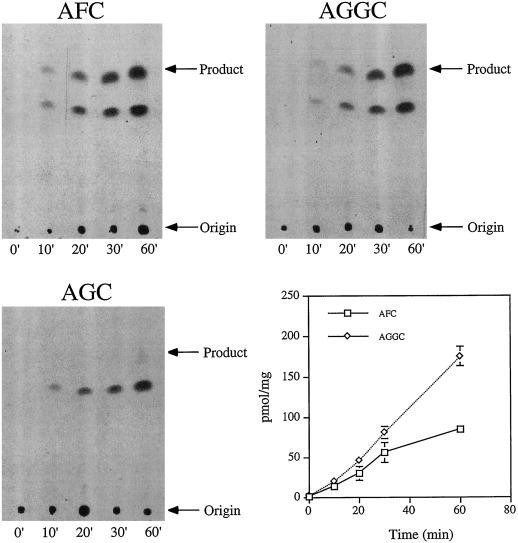
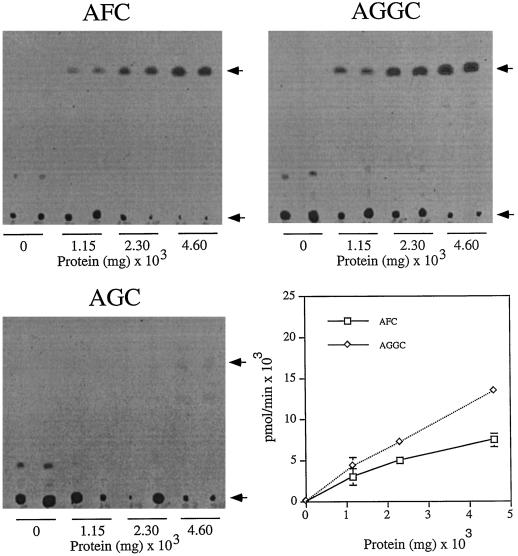
To demonstrate that the tobacco farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities were enzymatic, time-course and protein-dilution experiments were performed to ensure linear formation of product with time and tobacco protein (see Figs. 5 and 6). As shown in Figure 5, the formation of product was linear with time over a 60-min period at 30°C. Furthermore, as shown in Figure 6, product formation was linearly dependent on the amount of tobacco membrane protein in the assay. In both experiments product was formed in the presence of AFC and AGGC but not AGC.
Figure 5.
Time-dependent farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities. Product formation in the presence of 200 μm AFC, AGGC, or AGC was observed over a 60-min period at 30°C in 0.029 mg of tobacco membrane protein. Arrows indicate the positions of relevant methylated products and the origins at which samples were spotted before silica gel TLC. The plot shown was generated by subtracting the AGC background.
Figure 6.
Protein-dependent farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities. Product formation in the presence of 200 μm AFC, AGGC, or AGC was measured as a function of the amount of tobacco membrane protein in the 60-min assay at 30°C. Arrows indicate the positions of relevant methylated products and the origins at which samples were spotted before silica gel TLC. The plot shown was generated by subtracting the AGC background. The background is less obvious than in Figures 3–5 because the fluorograms shown represent relatively short exposures.
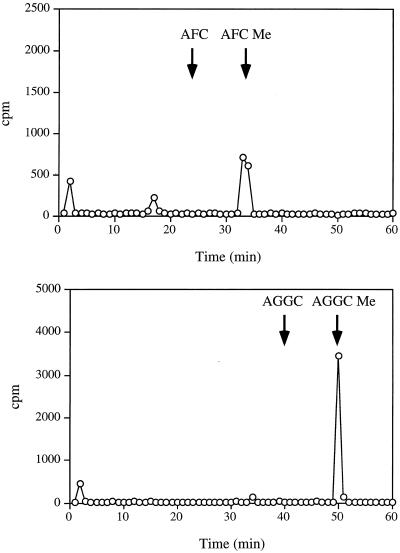
Given the data shown, the products shown in Figures 3–6 are likely to correspond to the α-carboxyl methyl esters of AFC and AGGC. These products form in the presence of AFC or AGGC but not AGC and possess base-labile methyl groups derived from S-adenosyl-l-[3H-methyl]Met. However, a more rigorous product identification was undertaken to rule out the possibility of nonrelevant methyl ester products. Accordingly, comigration of the radiolabeled enzymatic products with chemically synthesized AFC and AGGC methyl ester standards was demonstrated by HPLC (see Fig. 7). These results confirm the identities of the reaction products and therefore demonstrate the existence of bona fide farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities in cultured tobacco BY-2 cells.
Figure 7.
Comigration of reaction products with chemically synthesized AFC and AGGC methyl esters by HPLC. HPLC elution profiles are shown for tritiated reaction products generated in the presence of AFC (top) or AGGC (bottom). The positions of AFC, AFC methyl ester (AFC Me), AGGC, and AGGC methyl ester (AGGC Me) standards were determined by A214 and are indicated by arrows.
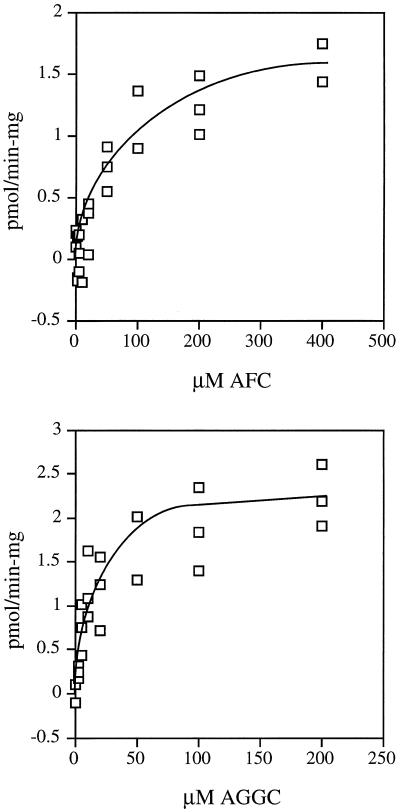
To characterize the tobacco farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities further, kinetic analyses were performed on BY-2 membrane preparations, and apparent Km and Vmax values were determined in the presence of AFC or AGGC. As shown in Figure 8, the apparent Km for AFC was 73 μm and the apparent Vmax in the presence of AFC was 1.7 pmol min−1 mg−1 tobacco membrane protein. In contrast, the apparent Km for AGGC was 21 μm and the apparent Vmax in the presence of AGGC was 2.7 pmol min−1 mg−1 tobacco membrane protein. These results are in reasonable agreement with published values for mammalian and yeast prenylcysteine α-carboxyl methyltransferase activities; however, the apparent Km values reported here are about 2-fold higher than published values. This difference is perhaps because of kinetic differences between the plant enzyme(s) and other prenylcysteine α-carboxyl methyltransferases or because of side reactions or incomplete partitioning that may reduce the availability of AFC and AGGC in the assay. In all known cases the Km for AFC has been found to be 2- to 3-fold higher than that for AGGC.
Figure 8.
Kinetic analyses of tobacco farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities. Activity curves are shown as a function of AFC (top) or AGGC (bottom) concentration. The plot shown was generated by subtracting the AGC background. Km and Vmax values were determined by Lineweaver-Burk analysis of the data (not shown).
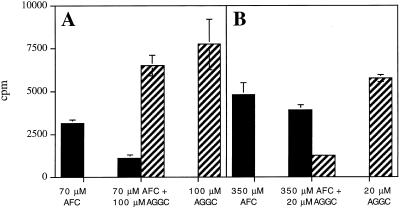
To determine whether the same enzyme was responsible for the observed farnesylcysteine and geranylgeranylcysteine α-carboxyl methyltransferase activities, competition analyses were performed. In the first experiment AFC was used at a concentration equal to its apparent Km and AGGC was used at a concentration equal to five times its apparent Km. As shown in Figure 9A, α-carboxyl methylation of AFC was greatly reduced when AFC and AGGC were mixed at these concentrations, subjected to in vitro α-carboxyl methylation in the presence of tobacco membranes, and analyzed by HPLC (the products of each reaction were resolved by HPLC and quantitated by liquid scintillation of collected fractions because the TLC system shown in Figs. 3–6 did not discriminate effectively between AFC and AGGC methyl ester formation). In the second experiment AFC was used at a concentration equal to five times its apparent Km and AGGC was used at a concentration equal to its apparent Km. As shown in Figure 9B, α-carboxyl methylation of AGGC was dramatically reduced under these reaction conditions. These results suggest that AFC and AGGC compete with one another, and are consistent with the hypothesis that the same enzyme accounts for tobacco farnesylcysteine and geranylgeranylcysteine αcarboxyl methyltransferase activities.
Figure 9.
Competition analyses suggest that AFC and AGGC are α-carboxyl methylated by the same enzyme. Prenylcysteine α-carboxyl methyltransferase assays were performed in the presence of AFC, AGGC, or both at the concentrations indicated below the graph. In A, AFC was used at its apparent Km and AGGC at five times its apparent Km. In B, AFC was used at five times its apparent Km and AGGC at its apparent Km. Samples were analyzed by quantitative HPLC. The black bars represent AFC α-carboxyl methylation and the striped bars represent AGGC α-carboxyl methylation.
DISCUSSION
Protein isoprenylation has been shown to be involved in cell-cycle progression in synchronized cultures of tobacco BY-2 cells (Qian et al., 1996). Furthermore, mutations in a farnesyl:protein transferase β-subunit gene have been shown to cause an enhanced response to exogenous ABA in Arabidopsis, suggesting possible farnesylation of a negative regulator of ABA responsiveness (Cutler et al., 1996). Although these exciting discoveries implicate protein isoprenylation in a variety of fundamental processes in plant growth and development, the precise role of protein isoprenylation and subsequent modifications in the targeting and function of the relevant proteins remains a mystery. Consistent with the hypothesis that isoprenylated plant proteins undergo further processing, we have demonstrated that plant cells contain a prenylcysteine α-carboxyl methyltransferase capable of catalyzing the methylation of farnesylated and geranylgeranylated Cys residues.
The formation of AFC and AGGC methyl esters in the presence of tobacco membranes provides compelling evidence for prenylcysteine α-carboxyl methyltransferase in plants. The identification of the methyl esters was based on: (a) the knowledge that S-adenosyl-l-[3H-methyl]Met was used as a methyl donor, (b) the observation that base hydrolysis of the methyl esters released volatile radioactivity, and (c) the observed HPLC comigration of the methyl esters with authentic AFC and AGGC methyl ester standards. These methyl ester products formed in the presence of AFC or AGGC but not AGC, suggesting that the enzyme responsible recognizes only biologically relevant prenylcysteine residues. Data from competition experiments suggest that the same enzyme catalyzes the α-carboxyl methylation of both AFC and AGGC, and kinetic analyses suggest that this enzyme uses AFC with an apparent Km of 73 μm, whereas AGGC is used with an apparent Km of 21 μm. These Km values are approximately 2-fold higher than published values for mammalian and yeast prenylcysteine α-carboxyl methyltransferase activities, suggesting that the plant enzyme may exhibit somewhat different kinetics. However, this question remains open, because kinetic analyses have not been done on pure preparations of prenylcysteine α-carboxyl methyltransferase from any source, presumably because of the detergent-sensitive nature of the enzyme and the difficulty of purifying it to homogeneity.
The functional significance of prenylcysteine α-carboxyl methyltransferase in the targeting and function of isoprenylated plant proteins remains to be explored. It seems likely, given what is known in mammalian and yeast cells, that α-carboxyl methylation will be found to be necessary for the membrane association of some, but not all, isoprenylated plant proteins. In some cases α-carboxyl methylation may be found to be required for protein function. Careful use of AFC and AGGC as competitive inhibitors of prenylcysteine α-carboxyl methyltransferase in vivo will shed light on these important issues, provided that the effects caused by inhibition of methyltransferase activity can be distinguished from the effects caused by possible competition for binding of isoprenylated proteins in vivo or inhibition of protein isoprenylation. These studies will provide new insights into the role of isoprenylation and methylation in protein targeting and function in plants.
ACKNOWLEDGMENT
The authors are indebted to Steven Roach (Department of Chemistry, Indiana University-Purdue University, Indianapolis), who participated in many helpful discussions.
Abbreviations:
- AFC
N-acetyl-S-trans,trans-farnesyl-l-Cys
- AGC
N-acetyl-S-trans-geranyl-l-Cys
- AGGC
N-acetyl-S-all-trans-geranylgeranyl-l-Cys
Footnotes
This work was supported by National Science Foundation grant no. MCB-9601064.
LITERATURE CITED
- Beranger F, Cadwallader K, Porfiri E, Powers S, Evans T, de Gunzberg J, Hancock JF. Determination of structural requirements for the interaction of Rab6 with RabGDI and Rab geranylgeranyltransferase. J Biol Chem. 1994;269:13637–13643. [PubMed] [Google Scholar]
- Biermann BJ, Morehead TA, Tate SE, Price JR, Randall SK, Crowell DN. Novel isoprenylated proteins identified by an expression library screen. J Biol Chem. 1994;269:25251–25254. [PubMed] [Google Scholar]
- Boyartchuk VL, Ashby MN, Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–1800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Clarke S, Vogel JP, Deschenes RJ, Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci USA. 1988;85:4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- Fujiyama A, Tsunasawa S, Tamanoi F, Sakiyama F. S-Farnesylation and methyl esterification of C-terminal domain of yeast RAS2 protein prior to fatty acid acylation. J Biol Chem. 1991;266:17926–17931. [PubMed] [Google Scholar]
- Fukada Y. Prenylation and carboxylmethylation of G-protein γ subunit. Methods Enzymol. 1995;250:91–105. doi: 10.1016/0076-6879(95)50065-0. [DOI] [PubMed] [Google Scholar]
- Glomset JA, Gelb MH, Farnsworth CC. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990;15:139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Magee AI, Marshall CJ, Hancock JF. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989;8:1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Marshall CJ. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B) EMBO J. 1991;10:641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hrycyna CA, Clarke S. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:5071–5076. doi: 10.1128/mcb.10.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycyna CA, Sapperstein SK, Clarke S, Michaelis S. The Saccharomyces cerevisiae STE14 gene encodes a methyltransferase that mediates C-terminal methylation of a-factor and RAS proteins. EMBO J. 1991;10:1699–1709. doi: 10.1002/j.1460-2075.1991.tb07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzoor-Akbar, Wang W, Kornhauser R, Volker C, Stock JB. Protein prenylcysteine analog inhibits agonist-receptor-mediated signal transduction in human platelets. Proc Natl Acad Sci USA. 1993;90:868–872. doi: 10.1073/pnas.90.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzoor-Akbar, Winegar DA, Lapetina EG. Carboxyl methylation of platelet rap1 proteins is stimulated by guanosine 5′-(3-O-thio)triphosphate. J Biol Chem. 1991;266:4387–4391. [PubMed] [Google Scholar]
- Hwang SB, Lai MMC. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J Virol. 1993;67:7659–7662. doi: 10.1128/jvi.67.12.7659-7662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Davey J, Kawagishi-Kobayashi M, Yamamoto M. Genes encoding farnesyl cysteine carboxyl methyltransferase in Schizosaccharomyces pombe and Xenopus laevis. Mol Cell Biol. 1997;17:1543–1551. doi: 10.1128/mcb.17.3.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M, Farnsworth CC, Yoshida Y, Gelb MH, Glomset JA, Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci USA. 1990;87:8960–8964. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev OG, Ermolaeva MV, Gautam N. A farnesylated domain in the G protein γ subunit is a specific determinant of receptor coupling. J Biol Chem. 1994;269:21399–21402. [PubMed] [Google Scholar]
- Klein Z, Ben-Baruch G, Marciano D, Solomon R, Altaras M, Kloog Y. Characterization of the prenylated protein methyltransferase in human endometrial carcinoma. Biochim Biophys Acta. 1994;1226:330–336. doi: 10.1016/0925-4439(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Suzuki N, Kataoka T. The effect of posttranslational modifications on the interaction of Ras2 with adenyl cyclase. Science. 1993;259:683–686. doi: 10.1126/science.8430318. [DOI] [PubMed] [Google Scholar]
- Li G, Kowluru A, Metz SA. Characterization of prenylcysteine methyltransferase in insulin-secreting cells. Biochem J. 1996;316:345–351. doi: 10.1042/bj3160345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang Y, Zhu J-k, Yang Z. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell. 1996;8:293–303. doi: 10.1105/tpc.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loraine AE, Yalovsky S, Fabry S, Gruissem W. Tomato Rab1A homologs as molecular tools for studying Rab geranylgeranyl transferase in plant cells. Plant Physiol. 1996;110:1337–1347. doi: 10.1104/pp.110.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-T, Gilbert BA, Rando RR. Farnesylcysteine analogs to probe role of prenylated protein methyltransferase. Methods Enzymol. 1995;250:226–234. doi: 10.1016/0076-6879(95)50075-8. [DOI] [PubMed] [Google Scholar]
- Marcus S, Caldwell GA, Miller D, Xue C-B, Naider F, Becker JM. Significance of C-terminal cysteine modifications to the biological activity of the Saccharomyces cerevisiae a-factor mating pheromone. Mol Cell Biol. 1991;11:3603–3612. doi: 10.1128/mcb.11.7.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom M, Haklai R, Ben-Baruch G, Marciano D, Egozi Y, Kloog Y. Selective inhibition of Ras-dependent cell growth by farnesylthiosalicylic acid. J Biol Chem. 1995;270:22263–22270. doi: 10.1074/jbc.270.38.22263. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Protein prenylation: a mediator of protein-protein interactions. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- Morehead TA, Biermann BJ, Crowell DN, Randall SK. Changes in protein isoprenylation during the growth of suspension-cultured tobacco cells. Plant Physiol. 1995;109:277–284. doi: 10.1104/pp.109.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol. 1992;132:1–30. [Google Scholar]
- Ong OC, Ota IM, Clarke S, Fung BK-K. The membrane binding domain of rod cGMP phosphodiesterase is posttranslationally modified by methyl esterification at a C-terminal cysteine. Proc Natl Acad Sci USA. 1989;86:9238–9242. doi: 10.1073/pnas.86.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota IM, Clarke S. Enzymatic methylation of 23–29-kDa bovine retinal rod outer segment membrane proteins: evidence for methyl ester formation at carboxyl-terminal cystenyl residues. J Biol Chem. 1989;264:12879–12884. [PubMed] [Google Scholar]
- Parish CA, Smrcka AV, Rando RR. Functional significance of βγ-subunit carboxymethylation for the activation of phospholipase C and phosphoinositide 3-kinase. Biochemistry. 1995;34:7722–7727. doi: 10.1021/bi00023a019. [DOI] [PubMed] [Google Scholar]
- Parmryd I, Shipton CA, Swiezewska E, Andersson B, Dallner G. Identification of spinach farnesyl protein transferase: dithiothreitol as an acceptor in vitro. Eur J Biochem. 1996;234:723–731. doi: 10.1111/j.1432-1033.1995.723_a.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Sala D, Gilbert BA, Tan EW, Rando RR. Prenylated protein methyltransferases do not distinguish between farnesylated and geranylgeranylated substrates. Biochem J. 1992;284:835–840. doi: 10.1042/bj2840835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Sala D, Tan EW, Cañada FJ, Rando RR. Methylation and demethylation reactions of guanine nucleotide-binding proteins of retinal rod outer segments. Proc Natl Acad Sci USA. 1991;88:3043–3046. doi: 10.1073/pnas.88.8.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips MR, Pillinger MH. Prenylcysteine-directed carboxyl methyltransferase activity in human neutrophil membranes. Methods Enzymol. 1995;256:49–63. doi: 10.1016/0076-6879(95)56009-2. [DOI] [PubMed] [Google Scholar]
- Philips MR, Pillinger MH, Staud R, Volker C, Rosenfeld MG, Weissmann G, Stock JB. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science. 1993;259:977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- Pillinger MH, Volker C, Stock JB, Weissmann G, Philips MR. Characterization of a plasma membrane-associated prenylcysteine-directed α carboxyl methyltransferase in human neutrophils. J Biol Chem. 1994;269:1486–1492. [PubMed] [Google Scholar]
- Porfiri E, Evans T, Chardin P, Hancock JF. Prenylation of Ras proteins is required for efficient hSOS1-promoted guanine nucleotide exchange. J Biol Chem. 1994;269:22672–22677. [PubMed] [Google Scholar]
- Qian D, Zhou D, Ju R, Cramer CL, Yang Z. Protein farnesyltransferase in plants: molecular characterization and involvement in cell cycle control. Plant Cell. 1996;8:2381–2394. doi: 10.1105/tpc.8.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall SK, Crowell DN (1997) Protein isoprenylation in plants. In EJ Parish, WD Nes, eds, Biochemistry and Function of Sterols. CRC Press, New York, pp 231–244
- Randall SK, Marshall MS, Crowell DN. Protein isoprenylation in suspension-cultured tobacco cells. Plant Cell. 1993;5:433–442. doi: 10.1105/tpc.5.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SJ, Rane MJ, Dean WL, Corpier CL, Hoffman JL, McLeish KR. Effect of gamma subunit carboxyl methylation on the interaction of G protein alpha subunits with betagamma subunits of defined composition. Cell Signal. 1998;10:131–136. doi: 10.1016/s0898-6568(97)00117-4. [DOI] [PubMed] [Google Scholar]
- Sapperstein S, Berkower C, Michaelis S. Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol Cell Biol. 1994;14:1438–1449. doi: 10.1128/mcb.14.2.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt D, Callan K, Gruissem W. Molecular and biochemical characterization of tomato farnesyl-protein transferase. Plant Physiol. 1996;112:767–777. doi: 10.1104/pp.112.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-Q, Rando RR. Kinetic mechanism of isoprenylated protein methyltransferase. J Biol Chem. 1992;267:9547–9551. [PubMed] [Google Scholar]
- Stephenson RC, Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990;265:16248–16254. [PubMed] [Google Scholar]
- Stephenson RC, Clarke S. Characterization of a rat liver protein carboxyl methyltransferase involved in the maturation of proteins with the -CXXX C-terminal sequence motif. J Biol Chem. 1992;267:13314–13319. [PubMed] [Google Scholar]
- Swiezewska E, Thelin A, Dallner G, Andersson B, Ernster L. Occurrence of prenylated proteins in plant cells. Biochem Biophys Res Commun. 1993;192:161–166. doi: 10.1006/bbrc.1993.1395. [DOI] [PubMed] [Google Scholar]
- Tan EW, Pérez-Sala D, Cañada FJ, Rando RR. Identifying the recognition unit for G protein methylation. J Biol Chem. 1991;266:10719–10722. [PubMed] [Google Scholar]
- Tan EW, Rando RR. Identification of an isoprenylated cysteine methyl ester hydrolase activity in bovine rod outer segment membranes. Biochemistry. 1992;31:5572–5578. doi: 10.1021/bi00139a021. [DOI] [PubMed] [Google Scholar]
- Trainin T, Shmuel M, Delmer DP. In vitro prenylation of the small GTPase Rac13 of cotton. Plant Physiol. 1996;112:1491–1497. doi: 10.1104/pp.112.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker C, Lane P, Kwee C, Johnson M, Stock J. A single activity carboxyl methylates both farnesyl and geranylgeranyl cysteine residues. FEBS Lett. 1991a;295:189–194. doi: 10.1016/0014-5793(91)81415-5. [DOI] [PubMed] [Google Scholar]
- Volker C, Miller RA, McCleary WR, Rao A, Poenie M, Backer JM, Stock JB. Effects of farnesylcysteine analogs on protein carboxyl methylation and signal transduction. J Biol Chem. 1991b;266:21515–21522. [PubMed] [Google Scholar]
- Volker C, Pillinger MH, Philips MR, Stock JB. Prenylcysteine analogs to study function of carboxylmethylation in signal transduction. Methods Enzymol. 1995;250:216–225. doi: 10.1016/0076-6879(95)50074-x. [DOI] [PubMed] [Google Scholar]
- Vorburger K, Kitten GT, Nigg EA. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J. 1989;8:4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Lutz R, Sinensky M, Macara IG. p23rab2, a ras-like GTPase with a -GGGCC C-terminus, is isoprenylated but not detectably carboxymethylated in NIH3T3 cells. Oncogene. 1992;7:467–473. [PubMed] [Google Scholar]
- Yalovsky S, Loraine AE, Gruissem W. Specific prenylation of tomato Rab proteins by geranylgeranyl type-II transferase requires a conserved cysteine-cysteine motif. Plant Physiol. 1996;110:1349–1359. doi: 10.1104/pp.110.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Trueblood CE, Callan KL, Narita JO, Jenkins SM, Rine J, Gruissem W. Plant farnesyltransferase can restore yeast Ras signaling and mating. Mol Cell Biol. 1997;17:1986–1994. doi: 10.1128/mcb.17.4.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth HKCC, Xie H, Howald W, Fung B, Yamane K-K, Clarke S, Gelb MH, Glomset JA. Brain G protein γ subunits contain an all-trans-geranylgeranyl-cysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci USA. 1990;87:5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Cramer CL, Watson JC. Protein farnesyltransferase in plants. Molecular cloning and expression of a homolog of the β subunit from the garden pea. Plant Physiol. 1993;101:667–674. doi: 10.1104/pp.101.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K, Bressan RA, Hasegawa PM. Isoprenylation of the plant molecular chaperone ANJ1 facilitates membrane association and function at high temperature. Proc Natl Acad Sci USA. 1993;90:8557–8561. doi: 10.1073/pnas.90.18.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]