Abstract
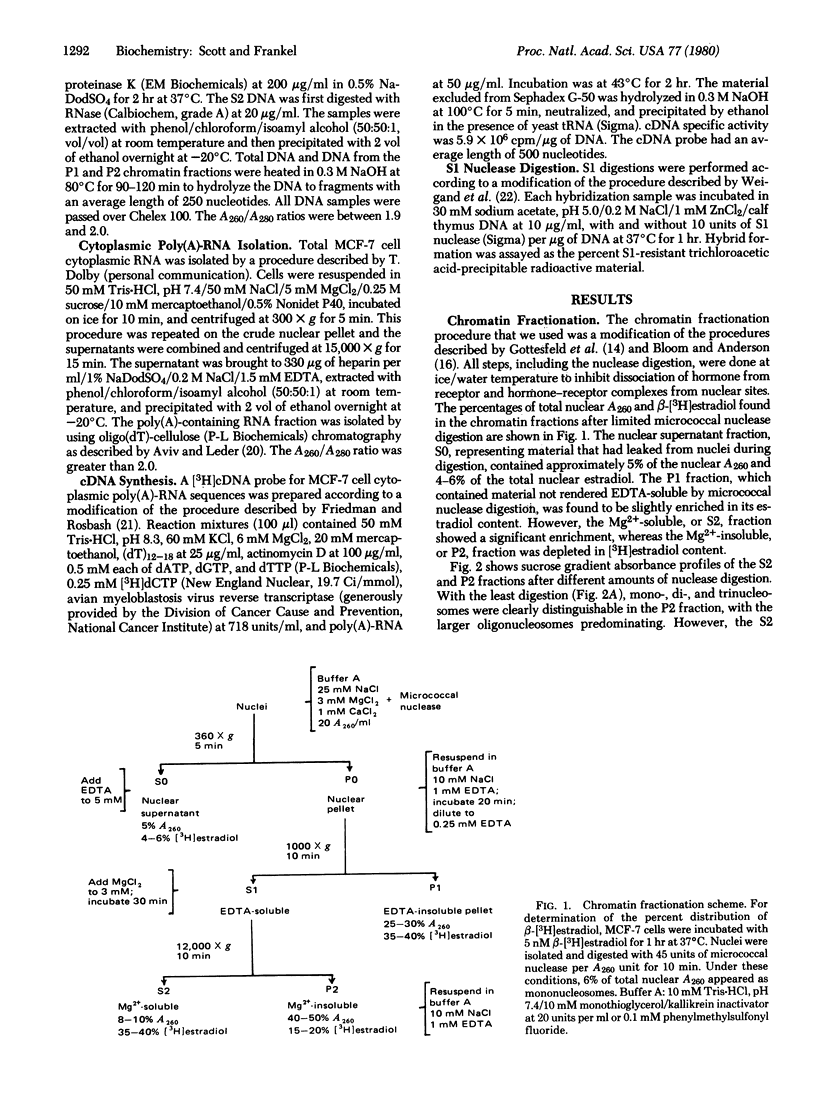
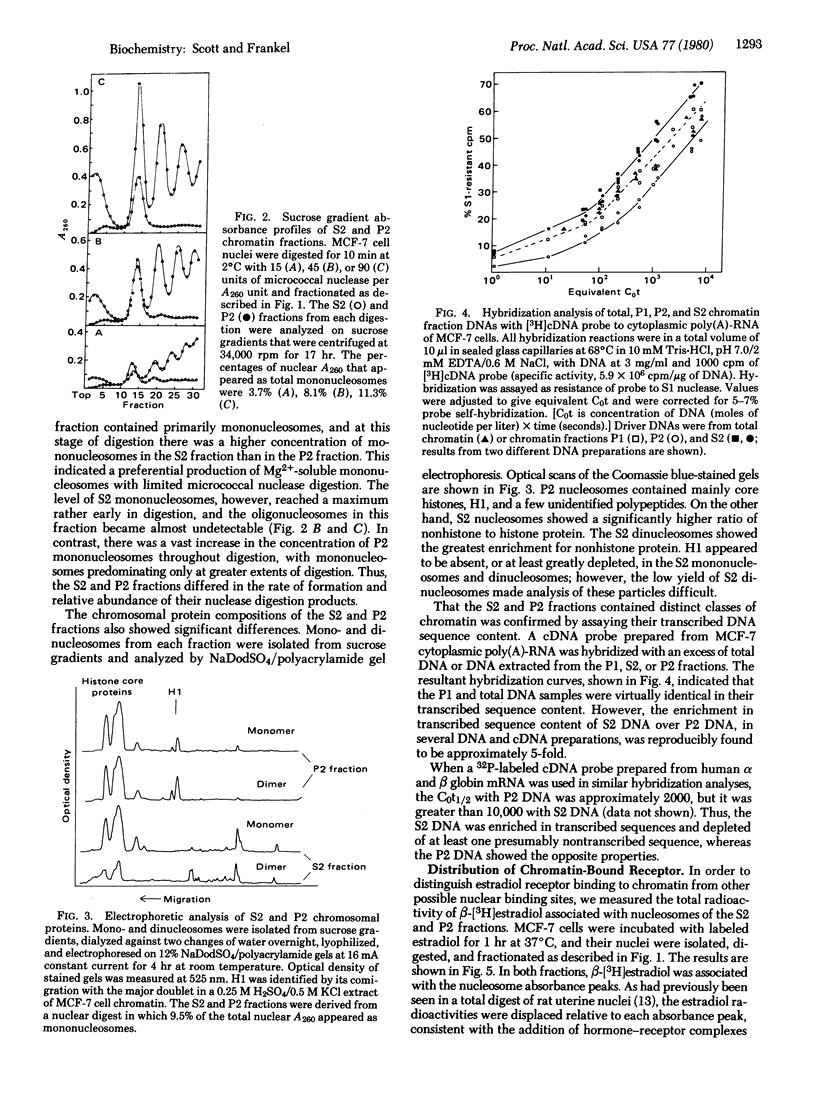
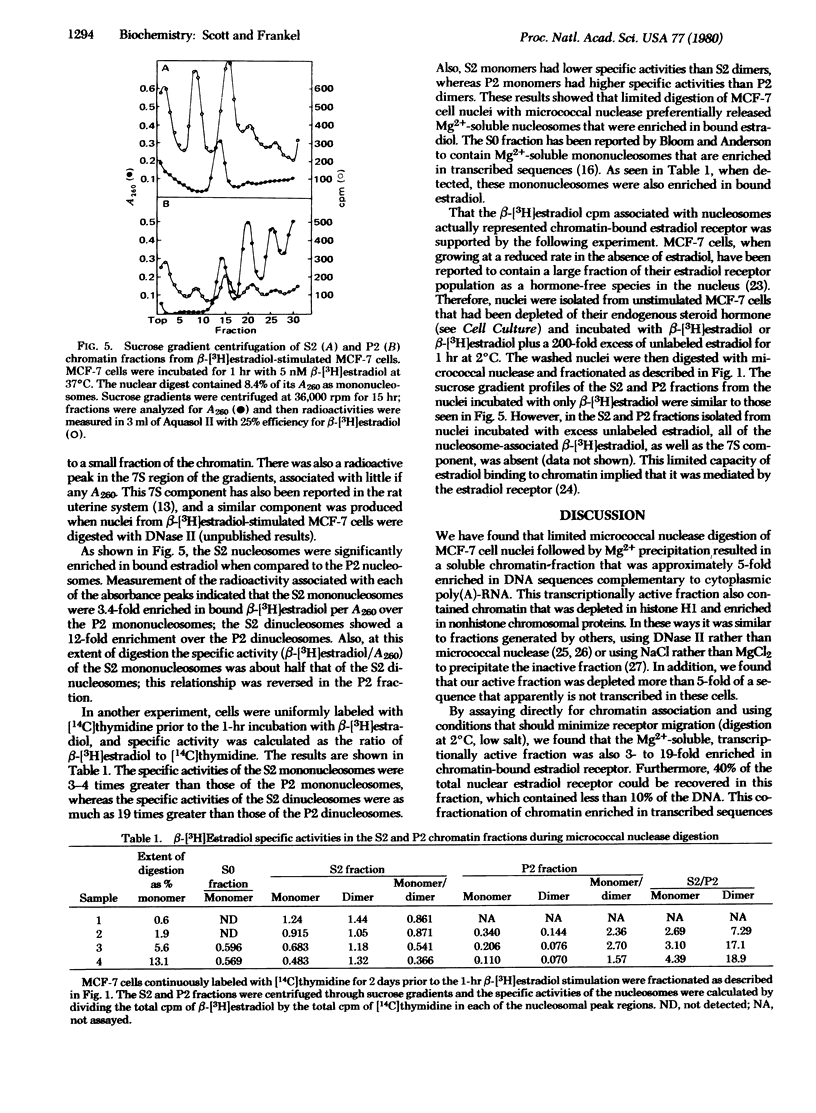
We have examined the interaction of the estradiol receptor molecule with chromatin in MCF-7 cells, a human breast tumor cell line responsive to estradiol. Receptor was found associated with the various nucleosomal products produced by digestion with micrococcal nuclease. In order to determine whether these receptor binding sites were distributed in a random or nonrandom manner within the chromatin, we have fractionated MCF-7 cell chromatin into transcriptionally active and inactive fractions by limited micrococcal nuclease digestion followed by Mg2+ precipitation. A comparison of the Mg2+-soluble and insoluble chromatin fractions showed that the Mg2+-soluble fraction: (i) was composed predominantly of mononucleosomes; (ii) was enriched in nonhistone proteins; (iii) apparently lacked histone H1; (iv) was enriched approximately 5-fold in transcribed sequences as measured by a cDNA probe to cytoplasmic poly(A)-RNA sequences; and (v) was depleted at least 5-fold of globin sequences, which is presumably a nontranscribed gene in these cells. When these cells were stimulated with β-[3H]estradiol, the Mg2+-soluble fraction showed a significant enrichment in chromatin-bound estradiol receptor: the Mg2+-soluble mononucleosomes showed a 3- to 4-fold enrichment and the di- and trinucleosomes, a 7- to 19-fold enrichment, when compared to the corresponding subunits in the Mg2+-insoluble chromatin fraction. This cofractionation of chromatin enriched in transcribed sequences and bound estradiol receptor indicated that receptor binding to MCF-7 cell chromatin was not random but, rather, occurred preferentially in specific regions of the chromatin.
Keywords: chromatin binding, chromatin fractionation, mammary tumor cells, nucleosomes, steroid hormone
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N., Peck E. J., Jr, Clark J. H. Nuclear receptor-estrogen complex: relationship between concentration and early uterotrophic responses. Endocrinology. 1973 May;92(5):1488–1495. doi: 10.1210/endo-92-5-1488. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrack E. R., Hawkins E. F., Allen S. L., Hicks L. L., Coffey D. S. Concepts related to salt resistant estradiol receptors in rat uterine nuclei: nuclear matrix. Biochem Biophys Res Commun. 1977 Dec 7;79(3):829–836. doi: 10.1016/0006-291x(77)91186-x. [DOI] [PubMed] [Google Scholar]
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Brooks S. C., Locke E. R., Soule H. D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973 Sep 10;248(17):6251–6253. [PubMed] [Google Scholar]
- Chamness G. C., Jennings A. W., McGuire W. L. Estrogen receptor binding to isolated nuclei. A nonsaturable process. Biochemistry. 1974 Jan 15;13(2):327–331. doi: 10.1021/bi00699a016. [DOI] [PubMed] [Google Scholar]
- Franceschi R. T., Kim K. H. Isolation of estrogen receptor in complex with a discrete nuclear subfraction from hen oviduct. J Biol Chem. 1979 May 10;254(9):3637–3646. [PubMed] [Google Scholar]
- Friedman E. Y., Rosbash M. The syntheiss of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res. 1977 Oct;4(10):3455–3471. doi: 10.1093/nar/4.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Butler P. J. Structure of transcriptionally-active chromatin subunits. Nucleic Acids Res. 1977 Sep;4(9):3155–3173. doi: 10.1093/nar/4.9.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Partington G. A. Distribution of messenger RNA-coding sequences in fractionated chromatin. Cell. 1977 Dec;12(4):953–962. doi: 10.1016/0092-8674(77)90160-x. [DOI] [PubMed] [Google Scholar]
- Hemminki K., Vauhkonen M. Distribution of estrogen receptors in hen oviduct chromatin fractions in the course of DNAase II digestion. Biochim Biophys Acta. 1977 Jan 3;474(1):109–116. doi: 10.1016/0005-2787(77)90218-0. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Jackson V., Chalkley R. The cytoplasmic estradiol receptors of bovine uterus. Their occurrence, interconversion, and binding properties. J Biol Chem. 1974 Mar 10;249(5):1627–1636. [PubMed] [Google Scholar]
- Levy-Wilson B., Dixon G. H. Limited action of micrococcal nuclease on trout testis nuclei generates two mononucleosome subsets enriched in transcribed DNA sequences. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1682–1686. doi: 10.1073/pnas.76.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Levy B., Baxter J. D. Distribution of thyroid and glucocorticoid hormone receptors in transcriptionally active and inactive chromatin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1045–1051. doi: 10.1016/0006-291x(76)90301-6. [DOI] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Partial purification of transcriptionally active nucleosomes from trout testis cells. Nucleic Acids Res. 1978 Nov;5(11):4155–4163. doi: 10.1093/nar/5.11.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T., Liao S. Association of the uterine 17beta-estradiol-receptor complex with ribonucleoprotein in vitro and in vivo. J Biol Chem. 1974 Aug 10;249(15):4671–4678. [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Maurer H. R., Chalkley G. R. Some properties of a nuclear binding site of estradiol. J Mol Biol. 1967 Aug 14;27(3):431–441. doi: 10.1016/0022-2836(67)90049-6. [DOI] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Notides A. C., Nielsen S. The molecular mechanism of the in vitro 4 S to 5 S transformation of the uterine estrogen receptor. J Biol Chem. 1974 Mar 25;249(6):1866–1873. [PubMed] [Google Scholar]
- Senior M. B., Frankel F. R. Evidence for two kinds of chromatin binding sites for the estradiol-receptor complex. Cell. 1978 Aug;14(4):857–863. doi: 10.1016/0092-8674(78)90341-0. [DOI] [PubMed] [Google Scholar]
- Socher S. H., Krall J. F., Jaffe R. C., O'Malley B. W. Distribution binding sites for the progesterone receptor within chick oviduct chromatin. Endocrinology. 1976 Sep;99(3):891–900. doi: 10.1210/endo-99-3-891. [DOI] [PubMed] [Google Scholar]
- Sutherland R. L., Baulieu E. E. Quantitative estimates of cytoplasmic and nuclear oestrogen receptors in chick oviduct. Effect of oestrogen on receptor concentration and subcellular distribution. Eur J Biochem. 1976 Nov 15;70(2):531–541. doi: 10.1111/j.1432-1033.1976.tb11045.x. [DOI] [PubMed] [Google Scholar]
- Toft D., Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D., Shyamala G., Gorski J. A receptor molecule for estrogens: studies using a cell-free system. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1740–1743. doi: 10.1073/pnas.57.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft D. The interaction of uterine estrogen receptors with DNA. Adv Exp Med Biol. 1973;36(0):85–96. doi: 10.1007/978-1-4684-3237-4_5. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Godson G. N., Radding C. M. Specificity of the S1 nuclease from Aspergillus oryzae. J Biol Chem. 1975 Nov 25;250(22):8848–8855. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. On the specificity of the binding of the estradiol receptor protein to deoxyribonucleic acid. J Biol Chem. 1974 Nov 25;249(22):7076–7086. [PubMed] [Google Scholar]
- Zava D. T., McGuire W. L. Estrogen receptor. Unoccupied sites in nuclei of a breast tumor cell line. J Biol Chem. 1977 Jun 10;252(11):3703–3708. [PubMed] [Google Scholar]



