Abstract
This study was designed to assess the safety, acceptability, pharmacokinetic (PK), and pharmacodynamic (PD) responses to rectal administration of tenofovir (TFV) 1% vaginally formulated gel and oral tenofovir disoproxil fumarate (TDF). This study was designed as a phase 1, randomized, two-site (United States), double-blind, placebo-controlled study of sexually abstinent men and women. Eighteen participants received a single 300-mg exposure of oral TDF and were then randomized 2:1 to receive a single and then seven daily exposures of rectal TFV or hydroxyethyl cellulose (HEC) placebo gel. Safety endpoints included clinical adverse events (AEs) and mucosal safety parameters. Blood and colonic biopsies were collected for PK analyses and ex vivo HIV-1 challenge. No serious AEs were reported. However, AEs were significantly increased with 7-day TFV gel use, most prominently with gastrointestinal AEs (p=0.002). Only 25% of participants liked the TFV gel. Likelihood of use “if somewhat protective” was ∼75% in both groups. Indices of mucosal damage showed minimal changes. Tissue TFV diphosphate (TFV-DP) Cmax 30 min after single rectal exposure was 6–10 times greater than single oral exposure; tissue TFV-DP was 5.7 times greater following 7-day versus single rectal exposure. In vivo exposure correlated with significant ex vivo tissue infectibility suppression [single-rectal: p=0.12, analysis of covariance (ANCOVA) p=0.006; 7-day rectal: p=0.02, ANCOVA p=0.005]. Tissue PK–PD was significantly correlated (p=0.002). We conclude that rectal dosing with TFV 1% gel resulted in greater TFV-DP tissue detection than oral dosing with reduced ex vivo biopsy infectibility, enabling PK–PD correlations. On the basis of increased gastrointestinal AEs, rectally applied, vaginally formulated TFV was not entirely safe or acceptable, suggesting the need for alternative rectal-specific formulations.
Introduction
Data from North America1 and Europe2 have demonstrated increased rates of HIV infection in men who have sex with men (MSM) and a notable prevalence of anal intercourse (AI) by men and women in the developed3–5 and developing world.6–8 These reports provide a clear rationale to focus HIV prevention efforts on rectal microbicides (RMs) as one strategy to reduce high transmission rates9 via unprotected receptive anal intercourse (RAI).10–12
Vaginal tenofovir (TFV) 1% gel is being developed and oral tenofovir/emtricitabine (Truvada) has been approved by the U.S. Food and Drug Administration for preexposure prophylaxis (PrEP) of HIV infection.13 Women receiving TFV 1% gel in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 study had 39% protection,14 MSM in the iPrEx study who were given tenofovir disoproxil fumarate (TDF)/emtricitabine (Truvada) had 44% protection,15 and serodiscordant men and women in the Partners PrEP Study given TDF or TDF/emtricitabine had between 62 and 73% protection.16 In CAPRISA 004 and iPrEx, the level of protection was related to adherence and drug concentration.15,17 Emphasizing the importance of this trial (as well as the related MTN-007 trial), there have been no prior studies evaluating rectal safety of the vaginally formulated 1% TFV gel product.
The vaginal formulation of TFV 1% gel used in CAPRISA 004 and other vaginal studies is extremely hyperosmolar (3111 mOsmol/kg), almost 10-fold more than human semen.18 Despite this, there were no significant differences in genital tract adverse events (AEs) in clinical trials of this formulation.14,19 However, given that hyperosmolar products can induce mucosal damage in the colon,20,21 they require careful phase 1 evaluation if they are to be considered as candidate RMs.
The RMP-02/MTN-006 study design was complex, built on the prior phase 1 RM trial (UC781)22 evaluating clinical and mucosal safety, acceptability, multicompartmental pharmacokinetic and ex vivo biopsy infectibility of in vivo exposed tissues (as a potential surrogate biomarker of product efficacy). This trial demonstrates that sufficient data can be collected in a small phase 1 trial to model pharmacokinetic–pharmacodynamic (PK–PD) correlations for RM efficacy. As phase 2B/3 studies are large, expensive, and time-consuming, demonstrating preliminary evidence of product efficacy in phase 1 trials would be very valuable in potentially reducing the risk of subsequent failure in phase 2B/3 studies.
Materials and Methods
Objectives
The primary objective was to evaluate the systemic safety of TFV 1% gel when applied rectally. Secondary objectives included evaluating acceptability and mucosal immunotoxicity of the TFV 1% gel, comparing systemic and compartmental PK among oral TDF and rectal TFV 1% gel users. An exploratory objective assessed the efficacy of TFV 1% gel in preventing ex vivo infectibility of rectal tissue biopsies with HIV-1. The trial was approved by the institutional review board at each site [University of California at Los Angeles (UCLA), Los Angeles, CA; Magee-Womens Research Institute (MWRI), Pittsburgh, PA]; all participants provided written informed consent. RMP-02/MTN-006 is registered at ClinicalTrials.gov (NCT00984971) and is in compliance with the CONSORT (Consolidated Standards of Reporting Trials) 2010 trial reporting recommendations (www.consortstatement.org).
Design
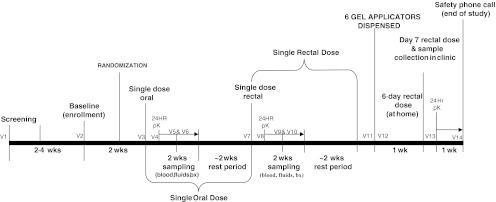
RMP-02/MTN-006 was a phase 1, double-blind, randomized, placebo-controlled comparison of oral TDF (300 mg), rectally applied TFV 1% gel, and the hydroxyethyl cellulose (HEC) placebo gel (Fig. 1). All participants received a single dose of oral TDF (stage 1) followed 4 weeks later (2 weeks for sample collection postexposure and then 2 weeks for healing) by rectally applied TFV 1% gel or the HEC gel given as a single dose (stage 2) and, 4 weeks later, seven daily doses (stage 3), during which six doses of the gel products were taken at home on sequential days (with reminder calls/daily journals) with the final dose administered in the clinic under supervision. Randomization, by study pharmacist, was a two-part process. After enrollment, each subject was assigned to either the treatment or placebo arm (2:1; TFV 1% gel:HEC gel) and also to one of two postexposure biopsy sampling arms for mucosal safety after each exposure stage. This reduced the number of sigmoidoscopic procedures per participant from five to three per study stage (single oral, single rectal, 7-day rectal) with at least 5–8 days between biopsy collections for mucosal healing: Group “A” biopsied on days 1–3 and 7–9 or group “B” biopsied on days 4–6 and 10–12. Each 2 weeks of biopsy sampling was followed by a 2-week resting period between stages. Sample size (n=18) was based on similar phase 1 studies of topical microbicide studies.22 Enrollment began November 2009 (Fig. 2) and was completed July 2010.
FIG. 1.
Study flow diagram.
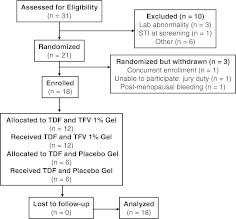
FIG. 2.
CONSORT (Consolidated Standards of Reporting Trials) enrollment summary. STI, sexually transmitted infection.
Study participants
Study participants were healthy HIV-1-seronegative men and women with a history of consensual RAI, willing to abstain from vaginal and rectal sex during active protocol phases. Female participants were required to be using an acceptable form of contraception.
Study products
TDF tablets (300 mg) were supplied by Gilead Sciences (Foster City, CA). TFV 1% gel and HEC gel were supplied by CONRAD (Arlington, VA). TFV 1% gel (weight/weight) is tenofovir (PMPA, 9-[(R)-2-(phosphonomethoxy)propyl]adenine monohydrate), formulated in purified water with edetate disodium, citric acid, glycerin, methylparaben, propylparaben, and hydroxyethyl cellulose with pH adjusted to 4–5 with an osmolarity of 3111 mOsmol/kg. The HEC placebo gel contained hydroxyethyl cellulose as the gel thickener, purified water, sodium chloride, sorbic acid, and sodium hydroxide.23 The gel was isotonic with a pH of 4.4, osmolarity of 304 mOsmol/kg,24 and viscosity similar to the other microbicide gel candidates. Both TFV and HEC gels were prefilled into single-use, opaque applicators (HTI Plastics, Lincoln, NE) containing approximately 4 ml of gel.
Study procedures
Screening included medical history, physical examination including digital rectal examination, and collection of rectal swabs and urine for Chlamydia trachomatis and Neisseria gonorrhea (CT and GC) nucleic acid amplification testing (NAAT). Pregnancy testing in female participants [urine human chorionic gonadotrophin (hCG)] was conducted at screening and all subsequent visits. Blood was collected for clinical safety evaluation (complete blood count, alanine aminotransferase, aspartate aminotransferase, creatinine, phosphate) and serology [syphilis, HIV-1, hepatitis B surface antigen (HBsAg), herpes simplex 1 and 2]. Participants meeting inclusion/exclusion criteria at the screening visit (V1) proceeded to the enrollment visit (V2) for a focused physical/rectal examination, randomization (both for drug/placebo gel product and biopsy schedule), and behavioral questionnaire completion. Safety and sexually transmitted infection (STI) blood/urine (urine hCG for female participants) samples were collected. Rectal swabs were collected to assess changes in rectal microflora; rectal sponges were inserted to collect rectal secretions for cytokines/chemokines and baseline (unexposed) pharmacokinetics (PK). Self-collected vaginal swabs were collected for pH, Gram stain, bacterial vaginosis (BV); vaginal sponges were used for baseline (unexposed) PK. Although results are not reported, positive STIs, pregnancy, and BV detection were exclusion criteria. Participants then received a Normosol-R pH 7.4 enema with a sample of feces collected from effluent for fecal calprotectin measurement. A flexible sigmoidoscope was then inserted into the rectum to ∼5 cm from the anal margin, where 50 cc of Normosol solution was slowly instilled. After 30 s, at least 25–30 ml of lavage fluid was collected via endoscopic aspiration to assess epithelial sloughing. The endoscope was then advanced to ∼15 cm, where ∼17 biopsies were collected for histology, flow cytometry, ex vivo tissue infectibility assays, and baseline PK.
After clinician-administered single-dose oral (V3; stage 1) and single-dose topical (V7; stage 2), participants received the same evaluations as V2 but endoscopic samples were collected approximately 30 min after product exposure. Additional blood and vaginal/rectal sponges were collected at 30 min, +2 h, +4 h, and +24 h for PK. On the basis of randomization, participants underwent further blood, vaginal/rectal sponges and sigmoidoscopic biopsy sampling for mucosal injury, flow cytometry, PK, and ex vivo tissue infectibility evaluations on either days 1–3, 4–6, 7–9, or 10–12 post-V3 and post-V7. For the 7-day exposure (V11; stage 3), vaginal/rectal sponges were collected for PK and participants received a 6-day supply of applicators. The seventh dose was administered by clinic personnel (V12) with sample collections for STIs, HIV serology, and PK 30 min later with 24-h PK collections as described above (V13).
Clinical safety
Emergent AEs were graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric AEs, version 1.0, December 2004 as well as Addenda 1 and 3 (Female Genital Grading Table for Use in Microbicide Studies and Rectal Grading Table for Use in Microbicide Studies, respectively) (http://rsc.tech-res.com/safetyandpharmacovigilance/). In cases in which an AE was covered in both tables, Addenda 1 and 3 were used.
Mucosal safety
Histology
A semiquantitative scoring system adapted for use in rectal microbicide trials22 was used to characterize potential product associated injury with a scale of 1 (normal) to 5 (mucosal erosion or ulceration).
Fecal calprotectin
Fecal calprotectin, an indirect index of mucosal inflammation, was measured with a commercial assay (Genova Diagnostics, Asheville, NC).25
Epithelial sloughing
Epithelial sloughing was evaluated by a modification of a previously described technique.26 Lavage samples were scored as either 0 or 1 (absence or presence of epithelial sheets) for a total score from 0 to 4.
Rectal microflora
Rectal microflora were characterized (baseline to V13) on the basis of previously described semiquantitative culture analysis.22
Mucosal T cell phenotype
Mucosal mononuclear cells were isolated from rectal biopsies, using mechanical/enzyme digestion as previously described.27 Monoclonal antibodies selection was the same as in RMP-01,22 supplied by BD Immunocytometry Systems (BDIS, Mountain View, CA). Once stained, all samples were shipped to UCLA for single-site analysis using a FACSCalibur flow cytometer (BDIS, Mountain View, CA) with analysis using CellQuest Pro software (BDIS).
Luminex analysis of rectal secretions
The MILLIPLEX MAP kit (Millipore, Billerica, MA) was used to detect RANTES (regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1α, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-12 (p-40), IL-6, and IL-1β, using Luminex 100 IS (Luminex, Austin, TX) as previously described.22
Product acceptability and adherence
Acceptability was measured as the proportion of participants who reported via computer-assisted self-interview (CASI): (1) overall liking of gel, (2) ease of use, and (3) likelihood of future use. Quantitative measures used a 10-point Likert scale (1, low; 10, high). Participants completed a diary documenting gel use during the 6-day home-use period. Returned diaries were cross-validated with counts of returned used/unused gel applicators. Adherence was defined as the proportion of times the product was used out of all possible uses.
PK analyses
Only plasma TFV and rectal tissue TFV-DP PK results are presented here. Data and PK correlations between the other compartments will be presented separately (unpublished results). Plasma TFV and tissue TFV-DP concentrations were determined by previously described liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods validated for all matrices at the Johns Hopkins Clinical Pharmacology Analytical Laboratory28,29 and met U.S. Food and Drug Administration (FDA) bioanalytical validation criteria.30 Analyses/interpretations of PK data were performed by the University of North Carolina Center for AIDS Research (UNC CFAR) Clinical Pharmacology/Analytical Chemistry Core.
PD analyses (ex vivo tissue infectibility)
At baseline and each specified time point after product exposure, endoscopic biopsies were collected in 50 ml of RPMI (with 1.125 μg/ml of Fungizone and 50 mg/ml of Zosyn), transported to the laboratory for ex vivo infection [2 h; common viral stock of R5 HIV-1BaL (104 TCID50)], as previously described.31–34 Supernatants for p24 quantification were collected every 3 days during each participant's 14-day infectibility studies (days 1, 4, 7, 11, and 14). Results are reported as cumulative p24 (National Cancer Institute, Bethesda, MD). The lower limit of quantification (LLOQ) of the assay was 10 pg of p24 per milliliter.
Analysis of study outcomes
Primary safety outcomes were protocol-defined as the frequency of AEs of grade 2 or higher. Between-group comparisons of numbers of subjects having any AEs were done using Fisher's exact test; for total number of AEs, analyses were done assuming a Poisson model. Given the small sample size, there was limited power to detect differences between study arms. Acceptability was defined as the proportion of participants reporting a high likelihood of future product use defined by a rating in the upper third of a 10-point Likert scale. Changes in mucosal safety parameters within groups were assessed by comparisons before (Visit 2-baseline) to 30 minutes after product exposure using two-sided paired t-tests or Wilcoxon tests with α=0.05. Cumulative day 14 changes in HIV-1 p24 tissue infectibility assays were assessed similarly with α=0.05. Differences in mucosal safety parameters and cumulative p24 values between experimental groups were assessed using ANCOVA models adjusting for the baseline measurements. Pharmacokinetic parameters were estimated using noncompartmental methods (Phoenix WinNonlin Pro 5.2; Certara, L.P., St. Louis, MO). Maximum concentration (Cmax), time at maximum concentration (Tmax), and concentration 24 h after dosing (C24h) were determined by visual inspection of subject profiles, and used the log-linear trapezoidal method to calculate the area under the time–concentration curve over 24 h (AUC24h). Detectable concentrations below the limit of quantification were imputed as 50% of the LLOQ. A linear regression was used to correlate detectable rectal tissue TFV-DP (log10 fmol/mg) in the TFV-treated group with concordant tissue infectibility (cumulative p24 log10 pg/ml levels, averaged across quadruplicate rectal biopsies) after single oral, single topical, and 7-day topical exposures.
Results
Enrollment; participant disposition
Twenty-one participants were randomized (15 at UCLA, 6 at MWRI), with 3 (UCLA) withdrawn before receiving any product (Fig. 2). The 18 enrolled participants received oral product and then rectal products according to their randomization (Fig. 1). All 18 completed all study visits (100% retention; 144 flexible sigmoidoscopies; >2300 biopsies). Participants were 14 males (78%) and 4 females (22%) with a median age of 45 years (range, 22–66 years); 11% African-American, 56% white, and 33% Hispanic (Table 1).
Table 1.
Baseline Demographics of Each Enrolled Treatment Group
| Oral tenofovir | Tenofovir gel | HEC placebo gel | |
|---|---|---|---|
| Participants enrolled | 18 | 12 | 6 |
| Age (years) | |||
| Mean (STD) | 42.1 (11.4) | 41.3 (11.9) | 43.7 (10.2) |
| Gender | |||
| Male | 14 (78%) | 10 (83%) | 4 (67%) |
| Female | 4 (22%) | 2 (17%) | 2 (33%) |
| Latino or of Hispanic origin | |||
| Yes | 5 (28%) | 3 (25%) | 2 (33%) |
| No | 13 (72%) | 9 (75%) | 4 (67%) |
| Race | |||
| Asian | 0 | 0 | 0 |
| Black or African-American | 2 (11%) | 1 (8%) | 1 (17%) |
| Native Hawaiian or Pacific Islander | 0 | 0 | 0 |
| White | 15 (83%) | 10 (84%) | 5 (83%) |
| Other | 1 (6%) | 1 (8%) | 0 |
HEC, hydroxyethyl cellulose; STD, standard deviation.
Adverse events
There were no serious or procedure-related AEs. After product exposure, the nonrandomized, single oral exposure stage identified 30 grade 1, 5 grade 2, and 2 grade 3 AEs (Table 2). During the randomized, single topical exposure stage there were 20 grade 1, 10 grade 2, and 0 grade 3 AEs with no significant differences between groups in overall or gastrointestinal (GI)-specific AEs. During the randomized, 7-day topical exposure stage: 49 grade 1, 7 grade 2, and 6 grade 3 AEs were reported (all resolved). A significant difference in number of AEs, especially GI-related AEs, was present in the TFV-treated group (p=0.002 for GI AEs during 7-day exposure). Regarding the seven grade 2 AEs in the 7-day exposure, six occurred in three (of twelve) TFV-treated subjects and one occurred in 1 of 6 in a placebo-treated subjects (all minor, non–life-threatening) (p=1.0). All six grade 3 AEs, five of which were GI related, occurred in two of twelve TFV-treated subjects (p=0.53).
Table 2.
Number of Participants with Adverse Events
| |
|
TFV 1% gel (topical) |
HEC placebo gel (topical) |
||
|---|---|---|---|---|---|
| TDF (oral) | Single dose | 7-day dose | Single dose | 7-day dose | |
| Participants enrolled | 18 | 12 | 6 | ||
| Participants with one or more AEs (all) | |||||
| Grade 1: Mild | 11 (30) | 4 (11) | 12 (41) | 4 (9) | 4 (8) |
| Grade 2: Moderate | 3 (5) | 4 (6) | 3 (6) | 1 (4) | 1 (1) |
| Grade 3: Severe | 2 (2) | 0 | 2 (6) | 0 | 0 |
| Participants with gastrointestinal AEs | |||||
| Grade 1: Mild | 7 (15) | 4 (6) | 12 (34) | 3 (5) | 1 (5) |
| Grade 2: Moderate | 0 | 2 (4) | 2 (3) | 1 (4) | 1 (1) |
| Grade 3: Severe | 0 | 0 | 2 (5) | 0 | 0 |
| Participant's specific gastrointestinal AEs | |||||
| Abdominal bloating (G1) | 3 (3) | 0 | 4 (8) | 0 | 1 (1) |
| Abdominal bloating (G2) | 0 | 1 (1) | 0 | 1 (1) | 0 |
| Abdominal bloating (G3) | 0 | 0 | 1 (1) | 0 | 0 |
| Abdominal pain/cramps (G1) | 1 (1) | 2 (2) | 2 (2) | 1 (1) | 1 (1) |
| Abdominal pain/cramps (G2) | 0 | 1 (1) | 0 | 1 (1) | 1 (1) |
| Abdominal pain/cramps (G3) | 0 | 0 | 1 (1) | 0 | 0 |
| Colon polyps (G1) | 4 (4) | 0 | 1 (1) | 0 | 0 |
| Constipation (G1) | 1 (1) | 0 | 0 | 0 | 0 |
| Defecation urgency (G1) | 0 | 0 | 4 (6) | 0 | 0 |
| Defecation urgency (G2) | 0 | 1 (1) | 1 (1) | 0 | 0 |
| Defecation urgency (G3) | 0 | 0 | 2 (2) | 0 | 0 |
| Diarrhea (G1) | 2 (3) | 1 (1) | 6 (7) | 0 | 0 |
| Diarrhea (G2) | 0 | 0 | 2 (2) | 0 | 0 |
| Fissure (anal) (G1) | 1 (1) | 0 | 0 | 0 | 0 |
| Flatulence (G1) | 0 | 0 | 2 (2) | 0 | 0 |
| Flatulence (G2) | 0 | 1 (1) | 0 | 0 | 0 |
| Hemorrhoid | 0 | 0 | 0 | 1 (1) | 0 |
| Loose stool (G1) | 0 | 0 | 0 | 1 (1) | 0 |
| Loss of appetite (G1) | 1 (1) | 0 | 0 | 0 | 0 |
| Mucosal red spots (G1) | 0 | 1 (1) | 0 | 2 (2) | 0 |
| Nausea (G1) | 1 (1) | 2 (2) | 3 (5) | 0 | 1 (1) |
| Nausea (G2) | 0 | 0 | 0 | 1 (1) | 0 |
| Rectal discharge (G1) | 0 | 0 | 1 (1) | 0 | 0 |
| Rectal pain (G1) | 0 | 0 | 1 (1) | 0 | 1 (1) |
| Tenesmus (G1) | 0 | 0 | 1 (1) | 0 | 1 (1) |
| Tenesmus (G2) | 0 | 0 | 0 | 0 | 0 |
| Tenesmus (G3) | 0 | 0 | 1 (1) | 0 | 0 |
| Vomiting (G2) | 0 | 0 | 0 | 1 (1) | 0 |
Only AEs following product exposure are reported. Some individuals report more than 1 AE. For convenience all Grade 3 AEs are listed in bold.
AE, adverse event; TDF, tenofovir disoproxil fumarate; TFV, tenofovir; HEC, hydroxyethyl-cellulose.
Mucosal safety
Epithelial sloughing, histopathology, rectal microflora, fecal calprotectin, and flow cytometry
No differences were observed within groups compared with baseline or between groups, at any time point postexposure.
Secreted rectal cytokines
There were no significant changes in HEC controls at any stage using either paired t-tests or Wilcoxon signed rank tests. Following oral TDF, there were no significant changes using paired t-tests; however, Wilcoxon tests identified IL-1β (p=0.02) and TNFα (p=0.03) suppression. Following single-rectal exposure, t-tests showed marginally significant suppression of IL-1β (p=0.046); with Wilcoxon tests, IL-1b suppression was more significant (p=0.003) and marginally significant changes emerge in MIP-1α (p=0.025) and TNFα (p=0.03). Following 7-day rectal exposure, t-tests show marginally significant suppression of IL-1β (p=0.04) and MIP-1α (p=0.03). When using Wilcoxon tests, the significance increased (IL-1β: p=0.002; MIP-1α: p=0.004) and marginally significant suppression of IL-6 (p=0.04) and TNFα (p=0.03) was identified. Inclusion of results from both methods is intended to provide full information from a small trial designed to identify any changes.
Product acceptability and adherence
Of the participants in the tenofovir group, 67% versus 83% in the HEC placebo group found the gel easy to use. However, only 25% of participants receiving TFV gel versus 50% in the HEC placebo group reported “liking the product very much.” Despite this, 75% of the TFV gel group and 67% in the HEC group stated that they would be “very likely” to use the gel in the future if it provided some protection. Self-reports of 6-day at-home use of dispensed applicators were cross-validated with returned used/unused applicator counts; these coincided in 16 of 18 cases. Using these cross-checks, 83% of all participants (92% in the tenofovir arm) were fully adherent during this stage.
Pharmacokinetics
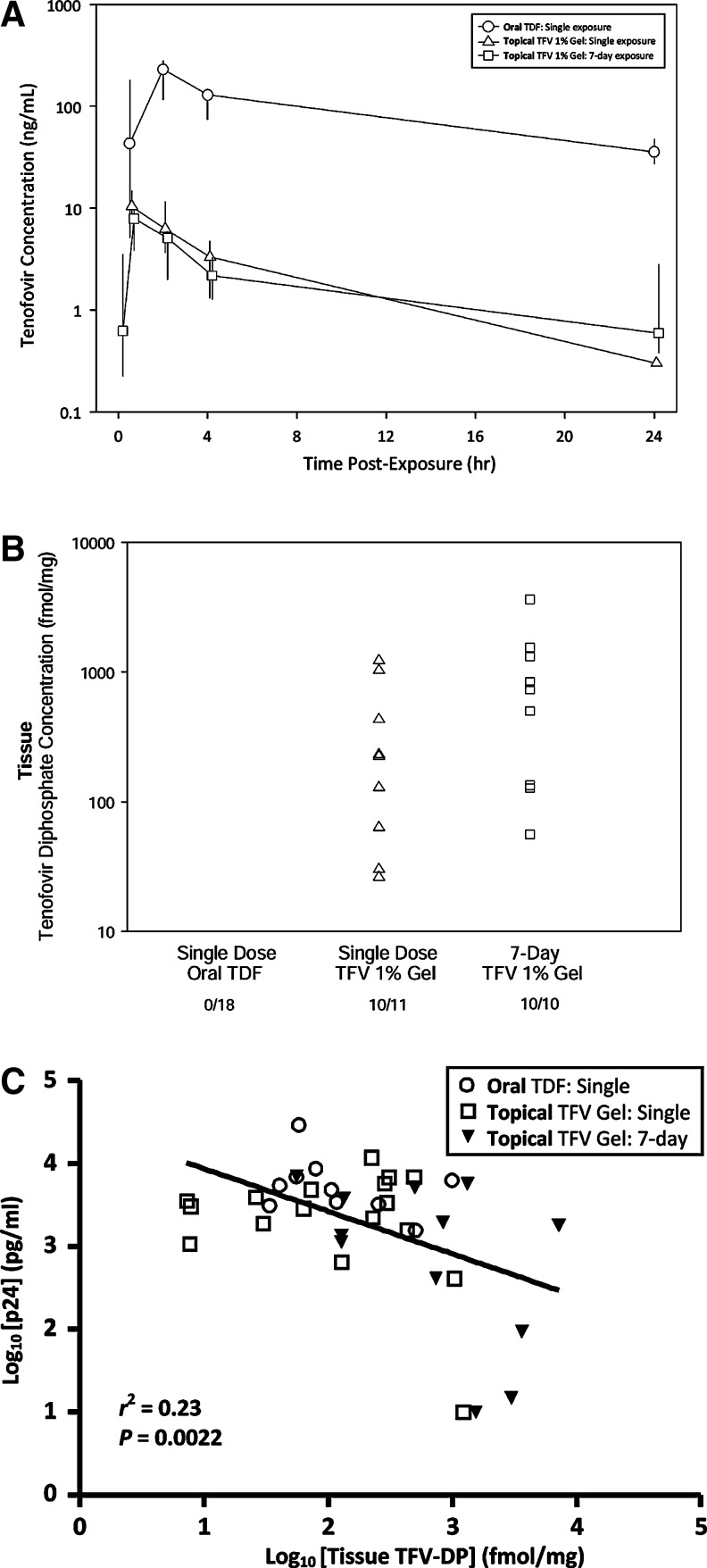
Plasma tenofovir (TFV)
Concentrations of plasma TFV Cmax and AUC24h were 23- and 30-fold higher after the single oral exposure compared with single rectal exposure. With oral dosing, the median AUC24h was 2003 ng·h/ml with a Cmax of 246 ng/ml at 2 h postdose. In contrast, for single rectal exposure, the AUC24h was 52 ng • h/ml with a Cmax of 8 ng/ml occurring 0.3 h postdose (Fig. 3A). Seven-day rectal exposures were similar to single-rectal exposures in plasma concentrations: AUC24h was 35 ng • h/ml with a Cmax of 5 ng/ml at 0.3 h postdose. Plasma C24h was detectable in 100% of single oral exposure subjects (median concentration, 36 ng/ml) but detectable in ∼70% of single rectal exposure subjects (median concentration. 0.3 ng/ml) and ∼88% after 7-day rectal exposure (median concentration, 0.9 ng/ml), indicating negligible accumulation during this time period.
FIG. 3.
Pharmacokinetics (PK): Tenofovir (TFV) levels in plasma and tenofovir diphosphate (TFV-DP) level in rectal tissue over 24 h postexposure. (A) Plasma (TFV): The Tmax and subsequent decay for detection of plasma TFV after single oral, single/topical–rectal, and 7-day topical–rectal are shown. Single oral exposures peak later and, at 24 h, plasma concentrations are ∼98% higher than either of the topical rectal exposures. (B) Tissue (TFV-DP): Detectable concentrations of TFV-DP from homogenized tissue biopsies acquired within the first 24 h for the single oral, single topical/rectal exposures and 30 min after the last of the 7-day topical rectal exposures are shown. (C) Pharmacokinetic–pharmacodynamic (PK–PD) correlation of tissue TFV-DP concentration with ex vivo tissue infectibility: The linear correlation of tissue TFV-DP (log10 fmol/mg) and infectivity [defined by log10-transformed cumulative p24 (pg/ml) on day 14] is shown for subjects with detectable tissue TFV-DP after single oral (n=18; 5 time points sampled over 14 days postexposure*), single topical exposure (n=12, five time points sampled over 14 days postexposure*), and 7 days topical exposure (n=12, 30 min after final exposure) to tenofovir product. [*Oral exposure: 18 subjects at 30 min and 9 subjects (group A or B) at 1–3, 4–6, 7–9, and 10–12 days; single topical/rectal: 12 subjects at 30 min and 6 subjects (group A or B) at 1–3, 4–6, 7–9, and 10–12 days.]
Tissue tenofovir diphosphate (TFV-DP)
After single oral and single rectal exposures, rectal biopsies from the 18 participants were collected at three time points from 30 min to 14 days (alternating days, for safety reasons). Tmax results reflect only the designated time points (30 min, 1–3 days or 4–6 days, 7–10 days or 10–12 days).
TFV-DP was not detected in any tissue samples 30 min after oral exposure (n=18) but was detected in 95% of single rectal exposed (n=11/12) as well as 7-day rectal exposed (n=12/12) tissue samples (Fig. 3B).
After 24 h, eight oral-exposed subjects had a median TFV-DP concentration of 29 fmol/mg (range, 0–992). This was Tmax for seven of eight subjects with detectable tissue TFV-DP; the eighth subject's Tmax occurred on day 7. Tissue concentrations of any TFV-DP after single oral exposure were detected in 3 of 18 subjects out to 6–7 days postexposure (median, 106 fmol/mg; range, 0–117).
In contrast, after single rectal exposure (n=12), the median 30-min TFV-DP concentration was 176 fmol/mg (range, 0–1229); by 24 h, five of six subjects sampled had a higher median TFV-DP tissue concentration of 285 fmol/mg (range, 0–490). These concentrations were approximately 6- to 10-fold higher than after the single oral exposure. Tmax occurred at 30 min in 9 of 12, with only 2 of 12 having their Tmax at 24h (1 of 12 never had measureable TFV-DP). TFV-DP concentrations were detected out to 6–7 days postexposure in 2 of 12 subject samples.
After 7-day rectal exposure, where tissue samples were only obtained 30 min after the seventh administered topical gel, TFV-DP was detected in all 12 subject biopsies (median, 789 fmol/mg; range, 56–7187). The accumulation ratios (TFV-DP tissue concentration at 30 min after seventh exposure/TFV-DP tissue concentration 30 min after single exposure) in 11 of 12 subjects yielded a median accumulation ratio of 5.7 (0.3–23) demonstrating TFV-DP accumulation over time.
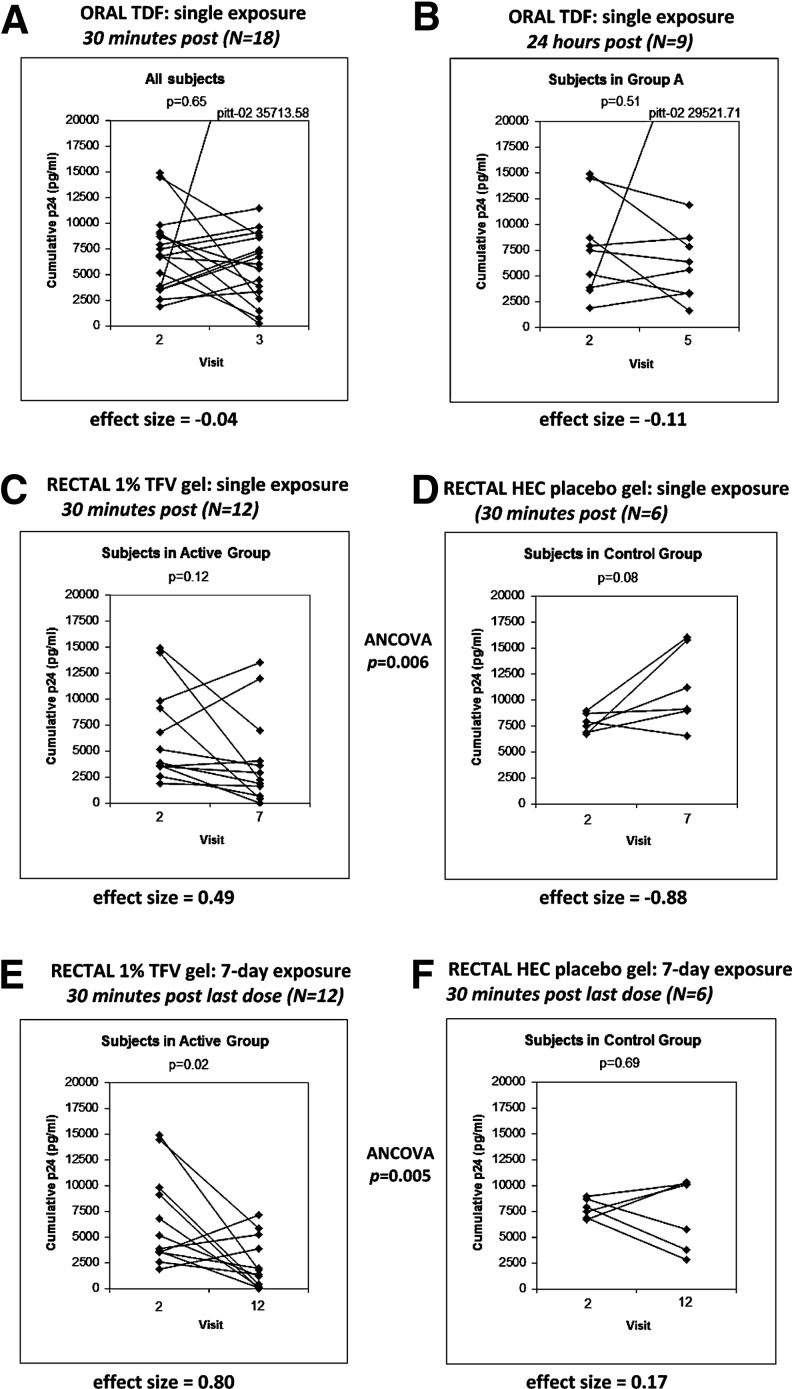
Pharmacodynamics
At baseline, all participants' rectal biopsies were infectible ex vivo with a viral titer of 104 TCID50 HIV-1BaL, with a known range of assay variability. No significant suppression of ex vivo infection was seen at any time point after single oral exposure (Fig. 4A and B show data for 30 min and 24hr post-exposure). After single rectal exposure, there was a nonsignificant declining p24 trend in the tenofovir group (p=0.12; effect size, 0.49) but the ANCOVA comparing suppression in TFV-exposed to placebo was significant at p=0.006 (Fig. 4C and D). Seven-day TFV rectal exposure showed significant suppression of ex vivo biopsy infectibility versus placebo (p=0.02; effect size, 0.80) with the ANCOVA comparing TFV-exposed to placebo significant at 30 min after final exposure (p=0.005) (Fig. 4E and F). Interestingly, all participants with reduced tissue infectibility ex vivo also showed suppression in IL-1β, TNFα and MIP-1α secreted rectal cytokines.
FIG. 4.
Changes in ex vivo infectibility by HIVBaL 104 TCID50 of rectal biopsies following TVF exposure. Graphs document the changes in cumulative p24 from ex vivo tissue infectibility at V2 (all baseline; no product exposure) compared to the same subject's tissue samples collected/exposed ex vivo following product exposure. (A and B) Show changes following single-oral exposure. As there was no placebo group during the oral-exposure stage, results from 30 minutes (A) and 24 hours (B) post-exposure are shown to demonstrate absence of delayed suppressive effects due to a systemically-delivered oral drug (B). (C and D) Show results at baseline (each graph: left side) and 30 minutes after (each graph: right side) single topical exposure to TFV gel (C) compared to HEC placebo (D). (E and F) Show the same arrangement 30 minutes following 7-days topical exposure. The points for the same individual are linked by a solid line. The p-value of a paired test (Wilcoxon for A,B; t-test for C–F) is displayed within each plot; effect sizes are displayed under each plot and ANCOVAs, showing differences between TFV-exposed and placebo groups, after adjusting for baseline measurements, are placed between respective graphs.
PK–PD correlation (tissue TFV-DP with ex vivo biopsy infectibility)
As shown in Fig. 3C, during each exposure stage, detectable rectal tissue TFV-DP concentrations (at least one of five time points sampled over 14 days postdosing) were found in 9 of 18 (50%) for single oral, 11 of 12 (95%) for single rectal, and 12 of 12 (100%) for 7-day rectal-exposed participants. Analyzing all paired TFV-exposed samples, tissue TFV-DP levels and ex vivo biopsy infectibility were correlated. A significant (p=0.0022), negative (slope=–0.5142±0.1561) linear dose–response relationship was found (Fig. 3C). Although the data were variable (r2=0.23), a clear relationship was found between decreased infectibility of ex vivo biopsies and increased tissue TFV-DP concentration.
Discussion
This study demonstrates that the vaginal formulation of TFV 1% gel is neither entirely safe (based on gastrointestinal AEs) nor entirely acceptable for rectal use, indicating the need for development of a rectal-specific formulation. This trial is also the first trial to compare single-exposure oral TDF with single-exposure rectal TFV for mucosal toxicity, PK, and ex vivo infectibility. Single rectal administration of TFV 1% gel was associated with >6–10 times greater local tissue concentration of TFV-DP than single oral administration, with minimal plasma TFV concentrations and with significantly lower levels of ex vivo tissue infection compared to the controls. After 7-day rectal exposure to TFV gel, rectal biopsies contained ∼5.7-fold more TFV-DP than single rectal exposure and also demonstrated significantly reduced tissue infectibility. This inversely correlated with the amount of drug found in the tissue. These latter points demonstrate the potential to generate PK–PD correlations in a phase 1 rectal microbicide trial.
Although there were no significant differences in total or gastrointestinal-related grade 2 AEs after single rectal exposure to TFV or HEC gels, 7-day exposure to TFV did produce more grade 2 and 3 AEs than placebo. Importantly, all six reported grade 3 AEs were in two (of 12) TFV gel participants; five of six were GI related. It is likely that these AEs, especially the grade 3 AEs, were caused by the high osmolality of the vaginally formulated TFV 1% gel.
Despite these clinical findings as well as publications associating the use of hyperosmolar products rectally with mucosal damage,20 TFV 1% gel was not associated with any significant index of mucosal damage overall.35 Using paired t-tests, some rectal-secretion cytokines (IL-1β, TNFα, IL-6, MIP-1α; all often classified as “pro-inflammatory” were shown to be reduced in the TFV-treated groups at different study stages. Given the small sample size it is of interest that when using the uncorrected cytokine results, all the subjects with reduced ex vivo infectibility at the end of the 7-day exposure period also expressed generally reduced mucosal cytokine levels (above). Although these two “proinflammatory” cytokines are usually associated with increased mucosal replication, their reduction in well-suppressed HIV-seropositive patients (even compared with healthy control subjects) has been reported.36,37 Studies seeking to elucidate the mechanisms of the antiviral effectiveness of tenofovir have reported the in vitro stimulation of a variety of chemokines and cytokines, including TNF-α and IL-1β.38 Combined with reports from MTN-007,39 these findings merit ongoing attention in future trials. Although the participants demonstrated high adherence by the measures used, it was quite clear that only a minority of TFV gel recipients liked the product, providing an additional, user-based rationale to develop/test rectal specific formulations.
Not surprisingly, single rectal administration of TFV 1% gel resulted in much higher, short-term tissue concentrations of intracellular TFV-DP than single oral TDF. Repeated rectal exposures further increased tissue TFV-DF. These findings are similar to the observations in MTN-001, where administration of vaginal TFV 1% gel resulted in significantly greater tissue concentrations of TFV-DP than oral dosing with Truvada.40
Administration of a single TDF oral exposure provided no reduction in ex vivo biopsy infectibility. However, both single-rectal and 7day-rectal TFV gel exposure showed significant ex vivo suppression of tissue infectibility. Although these infectibility assays are only potential, laboratory-based, efficacy indicators, the lack of significant ex vivo suppression after single oral exposure focuses attention on better characterizing the PK–PD relationships with regard to single, pericoital administration of either or both oral or topical–rectal tenofovir-based PrEP for HIV prevention.
The PK and PD data acquired during this trial, together with similar data from our previous rectal assessment of UC781 gel (RMP-01),22 provide an important contribution to the field of HIV prevention, demonstrating that meaningful PK–PD correlations can be generated in phase 1 microbicide trials. In the case of UC781 gel, these data have enabled the development of a PK–PD model to determine effective tissue concentrations (ECs) needed to reduce ex vivo tissue infectibility by 50–95% (EC50,90,95).41
Overall, this study suggests that optimization of the vaginally formulated TFV 1% gel is needed for use as a rectal microbicide.21 However, it is clear that rectal administration of an antiretroviral gel such as TFV is an extremely effective way to deliver high concentrations of active drug to highly vulnerable rectal mucosa. This study also demonstrates that intensified phase 1 trials including acceptability assessments, focused mucosal safety biomarkers, multicompartment PK, and ex vivo infectibility assays are feasible with high participant retention. The data from these early-in-development types of studies will allow optimization of product profiles before moving candidate microbicides into later-stage development.
Acknowledgments
Deep appreciation is offered to the dedicated participants who enrolled in this intensive study. Significant support at all stages of this study was provided by site staff and the study sponsors including UCLA (Charina McDonald, Terry Saunders, Karen Tanner, VaShira Rhodes, and Justin Akin), MWRI/University of Pittsburgh (Carol Oriss, Lorna Rabe, Kathryn Duffill, Lisa Rohan, and Sharon Hillier), JHU (Nicolette Louissaint, Nicole Anders, and Jianmeng Chen), CONRAD (Henry Gabelnick, Timothy McCormick, Marianne Callahan, and David Friend), Gilead Sciences (Jim Rooney), and NIH/NIAID/DAIDS (Jim Turpin, Jeanna Piper, and Grace Chow). Specific thanks is given to Cindy Jacobson, PharmD, Core Pharmacist-MTN, who was the study pharmacist overseeing study product receipt, distribution, and all randomizations.
Preliminary findings from this study were presented at the 18th Conference on Retroviruses and Opportunistic Infections (CROI), Boston in 2011. T.M., C.M., and H.G. are employed by CONRAD.
This study was funded by a U19 grant under the Integrated Preclinical-Clinical Program for HIV Topical Microbicides (IPCP-HTM), Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) (AI060614) and the NIAID's Microbicide Trials Network (5UM1AI068633). Additional support was provided by Gilead Sciences; the University of California, Los Angeles Center for AIDS Research (5P30 AI28697) Cores of Mucosal Immunology, Flow Cytometry, and Biostatistics; the University of North Carolina-Chapel Hill Center for AIDS Research, Clinical Pharmacology/Analytical Chemistry Core (P30 AI50410); the Johns Hopkins Clinical Pharmacology Analytical Laboratory; and CONRAD.
The RMP-02/MTN-006 study was registered at ClincialTrials.gov (NCT00984971) and the protocol can be found at www.mtnstopshiv.org.
P.A. was the protocol chair, oversaw the implementation of the study, and wrote the paper with I.M.; R.C. was the University of Pittsburgh site principal investigator and was responsible for implementation of the study at his site; A.K. analyzed and interpreted the PK data in the study; N.R.-H. analyzed the PK–PD correlations in the study; C.W.H. and N.N.B oversaw samples analysis for drug concentrations in the JHU Clinical Pharmacology Analytical Laboratory; J.E. was the laboratory manager at the UCLA site; L.J. was the laboratory manager at the University of Pittsburgh site; E.K. provided regulatory oversight for the study; R.D. developed the clinical trials database used in the study; W.C. developed the statistical plan and supervised analysis of the study data; C.J. conducted the statistical analysis of the study; A.C.-D. was responsible for behavioral assessments within the study; C.M. provided oversight for the protocol development; I.M. was the co-principal investigator for the MDP grant and wrote the paper with P.A.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Prejean J. Song R. Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansen IA. Geskus RB. Davidovich U, et al. Ongoing HIV-1 transmission among men who have sex with men in Amsterdam: A 25-year prospective cohort study. AIDS. 2011;25:493–501. doi: 10.1097/QAD.0b013e328342fbe9. [DOI] [PubMed] [Google Scholar]
- 3.Mosher WD. Chandra A. Jones J. Sexual behavior and selected health measures: Men and women 15–44 years of age, United States, 2002. Adv Data. 2005;362:1–55. [PubMed] [Google Scholar]
- 4.Misegades L. Page-Shafer K. Halperin D. McFarland W. Anal intercourse among young low-income women in California: An overlooked risk factor for HIV? AIDS. 2001;15:534–535. doi: 10.1097/00002030-200103090-00017. [DOI] [PubMed] [Google Scholar]
- 5.Gorbach PM. Manhart LE. Hess KL, et al. Anal intercourse among young heterosexuals in three sexually transmitted disease clinics in the United States. Sex Transm Dis. 2009;36:193–198. doi: 10.1097/OLQ.0b013e3181901ccf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karim SS. Ramjee G. Anal sex and HIV transmission in women. Am J Public Health. 1998;88:1265–1266. doi: 10.2105/ajph.88.8.1265-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane T. Pettifor A. Pascoe S. Fiamma A. Rees H. Heterosexual anal intercourse increases risk of HIV infection among young South African men. AIDS. 2006;20:123–125. doi: 10.1097/01.aids.0000198083.55078.02. [DOI] [PubMed] [Google Scholar]
- 8.Kalichman SC. Simbayi LC. Cain D. Jooste S. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Transm Infect. 2009;85:411–415. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) HIV transmission risk. http://www.cdc.gov/hiv/law/transmission.htm http://www.cdc.gov/hiv/law/transmission.htm
- 10.Anton P. Herold BC. HIV transmission: Time for translational studies to bridge the gap. Sci Transl Med. 2011;3:77ps11. doi: 10.1126/scitranslmed.3002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan I. Rectal microbicides: Can we make them and will people use them? AIDS Behav. 2011;15(Suppl 1):S66–S71. doi: 10.1007/s10461-011-9899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anton PA. Future prospects and perspectives on microbicides. Curr HIV Res. 2012;10:113–115. doi: 10.2174/157016212799304634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. FDA approval of Truvada for a PrEP indication: Label information. http://www accessdata fda gov/scripts/cder/drugsatfda/index cfm?fuseaction=Search Label_ApprovalHistory#apphist. 2012. http://www accessdata fda gov/scripts/cder/drugsatfda/index cfm?fuseaction=Search Label_ApprovalHistory#apphist
- 14.Abdool KQ. Abdool Karim SS. Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant RM. Lama JR. Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeten JM. Donnell D. Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim SS. Kashuba AD. Werner L. Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet. 2011;378:279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohan LC. Moncla BJ. Kunjara Na Ayudhya RP, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer KH. Maslankowski LA. Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs EJ. Lee LA. Torbenson MS, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: Potential implication for HIV transmission. J Infect Dis. 2007;195:703–710. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 21.Dezzutti CS. Rohan LC. Wang L, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother. 2012;67:2139–2142. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anton PA. Saunders T. Elliott J, et al. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS One. 2011;6:e23243. doi: 10.1371/journal.pone.0023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz JL. Ballagh SA. Kwok C, et al. Fourteen-day safety and acceptability study of the universal placebo gel. Contraception. 2007;75:136–141. doi: 10.1016/j.contraception.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.CONRAD. Investigator's Brochure: Universal placebo gel. UC-781. Version 3.0. 2006. Arlington, VA: CONRAD; 2006. [Google Scholar]
- 25.Gaya DR. Lyon TD. Duncan A, et al. Faecal calprotectin in the assessment of Crohn's disease activity. QJM. 2005;98:435–441. doi: 10.1093/qjmed/hci069. [DOI] [PubMed] [Google Scholar]
- 26.Patton DL. Cosgrove Sweeney YT. Rabe LK. Hillier SL. Rectal applications of nonoxynol-9 cause tissue disruption in a monkey model. Sex Transm Dis. 2002;29:581–587. doi: 10.1097/00007435-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 27.McGowan I. Elliott J. Cortina G, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056) J Acquir Immune Defic Syndr. 2007;46:417–425. doi: 10.1097/QAI.0b013e318156ef16. [DOI] [PubMed] [Google Scholar]
- 28.King T. Bushman L. Kiser J, et al. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843:147–156. doi: 10.1016/j.jchromb.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Keller MJ. Madan RP. Torres NM, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity after application of 1% tenofovir gel. PLoS One. 2011;6:e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Bioanalytical METHOD VALIDATION. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine; 2001. [Google Scholar]
- 31.Fletcher PS. Elliott J. Grivel JC, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS. 2006;20:1237–1245. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 32.Anton PA. Elliott J. Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14:1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz JL. Kovalevsky G. Lai JJ, et al. A randomized six-day safety study of an antiretroviral microbicide candidate UC781, a non-nucleoside reverse transcriptase inhibitor. Sex Transm Dis. 2008;35:414–419. doi: 10.1097/OLQ.0b013e318162c4d8. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JL. Poindexter A. Wheeless A. Mauck CK. Callahan MM. Safety evaluation of 1% tenofovir gel in healthy men. Int J STD AIDS. 2009;20:384–386. doi: 10.1258/ijsa.2008.008309. [DOI] [PubMed] [Google Scholar]
- 35.Melchjorsen J. Risor MW. Sogaard OS, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr. 2011;57:265–275. doi: 10.1097/QAI.0b013e3182185276. [DOI] [PubMed] [Google Scholar]
- 36.Gumbi PP. Nkwanyana NN. Bere A, et al. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T cell responses in the female genital tract during chronic HIV infection. J Virol. 2008;82:8529–8536. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGowan I. Elliott J. Fuerst M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 38.Zidek Z. Frankova D. Holy A. Activation by 9-(R)-[2-(phosphonomethoxy)propyl]adenine of chemokine (RANTES, macrophage inflammatory protein 1α) and cytokine (tumor necrosis factor α, interleukin-10 [IL-10], IL-1β) production. Antimicrob Agents Chemother. 2001;45:3381–3386. doi: 10.1128/AAC.45.12.3381-3386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGowan I. Hoesley C. Andrew P. Janocko L. Dai J. Carballo-Dieguez A, et al. MTN-007: A phase 1 randomized, double-blind, placebo-controlled rectal safety, acceptability study of tenofovir 1% gel. Presented at the 19th Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, Washington. Mar;2012 ; [Paper #34LB]. [Google Scholar]
- 40.Hendrix C. Minnis A. Guddera V, et al. MTN-001: A phase 2 cross-over study of daily oral and vaginal TFV in healthy, sexually active women results in significantly different product acceptability and vaginal tissue drug concentrations (#35LB). Presented at the 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 41.Richardson-Harman N. Mauck C. McGowan I. Anton P. Dose response relationship between tissue concentrations of UC781 and explant infectibility with HIV-1 in the RMP-01 rectal safety study. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/aid.2012.0073. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]