Abstract
Previously we showed that repeated vaginal application of a MIV-150/zinc acetate carrageenan (MIV-150/ZA/CG) gel and a zinc acetate carrageenan (ZA/CG) gel significantly protected macaques from vaginal simian human immunodeficiency virus reverse transcriptase (SHIV-RT) infection. Gels were applied either daily for 2 weeks or every other day for 4 weeks, and the animals were challenged 4–24 h later. Herein, we examined the effects of a single vaginal dose administered either before or after virus challenge. Encouraged by the vaginal protection seen with MIV-150/ZA/CG, we also tested it rectally. Vaginal applications of MIV-150/ZA/CG, ZA/CG, and CG gel were performed once 8–24 h before, 1 h after, or 24 h before and 1 h after vaginal challenge. Rectal applications of MIV-150/ZA/CG and CG gel were performed once 8 or 24 h before rectal challenge. While vaginal pre-challenge and pre/post-challenge application of MIV-150/ZA/CG gel offered significant protection (88%, p<0.002), post-challenge application alone did not significantly protect. ZA/CG gel reduced infection prechallenge, but not significantly, and the effect was completely lost post-challenge. Rectal application of MIV-150/ZA/CG gel afforded limited protection against rectal challenge when applied 8–24 h before challenge. Thus, MIV-150/ZA/CG gel is a highly effective vaginal microbicide that demonstrates 24 h of protection from vaginal infection and may demonstrate efficacy against rectal infection when given close to the time of HIV exposure.
Introduction
There is a pressing need for topical microbicides to prevent or significantly reduce the sexual transmission of HIV. The CAPRISA 004 study––the first Phase 2b clinical trial of a microbicide containing an anti-HIV drug to show efficacy—demonstrated that user adherence is critical to the success of a coitus-dependent microbicide.1 Microbicides with a longer duration of protection may prove more effective than a pericoital gel that has to be applied within minutes or a few hours of intercourse. Importantly, HIV is transmitted across both the rectal and vaginal mucosa, and microbicides that are developed for vaginal use may be used rectally. Because the disparity in the biology of the rectum and the vagina may require differences in the formulation of a microbicide for each route and since rectal transmission of HIV is more efficient than vaginal transmission, it is vital to test all formulations in preclinical development both vaginally and rectally.2
We are developing innovative microbicides that contain the nonnucleoside reverse transcriptase inhibitor (NNRTI) MIV-150 and zinc acetate (ZA) in a 3% carrageenan (CG) gel.3 MIV-150 binds tightly to the HIV reverse transcriptase (RT), has strong antiviral and virucidal properties, and blocks transmission of both R5 and X4-tropic isolates.3,4 Moreover, MIV-150 is not used to treat HIV-infected individuals and requires two to three amino acid mutations in the HIV-1 RT for a profound decrease in susceptibility to be observed in vitro compared to one or two for nevirapine and efavirenz, first line therapeutic NNRTIs.5 ZA is a nonantiretroviral drug added to boost antiviral activity without contributing to the development of resistance. There is evidence that zinc salts have activity against HIV as well as other viruses, including herpes simplex virus 2 (HSV-2),6–8 and we recently demonstrated potent activity of a ZA/CG gel against a lethal vaginal or rectal dose of HSV-2 in the mouse model.9
Gels containing 50 μM MIV-150 and 14 mM ZA in CG (MIV-150/ZA/CG) and 14 mM ZA in CG (ZA/CG) have shown great promise in preventing vaginal acquisition of simian human immunodeficiency virus reverse transcriptase (SHIV-RT) in a stringent macaque model.10 Daily vaginal application for 2 weeks or every other day for 4 weeks provided significant protection (compared to CG) for up to 24 h after challenge. Additionally, rectal administration of a 50 μM MIV-150/CG gel applied once 0.5–4 h before challenge completely protected macaques from rectal SHIV-RT infection and still reduced infection (although not significantly) with a higher viral dose.11
Herein, we expanded our testing of the MIV-150/ZA/CG and ZA/CG gels, examining efficacy after a single dose before or after vaginal challenge and of the MIV-150/ZA/CG gel before rectal challenge. A single vaginal application of the MIV-150/ZA/CG gel, but not the ZA/CG gel, 24 h before challenge was as effective as repeated application. When applied rectally 8–24 h before challenge, MIV-150/ZA/CG was not significantly protective, although we observed a trend toward reduced infection frequency when the gel was applied closer to the time of challenge. Taken together, our studies demonstrate the safety and efficacy of the MIV-150/ZA/CG gel against vaginal SHIV transmission under single and repeated dosing regimens with the possibility for activity against rectal infection if the gel is used closer to the time of virus exposure.
Materials and Methods
Animal treatments and challenge
Adult female Chinese and Indian rhesus macaques (Macaca mulatta) were housed and cared for at Tulane National Primate Research Center (TNPRC; Covington, LA) in compliance with the regulations detailed in the Animal Welfare Act,12 and the Guide for the Care and Use of Laboratory Animals.13 TNPRC has full accreditation from the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC#000594), and all studies were approved by the TNPRC Animal Care and Use Committee (#A4499-01) in compliance with animal care procedures. The animals ranged in age from 4 to 12 years old and ranged in weight from 4 to 10 kg. Prior to the start of the study, all animals tested negative for simian type D retroviruses, simian T cell leukemia virus-1, and SIV. For all procedures, animals were anesthetized with ketamine-HCl (10 mg/kg) and given postprocedural analgesia. Five weeks before vaginal virus challenge, animals received a single 30 mg intramuscular injection of Depo-Provera.
In the vaginal studies, 2 ml of each gel was introduced atraumatically into the vaginal vault before or after challenge with 103 TCID50 SHIV-RT (SIVmac239 expressing HIV-1HxB2 RT). In rectal studies, 3 ml of each gel was introduced into the rectum atraumatically prior to challenge with 103 TCID50 SHIV-RT. Individual animal information is summarized in Tables 1 and 2. Animals were randomly assigned to the various groups, while ensuring that there was relatively even distribution of animals of different origin across the groups when possible (dependent on animal availability). There appeared to be no impact of the animals' origin on their susceptibility to infection. This is further supported by data from historical and ongoing microbicide studies that show 63% of Indian macaques (15/24) and 60% (33/55) of Chinese macaques in placebo (no gel and placebo gel/ring) groups become infected after challenge with this virus inoculum. Real time CG-treated controls were included with each challenge (Tables 1 and 2) and these were combined with historical controls for the vaginal study (11/16 infected) for the complete analyses (since there was no difference between the different CG treatment groups; p=1, Fisher's exact test).
Table 1.
Summary of Vaginally Challenged Animals
| Animal ID | Origin | Gel | Application time | Infectiona | SIV Ab responseb |
|---|---|---|---|---|---|
| DF20 | Indian | CG | 8 h pre | − | − |
| GR57 | Indian | CG | 24 h pre | + | + |
| HL51 | Chinese | CG | 24 h pre | + | + |
| IR45 | Chinese | CG | 24 h pre | − | − |
| IR30c | Chinese | CG | 1 h post | + | + |
| DE23 | Indian | CG | 1 h post | + | + |
| IR47 | Chinese | CG | 1 h post | − | − |
| DR71d | Indian | CG | 24 h pre+1 h post | + | + |
| HL53 | Chinese | CG | 24 h pre+1 h post | − | − |
| HL55 | Chinese | MIV-150/ZA/CG | 24 h pre | − | − |
| HL54 | Chinese | MIV-150/ZA/CG | 24 h pre | − | − |
| HL56 | Chinese | MIV-150/ZA/CG | 24 h pre | − | − |
| GA18 | Indian | MIV-150/ZA/CG | 24 h pre | − | − |
| IE79 | Chinese | MIV-150/ZA/CG | 24 h pre | − | − |
| IE81 | Chinese | MIV-150/ZA/CG | 24 h pre | − | − |
| HL67 | Chinese | MIV-150/ZA/CG | 24 h pre | − | − |
| GT64 | Chinese | MIV-150/ZA/CG | 1 h post | − | − |
| IC84 | Chinese | MIV-150/ZA/CG | 1 h post | − | − |
| GT60 | Chinese | MIV-150/ZA/CG | 1 h post | + | + |
| GT66 | Chinese | MIV-150/ZA/CG | 1 h post | − | − |
| GK16 | Indian | MIV-150/ZA/CG | 1 h post | + | + |
| HL63 | Chinese | MIV-150/ZA/CG | 1 h post | − | − |
| HL71 | Chinese | MIV-150/ZA/CG | 1 h post | + | + |
| GT58 | Chinese | MIV-150/ZA/CG | 24 h pre+1 h post | − | − |
| HL46 | Chinese | MIV-150/ZA/CG | 24 h pre+1 h post | − | − |
| IR27 | Chinese | MIV-150/ZA/CG | 24 h pre+1 h post | + | + |
| GM30 | Indian | MIV-150/ZA/CG | 24 h pre+1 h post | − | − |
| DE32 | Indian | MIV-150/ZA/CG | 24 h pre+1 h post | − | − |
| IE89 | Chinese | MIV-150/ZA/CG | 24 h pre+1 h post | − | − |
| HL69 | Chinese | MIV-150/ZA/CG | 24 h pre+1 h post | − | − |
| HM31 | Chinese | ZA/CG | 24 h pre | + | + |
| IR43 | Chinese | ZA/CG | 24 h pre | − | − |
| HM38 | Chinese | ZA/CG | 24 h pre | − | − |
| IR44 | Chinese | ZA/CG | 24 h pre | + | + |
| IC80 | Chinese | ZA/CG | 24 h pre | + | + |
| IR50 | Chinese | ZA/CG | 24 h pre | − | − |
| GA18 | Indian | ZA/CG | 24 h pre | − | − |
| DN72 | Indian | ZA/CG | 24 h pre | + | + |
| FH48 | Indian | ZA/CG | 8 h pre | − | − |
| CK44 | Indian | ZA/CG | 8 h pre | + | + |
| BV57 | Indian | ZA/CG | 8 h pre | − | − |
| CR36 | Indian | ZA/CG | 8 h pre | + | + |
| FI14 | Indian | ZA/CG | 8 h pre | − | − |
| DE32 | Indian | ZA/CG | 8 h pre | − | − |
| GJ29 | Chinese | ZA/CG | 1 h post | + | + |
| IR20 | Chinese | ZA/CG | 1 h post | + | + |
| IR40 | Chinese | ZA/CG | 1 h post | + | + |
| HL70 | Chinese | ZA/CG | 1 h post | + | + |
| IR38 | Chinese | ZA/CG | 1 h post | + | + |
| GJ79 | Chinese | ZA/CG | 1 h post | − | − |
All animals were concordant for plasma viral RNA and PBMC SIV DNA. DNA was tested at weeks 2–8.
ELISA for Abs to SIV was performed at weeks 4–8 and compared with baseline.
Animal was euthanized at week 4 as she exhibited self-mutilating psychoses.
Animal exhibited signs of respiratory issues and had enlarged lymph nodes, and was euthanized 28 weeks postchallenge (just after the completion of the study).
Table 2.
Summary of Rectally Challenged Animals
| Animal ID | Origin | Gel | Application time | Infectiona | SIV Ab responseb |
|---|---|---|---|---|---|
| CM24 | Chinese | CG | 24 h | + | + |
| DF62 | Chinese | CG | 24 h | + | + |
| DH23 | Chinese | CG | 24 h | + | + |
| DD56 | Indian | CG | 8 h | − | − |
| IR34 | Chinese | CG | 8 h | + | + |
| IR35 | Chinese | CG | 8 h | + | + |
| ER55 | Chinese | MIV-150/ZA/CG | 24 h | + | + |
| GD42 | Chinese | MIV-150/ZA/CG | 24 h | + | + |
| GR50 | Chinese | MIV-150/ZA/CG | 24 h | − | − |
| GT69 | Chinese | MIV-150/ZA/CG | 24 h | + | + |
| HH36 | Chinese | MIV-150/ZA/CG | 24 h | + | + |
| HH63 | Chinese | MIV-150/ZA/CG | 24 h | + | + |
| HH82 | Chinese | MIV-150/ZA/CG | 24 h | − | − |
| BR72 | Indian | MIV-150/ZA/CG | 8 h | + | + |
| DG41 | Indian | MIV-150/ZA/CG | 8 h | + | + |
| FM70 | Indian | MIV-150/ZA/CG | 8 h | − | − |
| IR25 | Chinese | MIV-150/ZA/CG | 8 h | − | − |
| IR26 | Chinese | MIV-150/ZA/CG | 8 h | + | + |
| IR32 | Chinese | MIV-150/ZA/CG | 8 h | + | + |
| IR33 | Chinese | MIV-150/ZA/CG | 8 h | − | − |
All animals were concordant for plasma viral RNA and PBMC SIV DNA. DNA was tested at weeks 2–8.
ELISA for Abs to SIV was performed at weeks 4–8 and compared with baseline.
EDTA blood was collected and transported overnight from TNPRC to our laboratories at the Population Council for processing and analysis. Peripheral blood mononuclear cells (PBMCs) and plasma were processed as previously described.10 Animals that became sick during the study were euthanized using methods consistent with recommendations of the American Veterinary Medical Association (AVMA) Panel on Euthanasia.
Virus stock
The in vivo challenge stock of SHIV-RT was grown from the original stock provided by DisaBottiger (Medivir AB, Sweden)4 using PHA-activated macaque PBMCs and titered before use.11
Microbicide formulations
CG (3% w/w, lot numbers 101015A515MR, 110509A515MR, and 110307A515ML) was used as the control gel. MIV-150/ZA/CG contained 3% CG, 50 μM MIV-150, 14 mM ZA (Lot numbers 101015A1005MR, 110606A1005MR, and 110307A1005ML), and 1% DMSO. ZA/CG (Lot number 110606A707MR) contained 3% CG and 14 mM ZA. Gels were stored at room temperature and used within 28 days of formulation. Gel viscosity and anti-HIV activity were verified for each lot prior to in vivo use (data not shown).
Determining SHIV-RT infection
Plasma viral RNA copies were measured by quantitative RT-PCR.11,14 The presence of PBMC DNA virus was evaluated with nested PCR as previously described.10,11 SIV-specific antibodies (Abs) were monitored by ELISA 4–8 weeks postchallenge as previously described.10,11,15 Percent protection was calculated using the formula: 1-[infected test(infected/challenged)/infected placebo(infected/challenged)] ×100.
RT gene sequencing
The HIV-1 RT gene of SHIV-RT was sequenced from plasma viral RNA taken at peak infection (unless otherwise noted) with minor modifications to the published method.10 The Ultra Sens Viral RNA kit (Qiagen) was used for extraction of viral RNA and the Blunt TOPO TA cloning kit (Invitrogen) was used to clone the RT genes.
LCMS/MS measurement of MIV-150 in plasma
Plasma samples were processed by adding acetonitrile (ACN) to precipitate proteins and inactivate viruses. The processed samples were vortexed for 30 min at room temperature and then centrifuged. The supernatant was diluted with water (1/4 volume) and MIV-150 quantified. MIV-160 (a related NNRTI with similar chemical properties) was used as the internal standard (IS). The compounds were separated using a Waters ACQUITY UPLC BEH analytical column (1.7 μm, 2.1×50 mm) using gradient elution with a mobile phase consisting of 5% ACN in water [A] and ACN [B], with 0.1% acetic acid being added to both [A] and [B], at a flow rate 0.5 ml/min. The retention times for MIV-150 and MIV-160 were 0.8 and 0.57 min, respectively, with a total run time of 5 min. The analytes were detected with a Xevo-TQs triple quadrupole mass spectrometer in positive electrospray ionization mode using multiple reaction monitoring (MRM). The extracted ions monitored following MRM transitions were m/z 369.296→224.114 for MIV-150 and m/z 343.2→198.06 for MIV-160 (IS). The assay was linear over the range of 0.02–20 ng/ml when 0.05 ml of plasma was used in the extraction. The overall intraday and interday assay variation was <15%.
Statistical analyses
Fisher's exact test (GraphPad Prism version 5.02 for Windows, GraphPad Software, San Diego, CA) was used for statistical comparison of the percentage of SHIV-RT-infected animals in the differently treated groups. p values<0.05 were considered statistically significant.
Results
A single application of the MIV-150/ZA/CG gel prevents vaginal SHIV-RT infection for up to 24 h
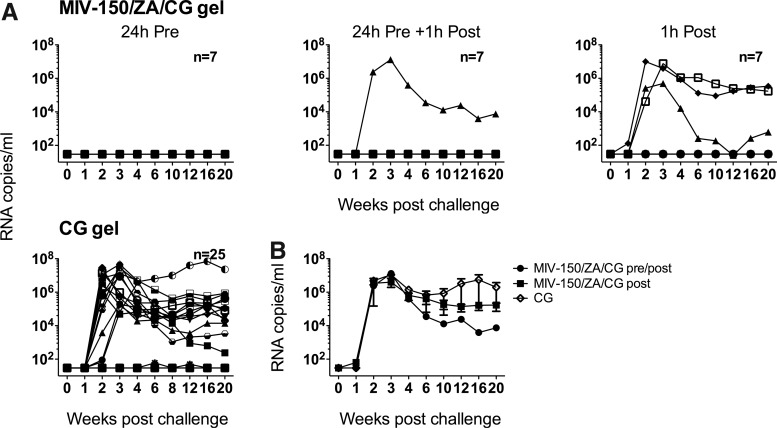
In previous studies, we showed that daily or every other day repeated application of MIV-150/ZA/CG gel provided macaques with a highly significant level of protection (88.9%, p<0.0002) from vaginal SHIV-RT infection for up to 24 h after the last gel application.10 To evaluate whether a single vaginal application of the gel was effective, we compared application of the MIV-150/ZA/CG gel once either 24 h before, 1 h after, or both 24 h before and 1 h after challenge with 103 TCID50 SHIV-RT, comparing the infection frequency with animals receiving the CG control gel. We observed a 64% (16 of 25) infection rate in animals receiving the CG gel, consistent with previous observations.4,10 When a single dose of the MIV-150/ZA/CG gel was applied 24 h before challenge, none of the seven animals became infected (100% protection vs. CG). When a single dose was applied 24 h before challenge and then 1 h after, one of seven animals became infected (78% protection vs. CG) (Fig. 1A and Table 1). Because we observed complete protection in the 24 h pre-group, we were unable to detect an effect of the 1 h post-exposure (no difference in infection frequency between these groups, p=1.00). Thus, we pooled the results for statistical analysis. Protection afforded by the MIV-150/ZA/CG gel applied 24 h pre or 24 h pre+1 h post was 89% compared to the CG gel, p<0.002 (Table 3).
FIG. 1.
A single dose of MIV-150/ZA/CG gel protects against vaginal simian human immunodeficiency virus reverse transcriptase (SHIV-RT) infection for up to 24 h. (A) MIV-150/ZA/CG or CG gel was administrated either 24 h before, 1 h after, or 24 h before and 1 h after vaginal SHIV-RT challenge. The number of animals in each treatment group is indicated. For CG gel this includes 9 real time and 16 historical controls. Plasma viral loads for each animal are shown over time (SIV RNA copies/ml). (B) Mean (±SEM) plasma viral load of infected animals from each group.
Table 3.
Statistics on Vaginally Challenged Animals
| Gel | Gel dosing relative to challenge | Infected/challenged | Protection vs. CG | p value vs. CG |
|---|---|---|---|---|
| MIV-150/ZA/CG | 24 h pre (±1 h post) | 1/14 | 89% | <0.002 |
| MIV-150/ZA/CG | 1 h post | 3/7 | 29% | <0.7 |
| ZA/CG | 8 h pre | 2/6 | 48% | <0.37 |
| ZA/CG | 24 h pre | 4/8 | 22% | <0.7 |
| ZA/CG | 1 h post | 5/6 | 0% | 1 |
| CG | 16/25a |
The number of challenged animals for CG included 9 real time (Table 1)and 16 historical controls.
To test whether the gel could be effective if used solely post-exposure, we applied the MIV-150/ZA/CG gel vaginally 1 h after SHIV-RT challenge. However, three of seven animals became infected (29% protection) (Fig. 1A and Table 1), which was not significantly different than CG (Table 3). In animals that received the MIV-150/ZA/CG gel and became infected, viremia was not significantly different from viremia in control animals (Fig. 1B). Sequencing of the plasma virus RNA revealed that all of the four animals that became infected after treatment with the MIV-150/ZA/CG gel carried wild-type RT (Table 4).
Table 4.
MIV-150/ZA/CG Applied Vaginally or Rectally Does Not Select for Nonnucleoside Reverse Transcriptase Inhibitor-Resistant Variants
| Challenge route | Animal ID | L100I, K101P, K103N, V108I, I178L, V179I, Y181C, Y188L, G190E, P225H |
|---|---|---|
| Vaginal | GK16 | 0 (6) |
| IR27 | 0 (9) | |
| GT60 | 0 (16) | |
| HL71 | 0 (16) | |
| Rectal | DG41 | 0 (18) |
| IR32 | 0 (20) | |
| ER55 | 0 (15) | |
| GD42 | 1 (17) V179G | |
| GT69 | 0 (19) | |
| HH36 | 0 (15) | |
| HH63 | 0 (15) | |
| IR26 | 0 (17) | |
| BR72 | 0 (14) |
The table lists the number of clones in which amino acid mutations conferring NNRTI resistance were detected. Parentheses indicate the total number of clones sequenced per animal.
Vaginal and rectal plasma samples were all sequenced at peak viremia except for GT69, which was sequenced at week 2 (81,000 copies/ml vs. 190,000 copies/ml at the week 3 peak viremia).
A single application of the ZA/CG gel has limited activity against vaginal SHIV-RT infection
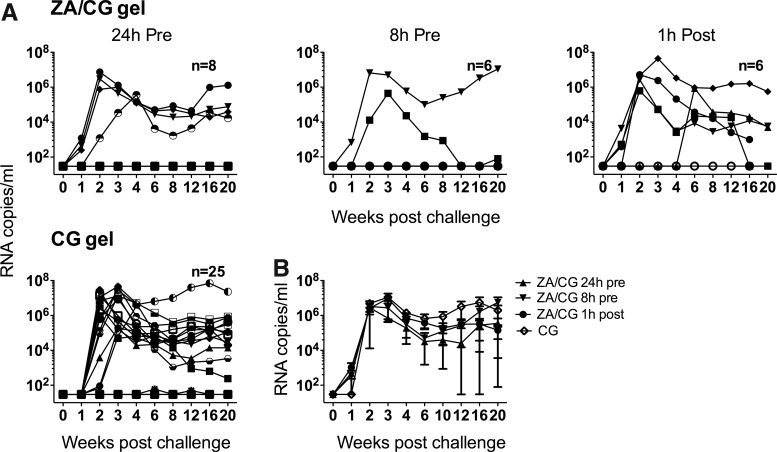
In previous work, we showed that repeated vaginal dosing of the ZA/CG gel was significantly protective (70.4% protection, p<0.017) against SHIV-RT challenge up to 24 h after the last gel.10 Testing the efficacy of a single gel application 24 h before vaginal SHIV-RT challenge, we found that four of eight animals became infected (22% protection) (Fig. 2A and Table 1). To see if protection would be increased with less time between gel application and challenge, we then applied the gel 8 h before challenge in another group of animals. In this group, the infection frequency was reduced to two of six infected (48% protection) (Fig. 2A and Table 1) but not significantly different from that at 24 h (p=0.63). Despite a trend toward reduced infection frequency when the gel was applied closer to the time of challenge, protection in neither the 8 h nor the 24 h group was significant (Table 3). Even comparing the pooled data from the 8 h and 24 h ZA/CG groups to the CG controls did not reveal a significant difference (33% protection, p<0.32). When we applied the gel once 1 h postchallenge, there was no protection at all, with five of six animals becoming infected (Fig. 2A and Tables 1 and 3). As was observed with the MIV-150/ZA/CG gel, all infected animals experienced similar viremia (Fig. 2B).
FIG. 2.
A single dose of ZA/CG gel has limited activity against vaginal SHIV-RT infection. (A) ZA/CG or CG gel was applied either 24 h before, 8 h before, or 1 h after vaginal SHIV-RT challenge. The number of animals in each treatment group is indicated. For CG gel this includes 9 real time and 16 historical controls. Plasma viral loads for each animal are shown over time (SIV RNA copies/ml of plasma. (B) Mean (±SEM) plasma viral load of infected animals from each group.
Limited activity of the MIV-150/ZA/CG gel against rectal challenge 8–24 h after gel application
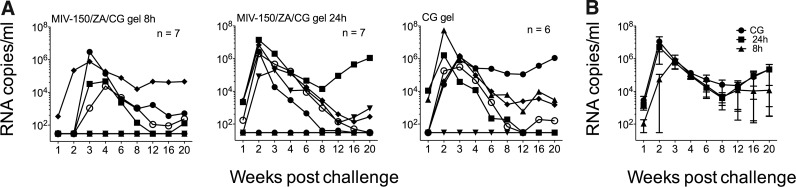
We also evaluated protection afforded by the MIV-150/ZA/CG gel against rectal infection with SHIV-RT. We had previously shown that a single dose of the MIV-150/CG gel 4 h before challenge completely protected macaques against rectal SHIV-RT infection.11 Since the MIV-150/ZA/CG gel is more effective vaginally than the MIV-150/CG gel,10 and to provide a comparison between the vaginal and rectal studies, we tested the efficacy of the MIV-150/ZA/CG gel rectally either 8 h or 24 h before rectal challenge. In the 8 h group, four of seven animals became infected (57.1%; 31% protection vs. CG) while in the 24 h group, five of seven animals became infected (71.4%; 14% protection vs. CG) (Fig. 3A and Table 2). Protection (relative to the CG gel group; five/six infected) was not significant in either group, nor was it significant when the MIV-150/ZA/CG gel groups were pooled together and compared to the CG gel group. Two animals that became infected in the group receiving the MIV-150/ZA/CG gel 8 h before challenge experienced delayed and reduced peak viremia compared to the other animals in this group. However, viremia was not significantly different in all infected animals that received the MIV-150/ZA/CG gel 8 h before challenge vs. the control overall (p=0.27) or at week 1 (p=0.56) or week 2 (p=0.11) (Fig. 3B). Of the nine animals infected in the MIV-150/ZA/CG groups all but one carried wild-type RT genes (GD42, 1 out of 17 clones tested had the V179G mutation; Table 4). Since two animals had delayed peak viremia at week 4, we additionally examined the virus at week 3 and verified that they carried wild-type RT genes (19/19 clones for DG41 and 16/16 clones for IR32 were wild type).
FIG. 3.
A single dose of MIV-150/ZA/CG gel has limited activity against rectal SHIV-RT challenge 8–24 h after gel application. (A) MIV-150/ZA/CG or CG gel was applied rectally either 8 or 24 h before rectal SHIV-RT challenge. The number of animals in each group is indicated. Plasma viral loads for each animal are shown over time (SIV RNA copies/ml of plasma). (B) Mean (±SEM) plasma viral load of infected animals from each group.
Discussion
Considering the importance of adherence in the effectiveness of an anti-HIV microbicide strategy,1 we think that the most efficacious products will be those that remain effective when used under diverse regimens according to the unique needs and desires of the users. Problems with adherence in the CAPRISA 004 trial (1% tenofovir gel) suggest that without user flexibility for dosing, gel may not be effective over the long term. Even though macaque studies are starting to address this by varying the timing of the tenofovir gel relative to virus in a repeat challenge model,16,17 more diverse regimens with additional and alternative APIs are needed. Building on previous data showing that repeated vaginal use of MIV-150/ZA/CG and ZA/CG gels reduced vaginal SHIV-RT acquisition in macaques,10 we evaluated the vaginal protection offered by a single application of these gels. Since vaginal gels might be used rectally and an ideal product could be used to prevent both vaginal and rectal HIV infection, we also obtained efficacy data on rectal application of the MIV-150/ZA/CG against rectal SHIV-RT infection.
Vaginal application of the MIV-150/ZA/CG gel was significantly protective compared to CG when applied once 24 h before challenge or both 24 h before and 1 h after challenge. Protection was the same as we previously observed for repeated application (1/14 infected for single vs. 2/28 infected for repeated10), indicating that the MIV-150/ZA/CG gel is as effective when used once prior to virus exposure as when used regularly over a 2- to 4-week period. Although a single vaginal application of the ZA/CG gel 8–24 h before challenge was not significantly protective compared to the CG gel, the effect of this regimen was also not statistically different from the significant protection we previously observed for repeated application of this gel (6/14 infected for single vs. 4/21 infected for repeated,10 p=0.16). Had we been able to test the ZA/CG gel in a larger group of animals, we might have been able to demonstrate a modest effect of a single dose. Nevertheless, our data clearly demonstrate that repeated application is needed for greater efficacy by the ZA/CG gel.
The benefit of repeated dosing for the ZA/CG gel that is not required for the MIV-150/ZA/CG gel may lie in the mechanism of action of ZA. Building on earlier reports,9,18,19 we have demonstrated that ZA has antiviral activity in vitro (Fernández-Romero, Hsu, and Robbiani, unpublished). A recent study suggests that this is at least partially mediated by zinc's ability to impede RT activity.20 In vivo, it is possible that ZA renders the mucosal surface more resistant to infection, and repeated application may provide positive feedback to promote or sustain this antiviral state.18,19,21,22 Additionally, the amount of zinc absorbed into the tissues may be greater with repeated application of the ZA/CG gel, and this may translate into greater efficacy (through antiviral and/or immunomodulatory mechanisms).
Importantly, limited protection was observed when the gels were applied only 1 h post challenge. This demonstrates that the gels are unsuitable for post exposure prophylaxis in a single dose and highlights that in the animals given the MIV-150/ZA/CG gel both 24 h pre-challenge and 1 h post challenge, the 24 h preexposure dose was the protective component. Preliminary data in an activated T cell system suggest greater in vitro antiviral activity of ZA after repeated dosing post virus exposure compared to a single co-exposure to ZA and virus (Fernández-Romero, unpublished). However, whether repeated dosing post virus exposure is effective in vivo remains to be determined.
We previously observed that a single rectal dose of the MIV-150/CG gel (which was only 56% protective vaginally compared with the 89% protection seen with the MIV-150/ZA/CG gel) completely protected macaques (zero of four infected, 0%) when given 4 h before rectal SHIV-RT challenge.11 Since vaginal gels will likely be used rectally, we wanted to establish the duration of protection afforded by the more potent MIV-150/ZA/CG gel when applied rectally (using the time points tested for vaginal challenge). However, we found that the MIV-150/ZA/CG gel had limited efficacy rectally when used 8–24 h before challenge, only slightly (and not significantly) reducing infection compared to the CG gel. Since the original rectal testing of the MIV-150/CG gel did not include a CG gel only group, we cannot be certain that there was no barrier effect of CG (within the MIV-150/CG gel) at the 4 h time point that contributed to the protection seen.11 Notably, there is basically no barrier effect of CG when given 8 h prior to challenge (compare the five/six infected in the CG group herein to the four/four infected in the methyl cellulose placebos in our earlier study11).
However, independently these data underscore that this gel can prevent rectal infection if used closer to the time of challenge (even if this might be contributed to by the CG barrier effect). In practice, rectal gel use is more likely to coincide with the timing of sex than a vaginal gel since a lubricant is often used to facilitate anal intercourse.23 Thus, the real world need for rectal gels that offer several hours of protection is limited. Moreover, knowing that a vaginal gel does not exacerbate rectal infection is important. Despite in vitro data suggesting a potential enhancing effect of CG on HIV infection,4,24,25 we have found no evidence of CG-mediated enhancement in macaques following vaginal or rectal application, even when challenging up to 24 h after gel dosing (data herein and unpublished observations).4,10,11,26 This also supports the earlier findings from the Phase 3 trial testing the efficacy of CG against HIV infection, where the HIV infection frequency was reduced, albeit not significantly, in the CG arm27 and that HIV shedding was not enhanced in HIV-infected women using CG.28
Although the MIV-150/ZA/CG gel had greater activity vaginally than the gels containing either only MIV-150 or ZA, specific controls of animals treated rectally with the MIV-150/CG or ZA/CG gels would be needed to verify the contribution of both agents rectally. It is possible that ZA may have more limited activity against rectal than vaginal SHIV-RT infection, which would lead the MIV-150/ZA/CG gel to perform much like the MIV-150/CG gel. However, the ZA/CG gel has been shown to effectively prevent high-dose rectal HSV-2 infection in mice,9 indicating that ZA is active in the presence of rectal enzymes and microflora.
Twelve of 13 animals that became infected after treatment with the MIV-150/ZA/CG gel (vaginally or rectally) had SHIV-RT carrying the wild-type RT gene. In one clone (of 17) from animal GD42 we detected the V179G mutation in the RT gene. V179 is a highly polymorphic position. V179F has been described as an important mutation frequently selected by etravirine, decreasing susceptibility to this drug when it is present in combination with Y181C/I/N.29 However, V179D/E are often found in treatment-naive individuals, with no substantial impact on NNRTI-containing treatment when present by themselves.29
A recent review noted that the prevalence of V179G in NNRTI-resistant vs. non-NNRTI-resistant samples is 0.15 vs. 0.03%, respectively, and that it resulted in an IC50 fold change of only 0.6 for efavirenz and etravirine.30 The effect of this mutation on MIV-150 susceptibility still needs to be tested phenotypically, but because of the polymorphism of this amino acid position, it is unlikely that this mutation will have a profound effect on MIV-150 antiviral activity. Finding predominantly wild-type virus (especially in the plasma) is not surprising since MIV-150 was not detected in the plasma after 24 h (<20 pg/ml), indicating that any MIV-150 absorbed into the blood was cleared rapidly (as we have seen in rats treated with the MIV-150/ZA/CG gel; Fernández-Romero and Zydowsky, unpublished). We were unable to perform pharmacokinetic studies to measure MIV-150 levels in the tissues at the time of challenge and so cannot rule out that there would have been differences between the various tissue drug levels (or earlier plasma levels) that might explain the differences in efficacy (vaginally vs. rectally). Additionally, the emergence of drug-resistant mutations in virus within the tissues (where virus might be exposed to drug over a longer period of time) is being investigated using SHIV-RT-infected macaques.
Expanding our original findings (i.e., repeated use of the MIV-150/ZA/CG gel prevented vaginal immunodeficiency virus infection), we provide evidence suggesting that even sporadic use of the MIV-150/ZA/CG gel blocks vaginal immunodeficiency virus and HSV-2 infections. Future work will address the window of protection for rectal use. Our findings encourage the advancement of this product for clinical testing.
Acknowledgments
We thank the veterinary staff at the TNPRC for continued support and Laiju (John) Zhang for statistical assistance. This work was supported by the United States Agency for International Development (USAID) Cooperative Agreement GPO-A-00-04-00019-00, the Swedish Ministry of Foreign Affairs, the Swedish International Development Cooperation Agency, the NIH base grant RR00164, and with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E. This research is made possible by the generous support of the American people through the USAID.
The contents of this manuscript are the sole responsibility of the Population Council and do not necessarily reflect the views or policies of USAID or the U.S. government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. None of the material in this article has been published or is under consideration elsewhere, including the Internet. M.R. is a 2001 Elizabeth Glaser scientist.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Karim QA, et al. Effectiveness, safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science Express. 2010 Jul 20; doi: 10.1126/science.1193748. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shattock RJ. Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Romero JA, et al. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sexually Transmit Dis. 2007;34:9–14. doi: 10.1097/01.olq.0000223287.46097.4b. [DOI] [PubMed] [Google Scholar]
- 4.Turville SG, et al. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One. 2008;3:e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren J. Stammers DK. Structural basis for drug resistance mechanisms for non-nucleoside inhibitors of HIV reverse transcriptase. Virus Res. 2008;134:157–170. doi: 10.1016/j.virusres.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Kumel G, et al. The mechanism of the antiherpetic activity of zinc sulphate. J Gen Virol. 1990;71:2989–2997. doi: 10.1099/0022-1317-71-12-2989. [DOI] [PubMed] [Google Scholar]
- 7.Haraguchi Y, et al. Inhibition of HIV-1 infection by zinc group metal compounds. Antivir Res. 1999;43:123–133. doi: 10.1016/s0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 8.Arens M. Travis S. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J Clin Microbiol. 2000;38:1758–1762. doi: 10.1128/jcm.38.5.1758-1762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Romero JA, et al. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob Agents Chemother. 2012;56:358–368. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney J, et al. An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLoS One. 2011;6:e15835. doi: 10.1371/journal.pone.0015835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer R, et al. The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J Virol. 2011;85:5504–5512. doi: 10.1128/JVI.02422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Animal Welfare Act and Regulation of 2001. U.S. Department of Agriculture; Beltsville, MD: Code of Federal Regulations, t., chapter 1, subchapter A: Animals and Animal Products. [Google Scholar]
- 13.Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals. U.S. Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 1985. Vol. Publication no. 85-23 1-83. [Google Scholar]
- 14.Cline AN, et al. Highly sensitive SIV plasma viral load assay: Practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith SM, et al. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: Predictive value for disease progression. AIDS Res Hum Retroviruses. 1999;15:1691–1701. doi: 10.1089/088922299309739. [DOI] [PubMed] [Google Scholar]
- 16.Parikh UM, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobard C, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86:718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankar AH. Prasad AS. Zinc and immune function: The biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 19.Mocchegiani E. Muzzioli M. Therapeutic application of zinc in human immunodeficiency virus against opportunistic infections. J Nutr. 2000;130:1424S–1431S. doi: 10.1093/jn/130.5.1424S. [DOI] [PubMed] [Google Scholar]
- 20.Fenstermacher KJ. DeStefano JJ. Mechanism of HIV reverse transcriptase inhibition by zinc: Formation of a highly stable enzyme-(primer-template) complex with profoundly diminished catalytic activity. J Biol Chem. 2011;286:40433–40442. doi: 10.1074/jbc.M111.289850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirano T, et al. Roles of zinc and zinc signaling in immunity: Zinc as an intracellular signaling molecule. Adv Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- 22.Rink L. Kirchner H. Zinc-altered immune function and cytokine production. J Nutr. 2000;130:1407S–1411S. doi: 10.1093/jn/130.5.1407S. [DOI] [PubMed] [Google Scholar]
- 23.Gross M, et al. Anal sex among HIV-seronegative women at high risk of HIV exposure. The HIVNET Vaccine Preparedness Study 2 Protocol Team. J Acquir Immune Defic Syndr. 2000;24:393–398. doi: 10.1097/00126334-200008010-00015. [DOI] [PubMed] [Google Scholar]
- 24.Pirrone V, et al. Application and removal of polyanionic microbicide compounds enhances subsequent infection by HIV-1. Virology. 2012;J9:33. doi: 10.1186/1743-422X-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirrone V, et al. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 2011;90:168–182. doi: 10.1016/j.antiviral.2011.03.176. [DOI] [PubMed] [Google Scholar]
- 26.Crostarosa F, et al. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection, the impact of HSV-2 on microbicide efficacy. PLoS One. 2009;4:e8060. doi: 10.1371/journal.pone.0008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skoler-Karpoff S, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 28.McLean CA, et al. HIV genital shedding, safety of Carraguard use by HIV-infected women: A crossover trial in Thailand. AIDS. 2010;24:717–722. doi: 10.1097/QAD.0b013e328333bf89. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DE, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4:e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tambuyzer L, et al. Compilation and prevalence of mutations associated with resistance to non-nucleoside reverse transcriptase inhibitors. Antivir Ther. 2009;14:103–109. [PubMed] [Google Scholar]