Abstract
Objective:
The aim of the present study was to examine the periodontal condition of an adult population in three isolated regions in Greece and to determine the association of periodontal disease with several demographic, behavioral and environmental factors.
Materials and Methods:
The study population consisted of 640 individuals, aged 20 to 69 years from three isolated regions. The following indices were assessed: Pocket Depth (PD), Clinical Attachment Level (CAL), Dental Plaque, Calculus and Bleeding on Probing (BOP). Statistical analysis was accomplished by multiple linear regression model which was used to assess the association between the mean clinical attachment loss and clinical, demographic and behavioral parameters.
Results:
The samples of the study showed high levels of dental plaque, dental calculus and BOP. The final multivariate model showed that age (p=0.000), gender (p=0.016) and presence of calculus (p=0.000) were associated with the mean clinical attachment loss. Age (p=0.000), gender (p=0.000) and dental plaque (p=0.027) were associated with gingival recession, while age (p=0.018) and gender (p=0.000) were associated with probing depth. Bleeding on probing, dental plaque, toothbrush frequency, level of education, tobacco consumption and reasons for dental visits were not associated with the mean clinical attachment loss.
Conclusion:
Periodontal disease consists of a complicated destructive condition of the Periodontal tissue with a. multi-factorial etiology. Oral hygiene instructions and a regular dental follow-up could play a significant role in the prevention of periodontal disease.
Keywords: Periodontal Disease, Epidemiology, Risk Factors
INTRODUCTION
Periodontal disease is a progressive inflammation of the periodontal tissue. It starts with bleeding gums, but can finally lead to tooth loss in case of negligence. Periodontal disease ranges from mild gingivitis to severe disease that results in the destruction of the supporting tissue and alveolar bone loss [1].
Previous epidemiological studies in developing countries have shown that the periodontal status of the population that determined by the presence of gingival inflammation, dental plaque and dental calculus was worse compared to countries with higher developmental levels [2,3].Other epidemiological studies [3–5] suggested that subjects in developing countries did not necessarily have a higher prevalence and severity of period on titis than subjects in industrialized nations even though they may have more dental plaque, calculus and gingival inflammation.
Previous studies have reported general conclusions regarding the assessment of periodontal status, as they were based on pre-selected population samples [6,7]. Miyazaki [5] reviewed the community periodontal index of treatment needs (CPITN) data according to the previous classification of this index from independent surveys conducted in 79 countries and reported little difference in the prevalence of periodontal pockets between developing and industrialized countries.
Very few epidemiological studies [4] that have been carried out in African countries showed low extent and severity of periodontal pockets or attachment loss, while more studies and reports are available from Eastern European and Scandinavian countries [8,9]. Similar investigations have not been carried out on a wide spectrum of population samples in Greece during the last decades. Comparisons with the 1985 findings indicate that severe periodontal diseases may be declining in Greece, whereas gingivitis may be increasing [10].
In order to test the characterization of prevalence and severity of periodontal disease, significance of differences between full-mouth examination and partial recording protocols is limited. For this reason, the mentioned protocols for assessment of periodontal status include several periodontal indices, such as Russell’s Index, Periodontal Disease Index, Gingival Periodontal Index, Gingival Bone Count Index, Extent and Severity Index and Community Periodontal Index [11].
Therefore, the purpose of the present study was to examine the periodontal condition of a sample of adults in three isolated regions in a city in Greece and estimate the association of demographic, behavioural and clinical factors with periodontal disease.
MATERIALS AND METHODS
Study population:
In the present study, 640 individuals, 300 males and 340 females, 20–69 years of age were selected. All the selected subjects were inhabitants of three isolated villages in N. W Achaia, one of the biggest municipalities in Greece, who visited a private practitioner for their regular dental follow-up which was organized by the Greek Dental Association for all the Greek population annually. The whole population of the mentioned villages was estimated as 1,023 inhabitants according to the local authorities.
The majority of the inhabitants worked in the capital of Achaia, Patra and attended the Local University. Inco-operation between the Greek Dental Association and the local authorities, they emphasized the necessity and importance for an annual dental follow-up.
An additional motivation for participation was the distribution of printed instructions on proper oral hygiene aspects to the individuals examined.
The sample was divided into five groups based on age; namely, group I (120 individuals), 20 to 29 years old; group II (130 individuals), 30 to 39 years old; group III (140 individuals), 40 to 49 years old; group IV (130 individuals), 50 to 59 years old; group V (120 individuals), 60 to 69 years old.
The participants of the study underwent an oral clinical and physical examination and filled a similar self-administered questionnaire that included several epidemiological variables. The oral health study presented here took place between May and October 2011 at the mentioned private practice.
Ethics:
The present study was not an experimental one. In Greece, only experimental studies must be reviewed and approved by authorized committees (such as the Greek Dental Association, Ministry of Health).
All participants were informed about the evaluation to which they would be submitted and gave their informed consent for participation.
Inclusion criteria:
If an index tooth was absent, the closest distal tooth was used as a substitute. In the absence of a tooth distal to the index tooth, the next tooth in the mesial location was measured. Molar or premolar index teeth were never replaced by the examination of an anterior tooth. In individuals with extensive destruction of the cemen to-enamel junction due to dental caries, erosion, abrasion, presence of prosthetic restoration or dental calculus, the location of the junction was estimated according to the location of adjacent teeth.
Exclusion criteria:
Individuals who had undergone a previous periodontal treatment, conservative or surgical, within the previous six months were excluded from the study sample.
Edentulous adults (n= 0) were excluded from the study due to the impossibility of evaluating the periodontal conditions in this population. Similarly, wisdom teeth and individuals with systemic disorders were excluded from the study.
Questionnaire:
Two independent physical examinations were performed, one blinded and one non-blinded to the medical history. Just before the physical examination, participants filled in a similar self-administered questionnaire that included several epidemiological variables such as age, gender, educational level (primary, secondary, college, university), smoking status (current regular smoker, occasional smoker and non-smoker), dental visits (frequency and reasons for the last dental visit), oral hygiene habits (tooth brush frequency, use of dental floss/mouthwash) and data regarding their variables of their general health. Socioeconomic status of the sample was excluded from the study because of the current economic crisis in Greece, as it could be a negative motivation for the individuals in order to participate. On the other hand, the study sample consisted of individuals who were permanent inhabitants in isolated areas and no significant differences regarding their socieconomic status would be expected.
Clinical Examination:
One well-trained and calibrated dentist who was also registered as an active member. In the Hellenic Society of Periodontology (HSP) and European Federation of Periodontology (EFP) performed the examinations of the participants and the clinical measurements.
The clinical measurements concerned the following teeth (according to Ramfjord Index): 16, 21, 24, 36, 41 and 44 [12].
The reason that Ramfjord Index was used is That it represents a simple, easy, reproducible and representative as possible periodontal index, especially in isolated societies with a low-level of oral hygiene and it is one of the most appropriate indices for partial-mouth recording protocols.
The variables that were measured clinically were the following:
-
Assessment of dental plaque and dental calculus (0= no calculus, 1= supra-gingival calculus, 2= subgingival calculus). Presence or absence of supra-gingival plaque was recorded after disclosing soft deposits using erythromycin solution (3%) for a period of 30 seconds; the teeth and gingival were dried with compressed air while dental unit Light was used as the light source for the inspections. Dental plaque and calculus Indices were recorded from the mesio-buccal site of each index tooth. The Plaque Index (PlI), which was used was a modification of the one determined by Löe [13].
Scores 2 and 3 were difficult to be distinguished from each other and therefore were Combined into a single category (i.e., 0= no dental plaque, 1= plaque detected after running the probe across the cervical area of the index tooth, 2= plaque that could be detected visually).
Probing depth (PD) assessment
Clinical attachment level (CAL)
Location of the free gingival margin (FGM) relative to the cemento-enamel junction(CEJ), FGM-CEJ
Bleeding on probing (BOP)
The above mentioned indices (PD), (CAL), (FGM-CEJ) and (BOP) were recorded at six sites on each index tooth.
A William’s probe (PCP10-SE, Hu-Friedy™ Mfg. Co. Inc., Chicago, IL, USA) color coded at 1, 2, 3, 5, 7, 8, 9, 10mm was used for periodontal examinations and PD and CAL were measured to the nearest millimeter.
Gingival inflammation was determined by the percentage of sites per individual that showed BOP.
Mean CAL (averaged across sites in each individual)was used to describe the cumulative amount of periodontal destruction. Prevalence of periodontal destruction was determined as the percent of individuals who showed a 4.0–6.0 mm or higher than 7.0 mm CAL at their most severely affected sites.
The extent of mild, moderate and severe periodontal destruction was estimated by the percent of sites per individual with a CAL of 2.0–4.0 mm, 4.0–6.0 mm and higher than 7.0 mm [3]. The reason why the mentioned classification was not based on the American Academy of Periodontology [14], is that, as mentioned in an isolated population, the oral hygiene level is poor enough and the presence of deep pockets and severity of clinical attachment loss could be expected extensively.
Reproducibility:
A randomly chosen sample of 128(20%) individuals was re-examined clinically by the same dentist in order to establish the intra-examiner variance. After consideration of the code numbers of the double examined participants no differences were recorded between the 1st and 2nd clinical assessments. It is obvious that in this case the in traexaminer consistency of clinical recordings per sextant was not estimated by k index.
Statistical analysis:
The individual was the statistical unit. For each participant, the mean values of the clinical parameters; namely, the dental plaque, dental calculus, PD, CAL and FGM-CEJ were calculated. Multiple linear regression analysis was used in order to assess the association of the mean CAL with demographic, behavioural and clinical parameters.
Associations were assessed by adding each independent variable to the fully adjusted model and testing whether the explained variable was increased significantly.
Tooth brushing frequency was classified into three levels (daily, occasionally, never).
Frequency of dental visits was dichotomized (yearly or more frequently vs. less frequently or never). Gender and smoking were classified as dichotomous variables and age, mean plaque and mean calculus scores (averaged across sites in each individual) were used as continuous variables.
The final multivariate model contained variables that were significant after adjusting for all other variables in the model.
The variables included in the final model for mean CAL were then included in regressions using mean PD and mean FGM-CEJ as dependent variables.
The purpose for using the above mentioned procedure was to assess whether the association of the independent variables with the mean CAL was related to variations in mean PD and/or to variations in the location of the FGM relative to the CEJ.
The data analysis was performed using the statistical package of SPSS ver.17.0 (SPSS Inc., Chicago, IL, USA).
A p value less than 5% (P<0.05) was considered as statistically significant.
RESULT
The total number of the participants who visited the mentioned practice was 712, of which 673 met the selection criteria and 640 of them accepted the invitation to participate in the study giving a response rate of 95.1%. The mean age of the sample of the study was 46.8 ± 2.5 years.
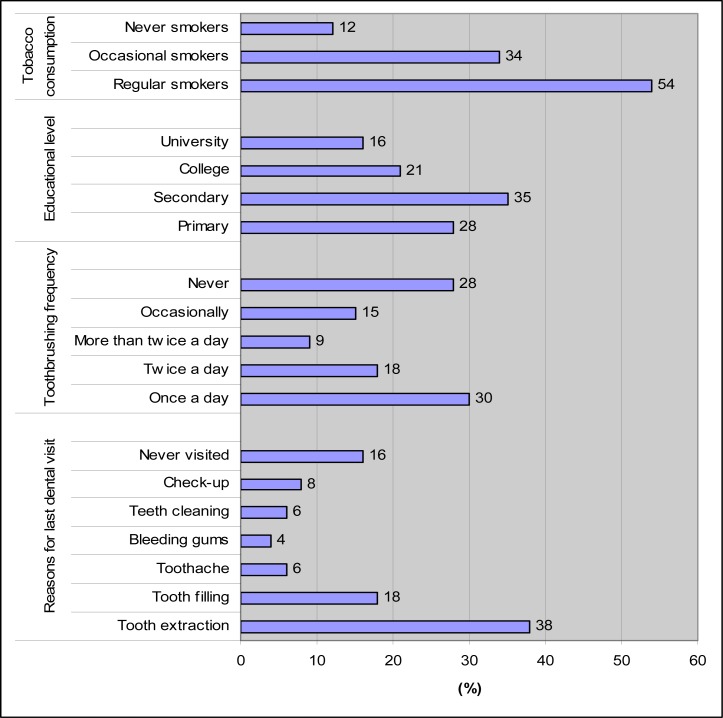
The educational level, tobacco consumption, reasons for seeking dental care and oral hygiene habits of the sample of the study are shown in figure 1.
Fig 1.
Answers from the questionnaire
Table 1 presents the extent (percent of affected sites per subject by age group and gender) of CAL, periodontal pockets, gingival recession, BOP, dental plaque and calculus. Prevalence of CAL, PD and gingival recession are shown in table 2. The final multivariate model (stepwise method) showed that age (p=0.000), gender(p=0.016) and presence of calculus (p=0.000) were associated with mean CAL. Age(p=0.018) and gender (p=0.000) were significantly associated with mean PD, while according to the same age model (p=0.000), gender (p=0.000) and the presence of dental plaque (p=0.027) were significantly associated with mean gingival recession (Table 3).
Table 1.
Extent (% of affected sites per subject by age group and gender) of clinical attachment loss, periodontal pockets, gingival recession, bleeding on probing, dental plaque and calculus
| Age (years) | N |
Periodontal parameters
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
CAL1
|
GR2 |
Probing Depth
|
BOP3 | Plaque | Calculus | |||||
| 2–3mm | 4–6mm | ≥7mm | 4–6mm | ≥7mm | ||||||
| 20–29 | 60 | 8.8% | 3.2% | 0.0% | 1.3% | 6.4% | 0.5% | 44.0% | 53.0% | 38.0% |
| 30–39 | 65 | 17.3% | 5.4% | 0.8% | 6.2% | 8.7% | 0.8% | 55.0% | 66.0% | 55.0% |
| 40–49 | 70 | 28.8% | 11.2% | 4.6% | 19.6% | 9.2% | 0.9% | 62.0% | 77.0% | 67.0% |
| 50–59 | 65 | 36.4% | 18.2% | 5.7% | 27.5% | 12.3% | 1.1% | 68.0% | 85.0% | 78.0% |
| 60–69 | 60 | 44.7% | 22.6% | 8.2% | 33.4% | 14.7% | 1.2% | 60.0% | 88.0% | 83.0% |
| Sex | ||||||||||
| Males | 150 | 23.1% | 8.4% | 1.8% | 8.8% | 11.7% | 1.1% | 59.4% | 71.5% | 67.7% |
| F/males | 170 | 21.3% | 7.8% | 0.6% | 7.2% | 8.8% | 0.7% | 56.2% | 76.1% | 60.7% |
| Total | 320 | 22.2% | 7.8% | 1.2% | 8.0% | 10.3% | 0.9% | 57.8% | 73.8% | 64.2% |
Clinical Attachment Loss
Gingival Recessions
Bleeding On Probing
Table 2.
Prevalence for clinical attachment loss, probing depth and gingival recession
| Age (years) | N |
Periodontal parameters
|
||||
|---|---|---|---|---|---|---|
| Clinical Attachment Loss | Probing Depth | Gingival Recession | ||||
|
| ||||||
| 4–6 mm | ≥7 mm | 4–6 mm | ≥7 mm | |||
| 20–29 | 60 | 21.5% | 1.4% | 62.1% | 7.3% | 19.8% |
| 30–39 | 65 | 37.8% | 6.2% | 71.6% | 8.1% | 36.5% |
| 40–49 | 70 | 48.6% | 15.8% | 78.4% | 11.4% | 51.4% |
| 50–59 | 65 | 54.7% | 22.4% | 64.6% | 12.7% | 69.6% |
| 60–69 | 60 | 68.2% | 27.6% | 60.8% | 10.4% | 78.3% |
| Sex | ||||||
| Males | 150 | 38.5% | 8.3% | 70.8% | 8.6% | 48.3% |
| Females | 170 | 38.1% | 7.9% | 64.2% | 7.2% | 42.1% |
| Total | 320 | 38.3% | 8.1% | 67.5% | 7.9% | 45.2% |
Table 3.
Final multivariate model for mean clinical attachment loss and regression on the explanatory variables ofmean probing depth and mean distance from the cement-enamel junction to the free gingival margin (gingival recession)
| Independent variables |
Periodontal parameters
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Attachment Loss (R2=0.382) | Probing Depth (R2=0.104) | CEJ-FGM1 (R2=0.307) | |||||||
|
| |||||||||
| β3 | SE4 | p-value | β | SE | p-value | β | SE | p-value | |
| Age | 0.30 | 0.00 | <0.000 | 0.13 | 0.01 | <0.01 | 0.33 | 0.01 | <0.000 |
| BOP2 | - | - | - | - | - | - | - | - | - |
| Dental plaque | - | - | - | - | - | - | 0.14 | 0.05 | <0.05 |
| Calculus | 0.37 | 0.11 | <0.000 | - | - | - | - | - | - |
| Gender | 0.15 | 0.10 | <0.01 | 0.31 | 0.04 | <0.000 | 0.54 | 0.05 | <0.000 |
Clinical attachment loss
Probing depth
Cement-enamel junction-Free gingival margin
Std.Er: Standard Error
DISCUSSION
The present study has important limitations because the sample was not randomly Drawn from the population but consisted of patients that visited a private dental practice for their regular dental follow-up which was organized by the Greek Dental Association annually.
On the other side similar studies have not been carried out in Greece and data from the present study are only comparable to those reported for similar studies which were carried out in other countries.
Mamai-Homata et al. [10] recorded the periodontal status of Greek adults aged 35–44 years in relation to socio-demographic and behavioral parameters and collected limited data comparable to those reported in the present study regarding few aspects only.
According to the results of the present study, the sample presented a relatively high Prevalence, but low extent of CAL.
Previous studies have recorded similar findings.
In a study by Baelum et al. [15], the prevalence of a CAL higher than 4mm was 92% among 30 to 39-year-old individuals and 100% among 50 to 59-year-old individuals, whereas contrary to the present study regarding the extent of CAL, the average percentage (%) of sites affected was 84% among 30 to 39-year-old individuals and 93% among 50 to 59-year-old individuals.
The high prevalence of the assessed CAL was 72.1% ( ≥ 5mm) and 60.9% ( ≥ 7 mm) [16].
In addition, 95% of the 35 to 44-year-old individuals and 99.2% of the 65 to 74-year-old individuals presented a CAL higher than 3mm [17] and 100% of the older than 50 years old individuals higher than showed a higher than 5 mm CAL [18], while the lower prevalence that was assessed was 19.7% (> 5 mm) [19]. The extent of a higher than 4mm CAL that was assessed was 11.8% in a Vietnamese population [20] and 20% in a younger adult population [21] showing a higher level than that of the present study. According to the above observations, the mean CAL and the percentage of sites/individual with CAL ≥ 2 mm in the present study are comparable to those mentioned studies that had regular access to dental care and preventive dentistry, while are less severe that those reported for populations in the European and other countries.
Those differences could be attributed to several factors such as the heterogeneous population samples, the different interests that have been showed by the population samples regarding the value of oral hygiene and the need for a regular dental follow-up, the origin of the sample collected (such as the dental hospital or private practice), the fact that most of the reviewed studies assessed the periodontal conditions using full-mouth clinical examinations with four or six sites per tooth, while others used two facialsites per tooth in half of the dentition.
These data collection protocols are comparable for the assessment of the mean CAL.
In addition, the sample of the present study concerned individuals who sought dental treatment in a private dental practice and we could not consider it as random one.
If it is supposed that a site with CAL 4–6 mm presents moderate periodontitis and that rates greater than 7 mm are consistent with a diagnosis of advanced periodontitis, according to table 2 which shows the prevalence of individuals with at least one affected site, at least 68.2% of the 60 to 69-year-old individuals showed sites with moderate periodontitis and at least 27.6% of the subjects of the same age group showed advanced periodontitis.
CAL was associated with gingival recession mainly rather than pocket deepening (p=0.018).
However, the final model showed that age, gender and dental plaque were the most important factors associated with gingival recession; whereas, age, gender and calculus were the factors that were associated with CAL. In a study by Corraini et al. [18], multivariate analysis identified dental plaque, calculus and age as risk indicators for CAL ≥ 5mm. Baelumetal. [15] found that an older age and a high percentage of sites with calculus were significant positive predictors of a high percentage of sites with CAL ≥ 4 mm while the same parameters were statistically significant predictors of a high percentage of sites with CAL ≥ 7mm.
Similar results regarding age and CAL were observed in a study by Holtfreter et al. [22] and also a study by Wang et al. [23]. However, Bouchard et al. [19] found that age and Gender were powerful independent predictors of CAL. In the present study, the prevalence of pocket depth increased with age up to the 40 to 49-years-old age group for a PD of 4–6 mm and then decreased, while for severe pockets (≥ 7mm) the prevalence increased with age up to the 50–59-years-old age group.
Previous studies have recorded higher prevalence rates of probing depths such as 75% (≥ 5mm) [24], 69.7%(≥ 4mm), 25.3%(≥ 6mm) [22] and 11.3%(> 5.5mm)[25], while other studies have recorded lower prevalence rates of probing depths such as 30% (≥ 4mm), less than 5 % (≥ 6mm) [15] and 43.3% (≥ 4mm) [26].
Similar findings with higher prevalence rates have been recorded and assessed; 84%(≥ 4mm) among 30–39-year-old individuals and 93% (PD≥ 4mm) among 50-59-year-old individuals [15] while in another study [17] 76.9% (≥ 4mm) was detected among 35-44-year-old individuals and 87.7% (≥ 4mm) among 65 to 74-year-old individuals.
The extent of the pocket depths in the present study was lower than the pocket depths in a study of a Vietnamese population [20] and another study including young adults [21], while it was higher than the pocket depths in a study in the Amazon rain forest [3].
As mentioned, gingival inflammation was determined by the percentage of sites per individual that showed BOP.
According to the results of the present study, gingivitis increased significantly with age except in the 60 to 69-year-old age group. This finding was consistent with other epidemiological studies of adult populations in other countries [16,20,25,26]. However, the above-mentioned finding was not in agreement with a previous study performed by Ronderos et al. [3]. BOP (57.8%) was lower than a study in the USA [25], which was estimated as 82.8%, while in another study in Brazil [26], it occurred in 97.9% of the samples. Similar observations were recorded in a study by Silva-Boghossian et al. [16] in Brazil, whereas in a study in Greece [10], BOP was 16.2%. According to the mentioned observations, the present study suggests that even without prior therapeutic or preventive period on talintervention, subjects with extensive dental plaques, gingivitis and calculus do not present severe loss of period on taltissue.
This finding is in agreement with a previous study which showed that destructive periodontal disease is not necessarily an inevitable consequence of long-standing gingival inflammation [3]. In addition, the present study does not support that periodontal disease is more extensive among poor societies and populations with minimal access to dental care and preventive dentistry, situations that are common in isolated villages/towns. This finding is in agreement with the findings of previous studies [3,5]. BOP was not associated with CAL, PD or gingival recession, while previous studies showed that BOP was significantly associated with CAL [16] and increased PD, but not with gingival recession [3].
Gingival recession was associated with age, gender and dental plaque. This finding Regarding age is in agreement with observations of other studies in several Age groups [27,28]. This relationship between the occurrence of gingival recession and age could be attributed to the longer period of exposure to factors that cause gingival recession and the cumulative effects of the lesion itself.
Regarding the role of dental plaque and gingival inflammation in the development of gingival recession, previous studies have showed that gingival inflammation was the most frequent precipitating etiological factor of gingival recession. They suggested that a localized inflammatory process causes the breakdown of connective tissue destruction and have also reported [27,29] that gingival recession was associated with a high level of dental plaque while a study by Goutoudi et al. [30] revealed that gingival margin recession was associated with both high inflammatory and plaque scores.
In addition, a significant association between gingival recession and periodontal disease [31] was recorded. A study by Slutzkey and Levin [32] showed a negative correlation between dental plaque on the buccal tooth aspect and gingival recession, while Loe et al. [2] emphasized the role of poor oral hygiene, dental plaque and calculus in gingival recession. PD was significantly associated with age and gender, but not dental plaque or calculus. Wang et al. [23] reported that PD increased with age while Brennan et al. [33] found that the prevalence of periodontal pockets greater than 6mm was associated with gender. The multivariate analysis indicated an association between dental plaque and gingival recession and this association was independent of calculus. Furthermore, tobacco consumption, educational level, tooth brushing frequency and reasons for dental visit were not significantly related to the mean attachment loss.
Bergström [34], Bokor-Bratic [35] and many investigators have shown the effects of smoking on the periodontium and periodontal health. These studies have shown that cigarette smoking is clearly established as one of the most significant risk factors in the development and the progression of periodontal disease. Regarding the role of dental hygiene habits, it is known that proper use of tooth Brush and use of dental floss are the most effective tools for removal of dental plaques from tooth surfaces, especially in terproximal surfaces [36,37]. Similarly, a regular dental follow-up can prevent the development, extent and severity of all forms of periodontal diseases.
The role of educational level regarding oral hygiene habits is also known, since more educated individuals have less gingival inflammation or other forms of periodontal diseases, which might be attributed to the fact that more educated individuals have realized the value and importance of preventive dentistry and oral hygiene procedures, applying proper habits and standards of oral hygiene, and following regular dental check-ups. Similar observations have been recorded in other studies, indicating that the level of education is the most important contributor to periodontal destruction. Paulander et al. [38] recorded that low educational subjects exhibited significantly more periodontal attachment loss and had significantly fewer healthy gingival units, while Zini et al. [39] showed that a lower level of education was associated with severe chronic periodontitis.
CONCLUSIONS
Periodontal disease of this sample was mainly associated with gingival inflammation and presence of dental plaque and calculus. However, the majority of the individuals showed CAL and gingival recession rather than deep pockets. Despite the extensive gingival inflammation and poor oral hygiene of the sample, it did not present destructive forms of periodontal disease. Study results showed that a strong need exists for improvement of the population’s self-awareness of oral hygiene and better oral health education mainly in rural regions of Greece.
In addition, it is important to focus more on periodontal aspects of dental care and effective prevention programmes and better control of periodontal disease are required.
Acknowledgments
The author would like to thank Mrs Vlassi Anthoula. for her contribution regarding the statistical procedure of the study.
REFERENCES
- 1.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol. 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 2.Löe H, Anerud A, Boysen H. The natural history of periodontal disease in man: prevalence, severity and extent of gingival recession. J Periodontol. 1992 Jun;63(6):489–95. doi: 10.1902/jop.1992.63.6.489. [DOI] [PubMed] [Google Scholar]
- 3.Ronderos M, Pihlstrom BL, Hodges JS. Periodontal disease among indigenous people in the Amazon rain forest. J ClinPeriodontol. 2001 Nov;28(11):995–1003. doi: 10.1034/j.1600-051x.2001.281102.x. [DOI] [PubMed] [Google Scholar]
- 4.Matthesen M, Baleum V, Aasrlev I, Fejerskov O. Dental health of children and adults in Guinea-Bissau, West Africa in 1986. Community Den Health. 1990 Jun;7(2):123–33. [PubMed] [Google Scholar]
- 5.Miyazaki H. A global overview of periodontal epidemiology. In: Pack ARC, Newman HN, editors. Periodontal needs of developing nations, International Academy of Periodontology. Sciences Reviews Limited; Middlesex: 1996. pp. 1–8NJ. [Google Scholar]
- 6.Micheelis W, Schiffner U. Institut der DeutschenZahnärzte (IDZ Materialreihe Band 31) DeutscherÄrzte-Verlag; Köln: 2006. Vierte Deutsche Mundgesundheitsstudie (DMS IV) [Google Scholar]
- 7.Gjermo P, Rosing CK, Susin C, Oppermann R. Periodontal diseases in Central and South America. Periodontol 2000. 2002;29:70–8. doi: 10.1034/j.1600-0757.2001.290104.x. [DOI] [PubMed] [Google Scholar]
- 8.Hugoson A, Norderyd O. Has the prevalence of periodontitis changed during the last 30 years? J ClinPeriodontol. 2008 Sep;35(8 Suppl):338–45. doi: 10.1111/j.1600-051X.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 9.Hugoson A, Sjodin B, Norderyd O. Trends over 30 years, 1973–2003, in the prevalence and severity of periodontal disease. J ClinPeriodontol. 2008 May;35(5):405–14. doi: 10.1111/j.1600-051X.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 10.Mamai-Homata E, Polychronopoulou A, Topitsoglou V, Oulis C, Athanassouli T. Periodontal diseases in Greek adults between 1985 and 2005-risk indicators. IntDent J. 2010 Aug;60(4):293–9. [PubMed] [Google Scholar]
- 11.Vettore MV, LamarcaGde A, Leão AT, Sheiham A, LealMdo C. Partial recording protocols for periodontal disease assessment in epidemiological surveys. Cad SaudePublica. 2007 Jan;23(1):33–42. doi: 10.1590/s0102-311x2007000100005. [DOI] [PubMed] [Google Scholar]
- 12.Ramfjord SP. Design of studies or clinical trials to evaluate the effectiveness of agents or procedures for the prevention or treatment of loss of the periodontium. J Periodont Res. 1974;9(Suppl):78–93. doi: 10.1111/j.1600-0765.1974.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 13.Löe H. The gingival index, the plaque index and there tention index systems. J Periodontol. 1967 Nov-Dec;38(6) Suppl:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 14.The American Academy of Periodontology . Proceedings of the World Workshop in Clinical Periodontics. Chicago: The American Academy of Periodontology; 1989. pp. I/23–I/24. [Google Scholar]
- 15.Baelum V, Pisuithanakan S, Teanpaisan R, Pithpornchaiyakul W, Pongpaisal S, Papapanou PN, et al. Periodontal conditions among adults in Southern Thailand. J Periodontal Res. 2003 Apr;38(2):156–63. doi: 10.1034/j.1600-0765.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 16.Silva-Boghossian CM, Luizz RR, Colombo AP. Periodontal status, socio-demographic and behavioural indicators in subjects attending a public dental school in Brazil: analysis of clinical attachment loss. J Periodontol. 2009 Dec;80(12):1945–54. doi: 10.1902/jop.2009.090242. [DOI] [PubMed] [Google Scholar]
- 17.Holtfreter B, Kocher T, Hoffmann T, Desvarieux M, Micheelis W. Prevalence of periodontal disease and treatment demands based on a German dental survey (DMS IV) J ClinPeriodontol. 2010 Mar;37(3):211–9. doi: 10.1111/j.1600-051X.2009.01517.x. [DOI] [PubMed] [Google Scholar]
- 18.Corraini P, Baelum V, Pannuti CM, Pustiglioni AN, Romito GA, Pustiglioni FE. Periodontal attachment loss in an untreated isolated population of Brazil. J Periodontol. 2008 Apr;79(4):610–20. doi: 10.1902/jop.2008.070294. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard P, Boutouyrie P, Mattout C, Bourgeois D. Risk assessment for severe clinical attachment loss in an adult population. J Periodontol. 2006 Mar;77(3):479–89. doi: 10.1902/jop.2006.050128. [DOI] [PubMed] [Google Scholar]
- 20.Do LG, Spencer JA, Roberts-Thomson K, Ha DH, Tran TV, Trinh HD. Periodontal disease among the middle-aged Vietnamese population. J IntAcadPeriodontol. 2003 Jul;5(3):77–84. [PubMed] [Google Scholar]
- 21.Thomson WM, Hashim R, Pack AR. The prevalence and intra oral distribution of periodontal attachment loss in a birth cohort of 26-years-old. J Periodontol. 2000 Dec;71(12):1840–5. doi: 10.1902/jop.2000.71.12.1840. [DOI] [PubMed] [Google Scholar]
- 22.Holtfreter B, Schwahn C, Biffar R, Kocher T. Epidemiology of periodontal diseases in the Study of Health in Pomerania. J ClinPeriodontol. 2009 Feb;36(2):114–23. doi: 10.1111/j.1600-051X.2008.01361.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang QT, Wu ZF, Wu YF, Shu R, Pan YP, Xia JL. Epidemiology and preventive direction of periodontology in China. J ClinPeriodontol. 2007 Nov;34(11):946–51. doi: 10.1111/j.1600-051X.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 24.Dowsett SA, Archila L, Segreto VA, Eckert GJ, Kowolik MJ. Periodontal diseasestatus of an indigenous population of Guatemala, Central America. J ClinPeriodontol. 2001 Jul;28(7):663–71. doi: 10.1034/j.1600-051x.2001.028007663.x. [DOI] [PubMed] [Google Scholar]
- 25.Hanson WL, Persson GR. Periodontal conditions and service utilization behaviours in a low income adult population. Oral Health Prev Dent. 2003;1(2):99–109. [PubMed] [Google Scholar]
- 26.Segundo TK, Ferreira EF, Costa JE. Periodontal disease in the Arturo’s black community in Contagen, Minas Gerais, Brazil. Cad Saude Publ. 2004 Mar-Apr;20(2):596–603. doi: 10.1590/s0102-311x2004000200029. [DOI] [PubMed] [Google Scholar]
- 27.Susin C, Haas AN, Oppermann RV, Hangejorden O, Albandar JM. Gingival recession: epidemiology and risk indicators in a representative urban Brazilian population. J Periodontol. 2004 Oct;75(10):1377–86. doi: 10.1902/jop.2004.75.10.1377. [DOI] [PubMed] [Google Scholar]
- 28.Arowojolu MO. Gingival recession at the University College Hospital, Ibadan-prevalence and effect of some aetiological factors. Afr J Med Med Sci. 2000 Sep-Dec;29(3–4):259–63. [PubMed] [Google Scholar]
- 29.Toker H, Ozdemir H. Gingival recession: epidemiology and risk indicators in a university dental hospital in Turkey. Int J Dent Hyg. 2009 May;7(2):115–20. doi: 10.1111/j.1601-5037.2008.00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Goutoudi P, Koidis PT, Konstantinidis A. Gingival recession: a cross-sectional clinical investigation. Eur J ProsthodontRestor Dent. 1997 Jun;5(2):57–61. [PubMed] [Google Scholar]
- 31.Tugnait A, Clerehugh V. Gingival recession-its significance and management. Rev J Dent. 2001 Aug;29(6):381–94. doi: 10.1016/s0300-5712(01)00035-5. [DOI] [PubMed] [Google Scholar]
- 32.Slutzkey S, Levin L. Gingival recession in young adults: occurrence, severity and relationship to past orthodontic treatment and oral piercing. Am J OrthodDentofacialOrthop. 2008 Nov;134(5):652–6. doi: 10.1016/j.ajodo.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 33.Brenann DS, Spencer AJ, Slade GD. Prevalence of periodontal conditions among public-funded dental patients in Australia. Aust Dent J. 2001 Jun;46(2):114–21. doi: 10.1111/j.1834-7819.2001.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 34.Bergström J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J ClinPeriodontol. 2000 Jan;27(1):61–8. doi: 10.1034/j.1600-051x.2000.027001061.x. [DOI] [PubMed] [Google Scholar]
- 35.Bokor-Bratic M. Effects of smoking on the periodontium. Medic Pregl. 2002 May-Jun;55(5–6):229–32. doi: 10.2298/mpns0206229b. [DOI] [PubMed] [Google Scholar]
- 36.Checchi L, Daprile G, Gatto MRA, Pelliccioni GA. Gingival recession and tooth brushing in an Italian School of Dentistry: a pilot study. J ClinPeriodontol. 1999 May;26(5):276–80. doi: 10.1034/j.1600-051x.1999.260502.x. [DOI] [PubMed] [Google Scholar]
- 37.Lobene RR, Soparkar PM, Newman MB. Use of dental floss. Effect on plaque and gingivitis. ClinPrev Dent. 1982 Jan-Feb;4(1):5–8. [PubMed] [Google Scholar]
- 38.Paulander J, Axelsson P, Lindhe J. Association between level of education and oral health status in 35-, 50-, 65- and 75-year-olds. J ClinPeriodontol. 2003 Aug;30(8):697–704. doi: 10.1034/j.1600-051x.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 39.Zini A, Sqan-Cohen HD, Marcenes W. Socio-economic position, smoking and plaque: a pathway to severe chronic periodontitis. J ClinPeriodontol. 2011 Mar;38(3):229–35. doi: 10.1111/j.1600-051X.2010.01689.x. [DOI] [PubMed] [Google Scholar]