Abstract
INTRODUCTION
Renal cell carcinoma (RCC) accounts for approximately 3% of adult malignancies and is responsible for over 13,000 deaths in the U.S. annually. The fatalities are largely due to distant metastasis, with lung, liver, bone and brain being most commonly affected organs. Gastric metastasis from RCC is a rare event (less than 20 cases reported in the English language literature) and usually presents as a large, solitary mass or ulcer (average size of 4.8 cm) resembling primary gastric cancer. Here we report the first case of metastatic RCC presenting as small gastric polyps.
PRESENTATION OF CASE
The patient was a 60-year-old African American woman with a history of clear cell RCC (pT1bNX). She underwent esophagogastroduodenoscopy and colonoscopy 5 months after nephrectomy due to anemia. Two non-ulcerated, 0.6-cm benign-appearing polyps were found at the greater curvature of the gastric body, which were subsequently removed endoscopically. Unexpectedly, histopathologic examination of the gastric polyps revealed nested collections of vacuolated epithelioid cells in a background of delicate, arborizing vasculature, immediately beneath the congested and hyperplastic foveolar epithelium. A diagnosis of metastatic RCC was rendered after confirming the renal epithelial origin by immunohistochemical stains.
DISCUSSION
Gastric metastasis from RCC usually presents as a large, solitary mass or ulcer, but it can be subtle and present as multiple, small benign-appearing polyps.
CONCLUSION
A careful follow up and thorough endoscopic and histopathologic examinations should be conducted in patients with a history of RCC who present with gastrointestinal manifestations.
Keywords: Renal cell carcinoma, Metastasis, Gastric polyp
1. Introduction
Renal cell carcinoma (RCC) accounts for approximately 3% of adult malignancies. The age-adjusted incidence of RCC has been increasing for the past 30 years in the United States and most European countries.1 More than 50% of RCCs are now detected incidentally and approximately one third of the patients have metastasis at the time of initial diagnosis.2 RCC is responsible for over 13,000 deaths in the United States annually,3 largely due to distant metastasis. Metastatic RCCs may occur in virtually all organ systems. The usual sites of metastasis include lung (75%), soft tissue (36%), bone (20%), liver (18%), cutaneous sites (8%), and central nervous system (8%).4 Gastric metastasis from RCC is extremely rare, with only 19 cases reported in the English language literature. Endoscopically, the gastric metastasis from RCC usually presents as a large, solitary mass or ulcer (average size of 4.8 cm) resembling primary gastric cancer. Here we report the first case of metastatic RCC presenting as multiple, small, benign-appearing gastric polyps.
2. Presentation of case
The patient, a 60-year-old African American woman, had a history of tobacco smoking, anemia, and chronic kidney disease secondary to hypertension. She underwent evaluation for increased renal insufficiency by ultrasound in July 2010. A large, solid, hypervascular mass was identified in the right kidney, which was confirmed by subsequent Magnetic Resonance Imaging (MRI). The patient underwent a laparoscopic radical nephrectomy in August 2010. On gross examination, a 6.4 cm × 5.1 cm × 3.5 cm, well circumscribed mass was identified near the upper pole of the kidney, distending the renal capsule. The cut surface of the lesion had a heterogeneous, golden yellow to tan appearance. Histological examination revealed a clear cell RCC, Fuhrman nuclear grade I/IV. The tumor was limited to the kidney. No lymph nodes were identified. The pathologic stage was pT1bNX. The patient's post-operative course was uneventful and she did not receive any neoadjuvant chemotherapy or immunotherapy.
In January 2011, the patient underwent esophagogastroduodenoscopy and colonoscopy due to iron deficiency anemia. The patient had no previous history of gastrointestinal disease. Two similar, non-ulcerated polyps were found arising from the greater curvature of the gastric body, each measuring 6 mm at the greatest dimension, which were completely removed (Fig. 1). The duodenum appeared unremarkable. Colonoscopy revealed two polyps arising from ascending and transverse colon, respectively, which were subsequently biopsied. Histologic examination of the gastric polyps revealed nodular and nested collections of vacuolated epithelioid cells interspersed with delicate, arborizing vasculature, immediately beneath the congested and hyperplastic foveolar epithelium. The lesions were confined to the lamina propria, with an infiltrative growth pattern at the periphery. The cells of interest exhibited clear cytoplasm, round to ovoid nuclei with finely granular open chromatin and small, inconspicuous nucleoli. Occasional mitotic figures, including atypical forms, were also noted. The lesional cells were immunoreactive with antibodies raised against vimentin and paired box protein PAX-2, and focally with broad spectrum cytokeratin (Fig. 2). The histomorphologic features and the immunophenotype resulted in a diagnosis of metastatic RCC. In addition, microscopic examination of colonic polyps revealed a tubular adenoma and a hyperplastic polyp. In February 2011, she was found to have multiple metastases in the lungs, bones, and right nephrectomy bed, and received 2 cycles of oral Sunitinib, a multi-targeted receptor tyrosine kinase inhibitor. She subsequently presented with headache, nausea, vomiting, blurry vision and dizziness, and was found to have a large metastasis in the left cerebellum for which she underwent surgical resection. In August 2011, the patient was started on Sorafenib, an inhibitor of several tyrosine protein kinases and Raf kinases. Due to multi-organ metastases, she expired 14 months after the initial diagnosis of RCC.
Fig. 1.
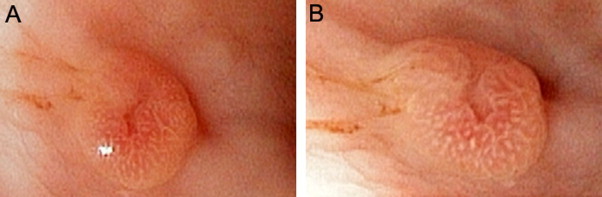
Endoscopic findings. Two non-ulcerated polyps with central indentation were found at the greater curvature of the gastric body, each measuring 6 mm in greatest dimension (A and B).
Fig. 2.
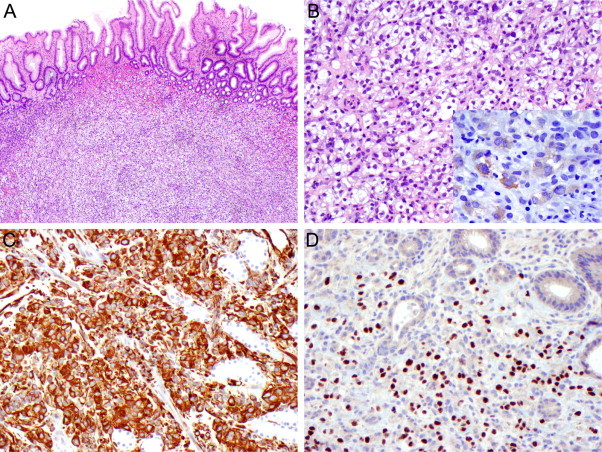
Histologic characteristics of the gastric polyps. Submucosal nodular collection of epithelioid clear cells (A, H&E, original magnification 40×). The lesional cell exhibited a nested growth pattern, in a background of delicate, arborizing vasculature (B, H&E, original magnification 200×). Tumor cells were focally immunoreactive with broad spectrum cytokeratin (2B inset), and diffusely expressed vimentin (2C, original magnification 200×) and PAX-2 (2D, original magnification 200×).
3. Discussion
Metastasis to the stomach is an uncommon event, with carcinomas of lung and breast and malignant melanoma being the most common origins.1 On the other hand, while metastatic RCC may involve any organ system including gastrointestinal tract, gastric metastasis from RCC is extremely rare, with only 19 cases reported in the English literature.
The majority of gastric metastases from RCCs occurred among male patients (72%), the average time of metastasis is approximately 7 years (ranged 0–20 years) for both genders (Table 1). In contrast, the mean interval between the diagnosis of the primary tumor other than RCC and the development of gastric metastasis is 1.3 years.5 Thus, gastric metastasis seems to be a late event in patients with RCC. An isolated gastric metastasis from RCC was reported even 20 years after radical nephrectomy, suggesting RCC has the potential for late solitary metastasis.6 On the other hand, 25–30% of patients with RCC had distant metastases at the time of diagnosis.7 Thus, it is not surprising that our patient developed gastric metastases 5 months after nephrectomy.
Table 1.
Characteristics of reported cases of metastatic RCC in the stomach.
| Case no. | Author | Age/sex | Interval (years) | Presenting symptoms | Endoscopic findings (location) | Histological type | Treatment | Outcomes (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Sullivan et al.10 | 69/M | 7 | UGI bleeding | Solitary large mass (antrum) | N/A | Antrectomy | N/A |
| 2 | Blake et al.11 | 63/M | 6 | UGI bleeding | N/A | N/A | Embolzsation | >5 |
| 3 | Picchio et al.12 | N/A | 14 | Anemia UGI bleeding | Solitary small polyp (body) | N/A | Subtotal gastrectomy | >6, NED |
| 4 | Mascarenhas et al.13 | 66/M | 4 | UGI bleeding | Ulcer (body) | N/A | Partial gastrectomy | >36, AWD |
| 5 | Kobayashi et al.14 | 78/M | 1.2 | Anemia | Solitary 5 cm mass (lower 1/3 of stomach) | N/A | Gastrectomy | 5, DOD |
| 6 | Kok Wee et al.6 | 60/M | 20 | UGI bleeding | Masses (10 cm and 3.5 cm) (body) | Clear cell | N/A | N/A |
| 7 | Lamb et al.15 | 69/F | 18 | UGI bleeding | Solitary large mass (posterior wall) | N/A | Embolization Octreotide | 23, DOD |
| 8 | Riviello et al.16 | 68/M | 10 | UGI bleeding | Solitary 5 cm mass (fundus) | Clear cell | Gastrectomy, Chemotherapy | 24, DOD |
| 9 | Pezzoli et al.17 | 78/M | 5 | Anemia | 2–3 cm polyps (body) | Clear cell | Electrosurgical snare resection | 6 |
| 10 | Saidi and Remine18 | N/A | 10 | Anemia UGI bleeding | 1 cm polyp (body) | N/A | Wedge resection | >18, NED |
| 11 | Pollheimer et al.1 | 69/M | 4.2 | Egigastric pain, nausea, emesis | Solitary 7.5 cm mass (body) | Clear cell | Tamoxifen | 19, DOD |
| 12 | Pollheimer et al.1 | 77/M | 6.3 | Asymptomatic | Solitary ulcerated 3 cm mass (antrum) | Clear cell | Interferon | 4, DOD |
| 13 | Pollheimer et al.1 | 83/F | 1.7 | UGI bleeding | Multiple mass, 4.5 cm (antrum) | Clear cell | Ablative endoscopic Interferon | 5, DOD |
| 14 | Pollheimer et al.1 | 65/F | 13.1 | UGI bleeding | Two lesions, 4 cm (stomach) | Clear cell | Ablative endoscopic | 3, DOD |
| 15 | Pollheimer et al.1 | 69/M | 9.3 | Anemia epigastric pain | Multiple, 5.4 cm (body) | Clear cell | Endoscopic Ablation, Sunitinib | >24, AWD |
| 16 | Yamamoto et al.8 | 74/M | 5 | UGI bleeding | Polypoid 7 cm mass (body) | N/A | Wedge resection | 1, DOOD |
| 17 | Tiwari et al.2 | 58/F | 0 | UGI bleeding | Polypoid 3–5 cm mass (antrum) | Clear cell | Subtotal gastrectomy | 2, DOD |
| 18 | García-Campelo et al.19 | 75/M | 3 | Asymptomatic | Multilobulated and polypoid masses (fundus and body) | N/A | Sunitinib | >6, NED |
| 19 | Sugasawa et al.20 | 69/M | 19 | Anemia | Elevated lesion with ulceration (fundus) | Clear cell | Wedge resection | >12, NED |
| 20 | Present case | 60/F | 0.4 | Anemia | 0.6 cm polyps (body) | Clear cell | Polypectomies, Sunitinib Sorafenib | 14, DOD |
AWD, alive with disease; DOD, dead of disease; DOOD, dead of other disease; NED, no evidence of disease; UGI, upper gastrointestinal.
The presenting symptoms in patients with gastric metastasis from RCC are mainly upper gastrointestinal bleeding (65%) and iron deficiency anemia (35%) (Table 1). Gastric metastases are more common in the body (63%), and are more likely to be large, solitary mass or ulcer (average size of 4.8 cm) resembling primary gastric cancer endoscopically. The present patient had two small (0.6 cm) gastric polyps, which were the smallest documented gastric metastases from RCC. The endoscopic findings of these two gastric polyps were benign-appearing, similar to her colonic polyps. Thus, a careful endoscopic examination is crucial for identification of early metastasis. Further, any unusual histological findings should prompt the pathologist to perform necessary ancillary studies to confirm/rule out metastasis.
Histologically, metastatic RCC are predominantly clear cell type (Table 1). Thus, the presence of clear cell morphology in any unknown lesion should prompt the pathologist to exclude the possibility of metastatic RCC, even in the absence of a prior diagnosis. Immunohistochemistry is a useful tool in this setting, especially vimentin and PAX-2. Vimentin is an intermediate filament protein expressed in normal renal tissues and in 87% of clear cell RCCs.8 PAX-2, a transcription factor required for development and proliferation of renal tubules, is expressed in 85% of metastatic clear cell RCCs.9
The management modalities for gastric metastasis from RCC include total or subtotal gastrectomy, endoscopic resection, embolization of the metastasis, chemotherapy, and immunotherapy (Table 1). The optimal treatment for gastric metastases from RCC remains controversial. In the absence of diffuse disease, aggressive therapy including surgical resection is appropriate for isolated gastric metastasis. In patients with gastrointestinal bleeding, surgical excision of gastric metastasis may be essential to prevent rebleeding.
4. Conclusion
The present case exemplifies that gastric metastases from RCC can be subtle and present as multiple, small benign-appearing polyps. This case also highlights the importance of a careful endoscopic examination as well as a thorough histopathologic examination in patients with a history of RCC who present with gastrointestinal manifestations, as minute and unexpected findings can directly impact patient care.
Conflict of interest statement
None.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author's contributions
All authors have contributed significantly and this work has been approved by all authors.
Acknowledgements
This study has been presented in part in the 2011 Annual Meeting of the College of American Pathologists, Grapevine, TX.
References
- 1.Pollheimer M.J., Hinterleitner T.A., Pollheimer V.S., Schlemmer A., Langner C. Renal cell carcinoma metastatic to the stomach: single-centre experience and literature review. BJU International. 2008;102:315–319. doi: 10.1111/j.1464-410X.2008.07617.x. [DOI] [PubMed] [Google Scholar]
- 2.Tiwari P., Tiwari A., Vijay M., Kumar S., Kundu A.K. Upper gastro-intestinal bleeding – rare presentation of renal cell carcinoma. Urology Annals. 2010;2:127–129. doi: 10.4103/0974-7796.68864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. Cancer statistics, 2009. CA-A Cancer Journal for Clinicians. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Maldazys J.D., deKernion J.B. Prognostic factors in metastatic renal carcinoma. Journal of Urology. 1986;136:376–379. doi: 10.1016/s0022-5347(17)44873-7. [DOI] [PubMed] [Google Scholar]
- 5.Tolia B.M., Whitmore W.F., Jr. Solitary metastasis from renal cell carcinoma. Journal of Urology. 1975;114:836–838. doi: 10.1016/s0022-5347(17)67155-6. [DOI] [PubMed] [Google Scholar]
- 6.Kok Wee L., Shyu R.Y., Sheu L.F., Hsieh T.Y., Yan J.C., Chen P.J. Metastatic renal cell cancer. Gastrointestinal Endoscopy. 2004;60:265. doi: 10.1016/s0016-5107(04)01542-1. [DOI] [PubMed] [Google Scholar]
- 7.Kibria R., Sharma K., Ali S.A., Rao P. Upper gastrointestinal bleeding revealing the stomach metastases of renal cell carcinoma. Journal of Gastrointestinal Cancer. 2009;40:51–54. doi: 10.1007/s12029-009-9074-y. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto D., Hamada Y., Okazaki S., Kawakami K., Kanzaki S., Yamamoto C. Metastatic gastric tumor from renal cell carcinoma. Gastric Cancer. 2009;12:170–173. doi: 10.1007/s10120-009-0519-6. [DOI] [PubMed] [Google Scholar]
- 9.Gokden N., Gokden M., Phan D.C., McKenney J.K. The utility of PAX-2 in distinguishing metastatic clear cell renal cell carcinoma from its morphologic mimics: an immunohistochemical study with comparison to renal cell carcinoma marker. American Journal of Surgical Pathology. 2008;32:1462–1467. doi: 10.1097/PAS.0b013e318176dba7. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan W.G., Cabot E.B., Donohue R.E. Metastatic renal cell carcinoma to stomach. Urology. 1980;15:375–378. doi: 10.1016/0090-4295(80)90473-2. [DOI] [PubMed] [Google Scholar]
- 11.Blake M.A., Owens A., O’Donoghue D.P., MacErlean D.P. Embolotherapy for massive upper gastrointestinal haemorrhage secondary to metastatic renal cell carcinoma: report of three cases. Gut. 1995;37:835–837. doi: 10.1136/gut.37.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picchio M., Paioletti A., Santini E., Iacoponi S., Cordahi M. Gastric metastasis from renal cell carcinoma fourteen years after radical nephrectomy. Acta Chirurgica Belgica. 2000;100:228–230. [PubMed] [Google Scholar]
- 13.Mascarenhas B., Konety B., Rubin J.T. Recurrent metastatic renal cell carcinoma presenting as a bleeding gastric ulcer after a complete response to high-dose interleukin-2 treatment. Urology. 2001;57:168. doi: 10.1016/s0090-4295(00)00877-3. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi O., Murakami H., Yoshida T., Cho H., Yoshikawa T., Tsuburaya A. Clinical diagnosis of metastatic gastric tumors: clinicopathologic findings and prognosis of nine patients in a single cancer center. World Journal of Surgery. 2004;28:548–551. doi: 10.1007/s00268-004-7216-8. [DOI] [PubMed] [Google Scholar]
- 15.Lamb G.W., Moss J., Edwards R., Aitchison M. Case report: octreotide as an adjunct to embolisation in the management of recurrent bleeding upper gastrointestinal metastases from primary renal cell cancer. International Urology and Nephrology. 2005;37:691–693. doi: 10.1007/s11255-005-0251-z. [DOI] [PubMed] [Google Scholar]
- 16.Riviello C., Tanini I., Cipriani G., Pantaleo P., Nozzoli C., Poma A. Unusual gastric and pancreatic metastatic renal cell carcinoma presentation 10 years after surgery and immunotherapy: a case report and a review of literature. World Journal of Gastroenterology. 2006;12:5234–5236. doi: 10.3748/wjg.v12.i32.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pezzoli A., Matarese V., Boccia S., Simone L., Gullini S. Gastrointestinal bleeding from gastric metastasis of renal cell carcinoma, treated by endoscopic polypectomy. Endoscopy. 2007;39(Suppl. 1):E52. doi: 10.1055/s-2006-945127. [DOI] [PubMed] [Google Scholar]
- 18.Saidi R.F., Remine S.G. Isolated gastric metastasis from renal cell carcinoma 10 years after radical nephrectomy. Journal of Gastroenterology and Hepatology. 2007;22:143–144. doi: 10.1111/j.1440-1746.2006.04335.x. [DOI] [PubMed] [Google Scholar]
- 19.García-Campelo R., Quindós M., Vázquez D.D., López M.R., Carral A., Calvo O.F. Renal cell carcinoma: complete pathological response in a patient with gastric metastasis of renal cell carcinoma. Anti-Cancer Drugs. 2010;21(Suppl. 1):S13–S15. doi: 10.1097/01.cad.0000361530.51675.60. [DOI] [PubMed] [Google Scholar]
- 20.Sugasawa H., Ichikura T., Ono S., Tsujimoto H., Hiraki S., Sakamoto N. Isolated gastric metastasis from renal cell carcinoma 19 years after radical nephrectomy. International Journal of Clinical Oncology. 2010;15:196–200. doi: 10.1007/s10147-010-0025-1. [DOI] [PubMed] [Google Scholar]


