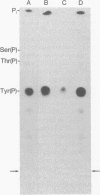
Abstract
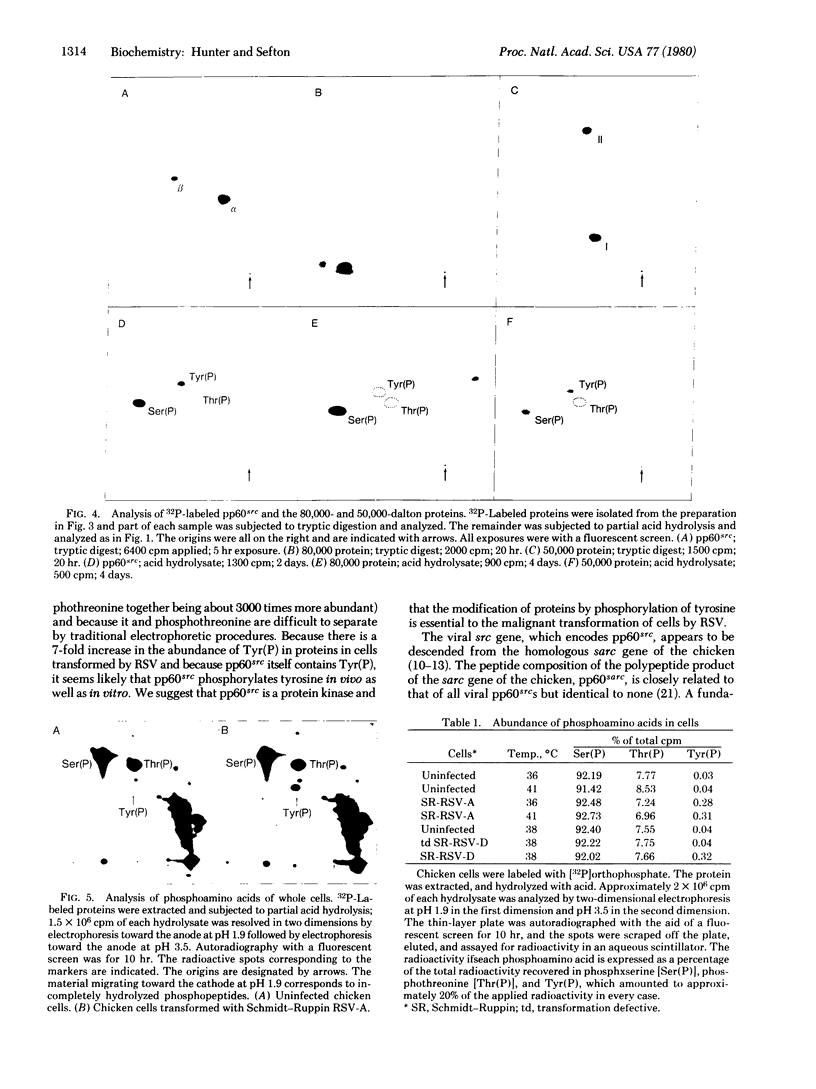
The protein kinase activity associated with pp60src, the transforming protein of Rous sarcoma virus, was found to phosphorylate tyrosine when assayed in an immunoprecipitate. Despite the fact that a protein kinase with this activity has not been described before, several observations suggest that pp60src also phosphorylates tyrosine in vivo. First, chicken cells transformed by Rous sarcoma virus contain as much as 8-fold more phosphotyrosine than do uninfected cells. Second, phosphotyrosine is present in pp60src itself, at one of the two sites of phosphorylation. Third, phosphotyrosine is present in the 50,000-dalton phosphoprotein that coprecipitates with pp60src extracted from transformed chicken cells. We infer from these observations that pp60src is a novel protein kinase and that the modification of proteins via the phosphorylation of tyrosine is essential to the malignant transformation of cells by Rous sarcoma virus. pp60sarc, the closely related cellular homologue of viral pp60src, is present in all vertebrate cells. This normal cellular protein, obtained from both chicken and human cells, also phosphorylated tyrosine when assayed in an immunoprecipitate. This is additional evidence of the functional similarity of these structurally related proteins and demonstrates that all uninfected vertebrate cells contain at least one protein kinase that phosphorylates tyrosine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitte L., Kabat D. Isotopic labeling and analysis of phosphoproteins from mammalian ribosomes. Methods Enzymol. 1974;30:563–590. doi: 10.1016/0076-6879(74)30056-0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Erikson E., Collett M. S., Erikson R. L. In vitro synthesis of a functional avian sarcoma virus transforming-gene product. Nature. 1978 Aug 31;274(5674):919–921. doi: 10.1038/274919a0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plimmer R. H. Esters of phosphoric acid: Phosphoryl hydroxyamino-acids. Biochem J. 1941 Apr;35(4):461–469. doi: 10.1042/bj0350461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Eisenman R. N., Leitch C. R. Identification of a Rous sarcoma virus transformation-related protein in normal avian and mammalian cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4479–4483. doi: 10.1073/pnas.76.9.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Product of in vitro translation of the Rous sarcoma virus src gene has protein kinase activity. J Virol. 1979 Apr;30(1):311–318. doi: 10.1128/jvi.30.1.311-318.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Varmus H. E., Bishop J. M. Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4102–4106. doi: 10.1073/pnas.75.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. Posttranslational covalent modification of proteins. Science. 1977 Dec 2;198(4320):890–896. doi: 10.1126/science.337487. [DOI] [PubMed] [Google Scholar]
- Vigne R., Breitman M. L., Moscovici C., Vogt P. K. Restitution of fibroblast-transforming ability in src deletion mutants of avian sarcoma virus during animal passage. Virology. 1979 Mar;93(2):413–426. doi: 10.1016/0042-6822(79)90245-9. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]