Abstract
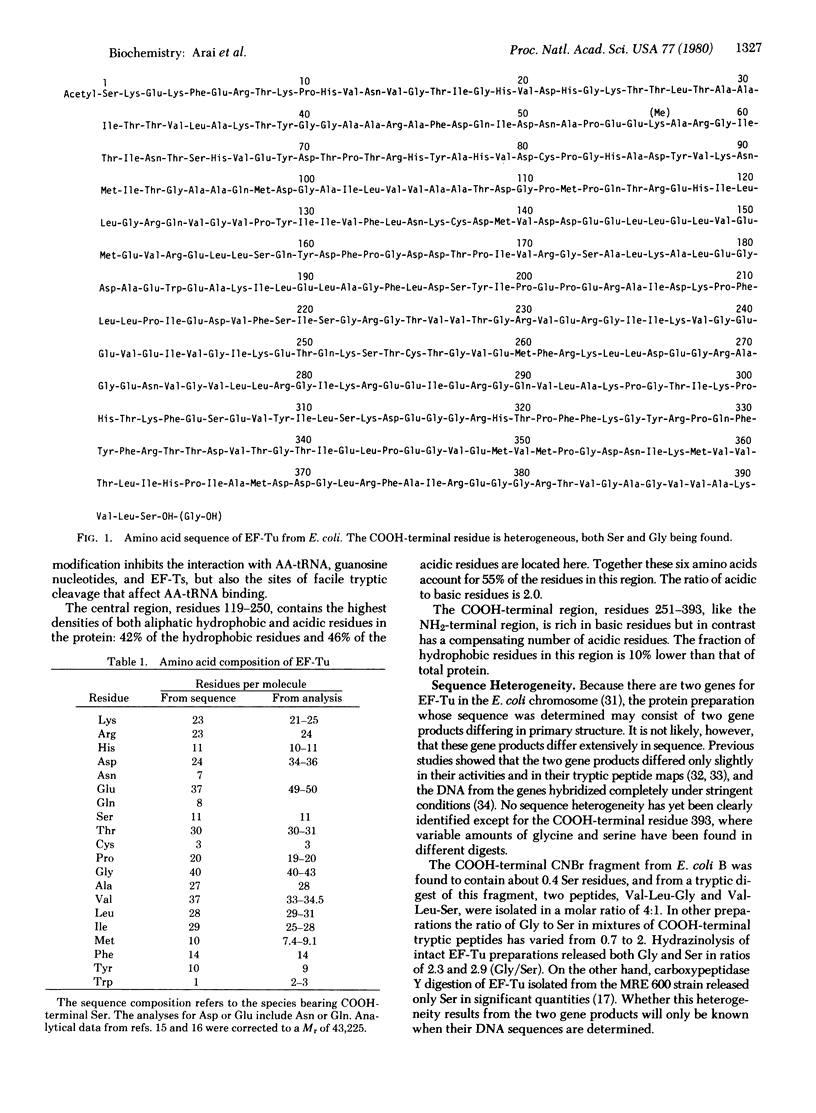
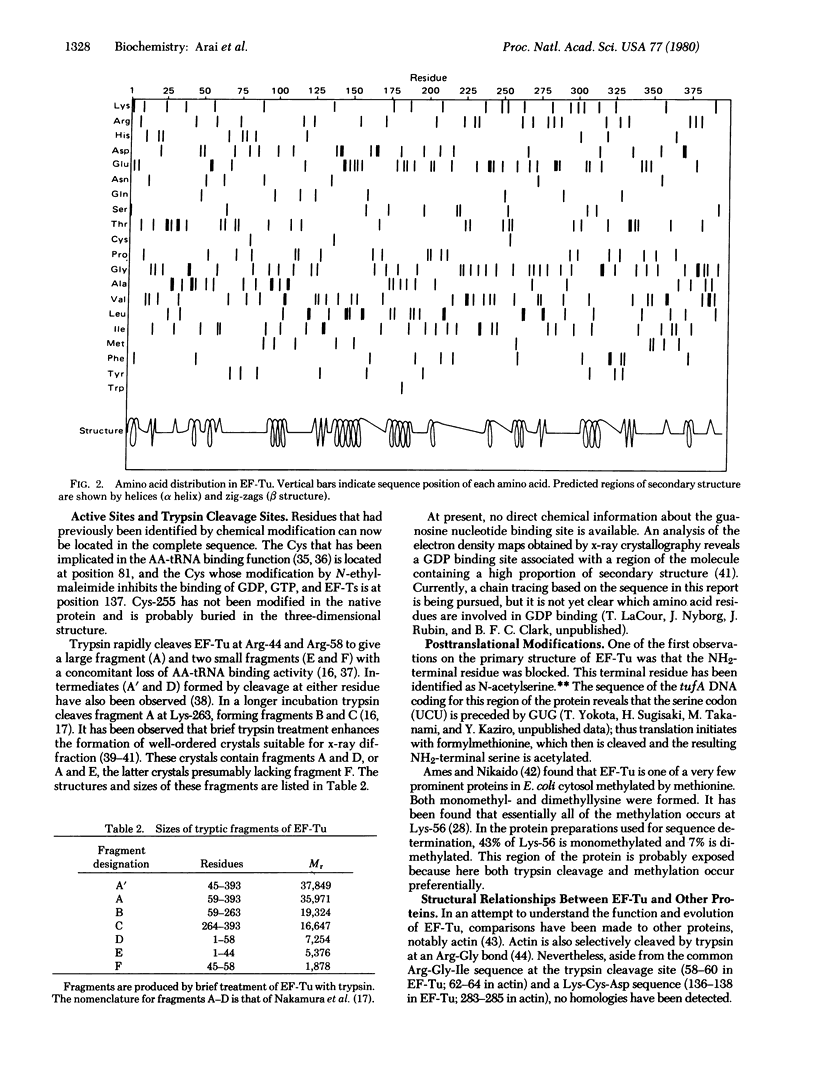
The amino acid sequence of elongation factor Tu (EF-Tu) from Escherichia coli has been determined. EF-Tu is a single-chain polypeptide composed of 393 amino acids (Mr 43,225 for the species bearing COOH-terminal serine). The NH2-terminal serine is acetylated, and lysine-56 is partially methylated. The sites of facile tryptic cleavage are at arginines 44 and 58 and at lysine-263. The cysteinyl residues associated with aminoacyl-tRNA and guanosine nucleotide binding activities are residues 81 and 137, respectively. The COOH-terminal amino acid is heterogenous in that analyses of the COOH-terminal peptides isolated from different EF-Tu preparations gave position 393 as glycine and serine in ratios (Gly/Ser) ranging from about 0.7 to 3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alakhov Y. B., Motuz L. P., Stengrevics O. A., Ovchinnikov Y. A. The primary structure of elongation factor G from Escherichia coli. Amino acid sequence of the region containing the GTP-binding center. FEBS Lett. 1978 Jan 15;85(2):287–290. doi: 10.1016/0014-5793(78)80475-x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Niakido K. In vivo methylation of prokaryotic elongation factor Tu. J Biol Chem. 1979 Oct 25;254(20):9947–9950. [PubMed] [Google Scholar]
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Arai K., Kawakita M., Kaziro Y., Kondo T., Ui N. Studies on the polypeptide elongation factors from E. coli. 3. Molecular characteristics of EF-Tu-guanosine diphosphate, EP-Ts, and EF-Tu-Ts complex. J Biochem. 1973 May;73(5):1095–1105. doi: 10.1093/oxfordjournals.jbchem.a130164. [DOI] [PubMed] [Google Scholar]
- Arai K., Kawakita M., Nakamura S., Ishikawa K., Kaziro Y. Studies on the polypeptide elongation factors form E. coli. VI. Characterization of sulfhydryl groups in EF-Tu and EF-Ts. J Biochem. 1974 Sep;76(3):523–534. doi: 10.1093/oxfordjournals.jbchem.a130596. [DOI] [PubMed] [Google Scholar]
- Arai K., Nakamura S., Arai T., Kawakita M., Kaziro Y. Limited hydrolysis of the polypeptide chain elongation factor Tu by trypsin. Isolation and characterization of the polypeptide fragments. J Biochem. 1976 Jan;79(1):69–83. doi: 10.1093/oxfordjournals.jbchem.a131060. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Douglass J., Smith D. Conformational alteration of protein synthesis elongation factor EF-Tu by EF-Ts and by kirromycin. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3264–3267. doi: 10.1073/pnas.74.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Crane L. J., Miller D. L. Guanosine triphosphate and guanosine diphosphate as conformation-determining molecules. Differential interaction of a fluorescent probe with the guanosine nucleotide complexes of bacterial elongation factor Tu. Biochemistry. 1974 Feb 26;13(5):933–938. doi: 10.1021/bi00702a017. [DOI] [PubMed] [Google Scholar]
- Furano A. V. Direct demonstration of duplicate tuf genes in enteric bacteria. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3104–3108. doi: 10.1073/pnas.75.7.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. The elongation factor Tu coded by the tufA gene of Escherichia coli K-12 is almost identical to that coded by the tufB gene. J Biol Chem. 1977 Mar 25;252(6):2154–2157. [PubMed] [Google Scholar]
- Gast W. H., Kabsch W., Wittinghofer A., Leberman R. Crystals of a large tryptic peptide (fragment A) of elongation factor EF-Tu from Escherichia coli. FEBS Lett. 1977 Feb 15;74(1):88–90. doi: 10.1016/0014-5793(77)80759-x. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Motoyoshi K., Nagata S., Kaziro Y. Purification and properties of a new polypeptide chain elongation factor, EF-1beta, from pig liver. J Biol Chem. 1976 Mar 25;251(6):1843–1845. [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. ATP binding to a protease-resistant core of actin. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2742–2746. doi: 10.1073/pnas.73.8.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Jurnak F., Rich A., Miller D. Preliminary x-ray diffraction data for tetragonal crystals of trypsinized Escherichia coli elongation factor. J Mol Biol. 1977 Sep;115(1):103–110. doi: 10.1016/0022-2836(77)90250-9. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- L'Italien J. J., Laursen R. A. Location of the site of methylation in elongation factor Tu. FEBS Lett. 1979 Nov 15;107(2):359–362. doi: 10.1016/0014-5793(79)80407-x. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Coupling techniques in solid-phase sequencing. Methods Enzymol. 1977;47:277–288. doi: 10.1016/0076-6879(77)47032-0. [DOI] [PubMed] [Google Scholar]
- Laursen R. A., Duffy L. The evolution of elongation factors Tu and G by gene duplication. FEBS Lett. 1978 Aug 15;92(2):200–202. doi: 10.1016/0014-5793(78)80753-4. [DOI] [PubMed] [Google Scholar]
- Laursen R. A., Nagarkatti S., Miller D. L. Amino acid sequence of elongation factor Tu. Characterization and alignment of the cyanogen bromide fragments and location of the cysteine residues. FEBS Lett. 1977 Aug 1;80(1):103–106. doi: 10.1016/0014-5793(77)80416-x. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Miller D. L. A comparison of the activities of the products of the two genes for elongation factor Tu. Mol Gen Genet. 1978 Feb 7;159(1):57–62. doi: 10.1007/BF00401748. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Hachmann J., Weissbach H. The reactions of the sulfhydryl groups on the elongation factors Tu and Ts. Arch Biochem Biophys. 1971 May;144(1):115–121. doi: 10.1016/0003-9861(71)90460-7. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Elongation factor Tu and the aminoacyl-tRNA-EFTu-GTP complex. Methods Enzymol. 1974;30:219–232. doi: 10.1016/0076-6879(74)30024-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Morikawa K., la Cour T. F., Nyborg J., Rasmussen K. M., Miller D. L., Clark B. F. High resolution x-ray crystallographic analysis of a modified form of the elongation factor Tu: guanosine diphosphate complex. J Mol Biol. 1978 Nov 5;125(3):325–338. doi: 10.1016/0022-2836(78)90406-0. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Arai K. i., Takahashi K., Kaziro Y. Alignment of the tryptic fragments and location of sulfhydryl groups of the polypeptide chain elongation factor Tu. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1418–1424. doi: 10.1016/s0006-291x(77)80137-x. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Arai K., Takahashi K., Kaziro Y. Amino acid sequences of two sulfhydryl-containing tryptic peptides of the polypeptide chain elongation factor Tu. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1069–1077. doi: 10.1016/0006-291x(75)90749-4. [DOI] [PubMed] [Google Scholar]
- Nakano A., Miyazawa T., Nakamura S., Kaziro Y. Involvement of histidine residues in the substrate binding of elongation factor Tu from Thermus thermophilus: proton nuclear magnetic resonance and photooxidation study. Arch Biochem Biophys. 1979 Aug;196(1):233–238. doi: 10.1016/0003-9861(79)90571-x. [DOI] [PubMed] [Google Scholar]
- Nomura M., Morgan E. A. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- Ohta S., Nakanishi M., Tsuboi M., Arai K. i., Kaziro Y. Structural fluctuation of the polypeptide-chain elongation factor Tu. A comparison of factors from Escherichia coli and Thermus thermophilus HB8. Eur J Biochem. 1977 Sep;78(2):599–608. doi: 10.1111/j.1432-1033.1977.tb11773.x. [DOI] [PubMed] [Google Scholar]
- Peterson J. D., Nehrlich S., Oyer P. E., Steiner D. F. Determination of the amino acid sequence of the monkey, sheep, and dog proinsulin C-peptides by a semi-micro Edman degradation procedure. J Biol Chem. 1972 Aug 10;247(15):4866–4871. [PubMed] [Google Scholar]
- Rosenbusch J. P., Jacobson G. R., Jaton J. C. Does a bacterial elongation factor share a common evolutionary ancestor with actin? J Supramol Struct. 1976;5(3):391–396. doi: 10.1002/jss.400050311. [DOI] [PubMed] [Google Scholar]
- Schulman R. G., Hilbers C. W., Miller D. L. Letters to the editor: Nuclear magnetic resonance studies of protein-RNA interactions. I. The elongation factor Tu-GTP aminoacyl-tRNA complex. J Mol Biol. 1974 Dec 15;90(3):601–607. doi: 10.1016/0022-2836(74)90237-x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Van Montagu M. Sequence analysis of fluorescamine-stained peptides and proteins purified on a nanomole scale. Application to proteins of bacteriophage MS2. Eur J Biochem. 1974 May 2;44(1):279–288. doi: 10.1111/j.1432-1033.1974.tb03483.x. [DOI] [PubMed] [Google Scholar]
- Wade M., Laursen R. A., Miller D. L. Amino acid sequence of elongation factor Tu. Sequence of a region containing the thiol group essential for GTP binding. FEBS Lett. 1975 Apr 15;53(1):37–39. doi: 10.1016/0014-5793(75)80676-4. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Wilson G. E., Jr, Cohn M. Magnetic resonance studies of the manganese guanosine di- and triphosphate complexes with elongation factor Tu. J Biol Chem. 1977 Mar 25;252(6):2004–2009. [PubMed] [Google Scholar]
- Wolf H., Assmann D., Fischer E. Pulvomycin, an inhibitor of protein biosynthesis preventing ternary complex formation between elongation factor Tu, GTP, and aminoacyl-tRNA. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5324–5328. doi: 10.1073/pnas.75.11.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Kirromycin, an inhibitor of protein biosynthesis that acts on elongation factor Tu. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4910–4914. doi: 10.1073/pnas.71.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eerd J. P., Takahshi K. Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry. 1976 Mar 9;15(5):1171–1180. doi: 10.1021/bi00650a033. [DOI] [PubMed] [Google Scholar]



