SUMMARY
A significant decline in human male reproductive function has been reported for the past 20 years but the molecular mechanisms remain poorly understood. However, recent studies showed that the gap junction protein connexin-43 (CX43; also known as GJA1) might be involved. CX43 is the predominant testicular connexin (CX) in most species, including in humans. Alterations of its expression are associated with different forms of spermatogenic disorders and infertility. Men with impaired spermatogenesis often exhibit a reduction or loss of CX43 expression in germ cells (GCs) and Sertoli cells (SCs). Adult male transgenic mice with a conditional knockout (KO) of the Gja1 gene [referred to here as connexin-43 (Cx43)] in SCs (SCCx43KO) show a comparable testicular phenotype to humans and are infertile. To detect possible signaling pathways and molecular mechanisms leading to the testicular phenotype in adult SCCx43KO mice and to their failure to initiate spermatogenesis, the testicular gene expression of 8-day-old SCCx43KO and wild-type (WT) mice was compared. Microarray analysis revealed that 658 genes were significantly regulated in testes of SCCx43KO mice. Of these genes, 135 were upregulated, whereas 523 genes were downregulated. For selected genes the results of the microarray analysis were confirmed using quantitative real-time PCR and immunostaining. The majority of the downregulated genes are GC-specific and are essential for mitotic and meiotic progression of spermatogenesis, including Stra8, Dazl and members of the DM (dsx and map-3) gene family. Other altered genes can be associated with transcription, metabolism, cell migration and cytoskeleton organization. Our data show that deletion of Cx43 in SCs leads to multiple alterations of gene expression in prepubertal mice and primarily affects GCs. The candidate genes could represent helpful markers for investigators exploring human testicular biopsies from patients showing corresponding spermatogenic deficiencies and for studying the molecular mechanisms of human male sterility.
INTRODUCTION
A significant decline in human male reproductive function associated with diseases of the male genital tract (azoospermia, cryptorchidism, testicular cancer) has been reported for the past two decades (Asklund et al., 2004; Povey and Stocks, 2010; Pointis et al., 2011). In addition, recent studies revealed that intercellular junctions, specifically gap junctions, might be structures of interest and be involved in this phenomenon (Steger et al., 1999; Brehm et al., 2002; Defamie et al., 2003; Pointis et al., 2011).
Gap junction channels connect neighboring cells in nearly all tissues; they permit an exchange of metabolites, signaling molecules and ions, and regulate numerous physiological functions such as cell proliferation, differentiation and apoptosis (Goodenough et al., 1996). Each channel is composed of two hemichannels, called connexons, separately contributed by the two participating cells. The connexons themselves are hexamers formed by self assembly of six connexins (CXs) (Bruzzone et al., 1996). To date, the family of CX genes consists of 20 members in the mouse and 21 members in the human genome (Sohl and Willecke, 2004).
In addition to their classical role, recent studies have reported on the channel-independent functions of gap junctions (Stout et al., 2004). These functions comprise, for example, extrinsic guidance of migrating cells owing to CX-mediated cell adhesion, as well as intracellular processes. Among the connexins, CX43 in particular has been shown to exert effects on migration by interfering with receptor signaling, cytoskeletal remodeling and tubulin dynamics (Kameritsch et al., 2011). Undocked gap junction hemichannels might also exert physiological roles because connexons are involved in: the release of, for example, ATP and NAD+, Ca2+ wave propagation, cell-volume control and the passage of survival signals within the tissue (Spray et al., 2006; Pointis et al., 2010). CXs might also function as scaffold proteins for the attachment of cytoplasmic partner molecules (Huang et al., 1998) and as signaling molecules (Kardami et al., 2007). CX43 interactions with the cytoskeleton have been suggested by several previous studies. The CX43 C-terminus has been shown to bind TJP1 (Giepmans et al., 2001a), a scaffold protein that facilitates linkage of the membrane with the actin cytoskeleton (Itoh et al., 1997; Hartsock and Nelson, 2008). CX43 can also directly bind to tubulin (Giepmans et al., 2001b). In a very recent study, CX43 was shown to modulate cell migration by regulating microtubule dynamics associated with abnormal actin stress fiber organization (Francis et al., 2011).
CX43 is the predominant testicular CX in most species, including humans (Risley et al., 1992; Steger et al., 1999; Batias et al., 2000; Bravo-Moreno et al., 2001; Brehm et al., 2002). In adult men, CX43 protein localization has been investigated in testes both with normal spermatogenesis and associated with different forms of spermatogenic impairment (Steger et al., 1999; Brehm et al., 2002; Defamie et al., 2003; Roger et al., 2004; Brehm et al., 2006; Steiner et al., 2011). As in rodent testes, CX43 immunoreactivity was generally present between Leydig cells (LCs) (Pérez-Armendariz et al., 1994) and, within the seminiferous epithelium, CX43 was found to form an integral component of the Sertoli cell (SC)-SC junctional complexes that represent the anatomical base of the blood-testis barrier (BTB) (Risley et al., 1992). Further components of the BTB, in addition to the CX-based gap junctions, are cadherin (CDH)-based adherens junctions and occludin (OCLN)-based tight junctions (Pointis et al., 2005). There is further evidence that CX43 plays a role in the coordination of changes in SC junctional permeability as well as in SC and germ cell (GC) differentiation (Pelletier, 1995). Furthermore, the synchronization of GC proliferation and differentiation is mediated through the gap junctional network (Decrouy et al., 2004).
Within the normal human (and murine) seminiferous epithelium, CX43 immunoreactivity is localized between SCs and GCs (spermatogonia and primary spermatocytes) and along the SC-SC junctional complexes (Steger et al., 1999; Brehm et al., 2002; Defamie et al., 2003). Some information is available concerning the presence of gap junctions and expression of CX43 in seminiferous tubules of pathologic human testes. By means of freeze fracture, no gap junctions were detected in feminized human testes (Nagano and Suzuki, 1976) and the presence of atypical testicular gap junctions was observed in infertile patients (Bigliardi and Vegni-Talluri, 1977). Gap-junction-like cell-membrane specializations were rare in hypospermatogenic and aspermatogenic testes (Schleiermacher, 1980). In addition, testicular histology of these infertile adult patients frequently reveals spermatogenic defects that are associated with heterogeneous SC populations. For example, tubules showing (1) spermatogenic arrest at the spermatogonial level and those with (2) SC-only (SCO) syndrome are characterized by immature populations of SCs expressing fetal differentiation markers, such as anti-Muellerian-hormone (AMH) and cytokeratin 18 (CK18) (Bergmann and Kliesch, 1994), and exhibit a reduction or even loss of CX43 in GCs and SCs (Steger et al., 1999; Brehm et al., 2002; Defamie et al., 2003). Finally, alterations of CX43 expression have been correlated to the development of human carcinoma in situ (CIS) of the testis and testicular tumors (Brehm et al., 2002; Roger et al., 2004; Brehm et al., 2006).
Taken together, there now exist many studies and review articles (e.g. Pointis and Segretain 2005; Pointis et al., 2005; Sridharan et al., 2007b; Pointis et al., 2010; Gilleron et al., 2011; Weider et al., 2011a; Pointis et al., 2011) that support functional roles of CX43 in the regulation of human spermatogenesis: (1) CX43 forms intercellular channels between SCs and proliferating GCs, indicating that this CX is involved in the physiological maturation and proliferation processes of these cell types; (2) alterations of CX43 expression are correlated with testicular disorders in men; for example, individuals with CIS and seminoma, the most frequent type of human GC tumors, show a reduction of CX43 expression in SCs and tumor cells. In CIS tubules, the reduction of CX43 expression was shown to be regulated at the transcriptional level and accompanied with a dedifferentiation of SCs (Brehm et al., 2006); (3) moreover, possible mutations in the human CX43 gene have been discussed because a recent study working with a mutant mouse model of oculodentodigital dysplasia (ODDD) showed impaired spermatogenesis, supporting the possibility of subfertility in ODDD human males (Gregory et al., 2011). However, to date, there is still a lack of information on the molecular mechanisms resulting in the impairment of human male reproductive function through CX disruption.
To further investigate the role of CX43, several knockout (KO) and knock-in (KI) mice have been generated. General KO of the Cx43 gene leads to cardiac malformation and prenatal death; thus, a functional analysis of spermatogenesis was not possible in this KO mouse line (Reaume et al., 1995). Nevertheless, a severe GC loss in the embryonic tissue has been detected, indicating an indispensable role of CX43-mediated cell coupling for GC development (Juneja et al., 1999). Testes of Cx43-null mutant embryos neither allow a normal GC proliferation nor differentiation when grafted under the kidney capsule of adult males (Roscoe et al., 2001). However, the detailed mechanisms that lead to sterility of the male Cx43 mice in the mentioned studies are still unknown (Pointis et al., 2005).
To study the effects of a deletion of Cx43 on spermatogenesis, a conditional SC-specific KO of the Cx43 gene (SCCx43KO) has been generated and revealed that Cx43 expression in SCs is an absolute requirement for normal testicular development and initiation of spermatogenesis (Brehm et al., 2007; Sridharan et al., 2007a). Compared with the generalized KO (Reaume et al., 1995), SCCx43KO mice are viable, but infertile (Brehm et al., 2007; Sridharan et al., 2007a). Weighing of animals and testes displays no differences in the body weight between SCCx43KO and wild type (WT); however, testes were significantly smaller in the KO males. Furthermore, adult SCCx43KO mice show a significantly reduced number of GCs per tubule, whereas the mean number of SCs per tubule is significantly higher compared with WT (Brehm et al., 2007). Interestingly, histological analysis of adult SCCx43KO mice revealed similar spermatogenic alterations as seen in infertile humans, such as an arrest of spermatogenesis at the level of spermatogonia, or SCO syndrome (Brehm et al., 2007; Sridharan et al., 2007a).
The number of GCs that can be supported by each SC is fixed for each species. This species-specific ratio of GCs to SCs is determined prepubertally by proliferation and apoptosis (Rodriguez et al., 1997; Sharpe et al., 2003). A disruption of cell-cell communication is thought to lead to increased apoptosis and GC loss, demonstrating the significant role of testicular CX to maintain the survival of GCs by regulating intercellular communications between GCs and adjacent supporting cells (Lee et al., 2006b). Dye-coupling studies demonstrated that CX43 participates in the coupling between SCs and between SCs and GCs, with the dye transfer from SCs to GCs being mostly unidirectional (Decrouy et al., 2004). Thus, the observed phenotype in adult SCCx43KO mice might, for instance, be due to: (1) a disrupted direct connection; for example, a disturbed flow of signaling molecules and high-energy metabolites from SCs to GCs via gap junction channels containing CX43 or (2) channel-independent functions.
To get a deeper insight into the molecular mechanisms and possible signaling pathways leading to the observed histological phenotype and infertility in adult SCCx43KO mice, a genome-wide gene expression profiling of 8-day-old WT and SCCx43KO mice was performed. Mice of that age have been chosen because (1) the morphology of the testis was still comparable between the two genotypes and (2) murine spermatogenesis has been initiated, indicated by the first appearance of type B spermatogonia (Bellvé et al., 1977).
RESULTS
GC number is significantly reduced in 8-day-old SCCx43KO mice
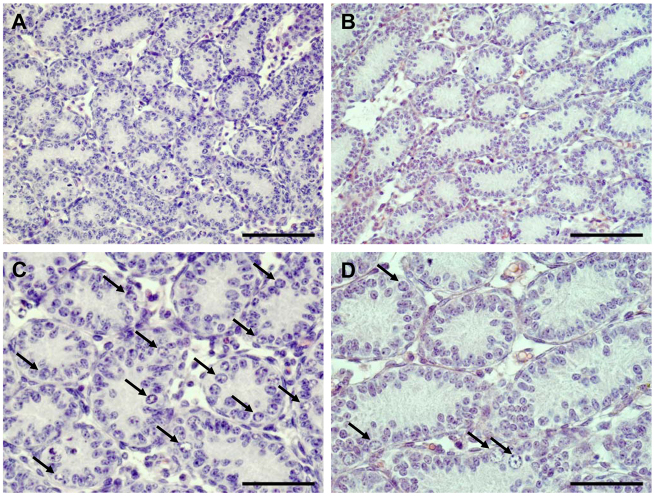
Comparison of testis weight revealed no significant differences between WT and SCCx43KO mice on day 8 post partum (p.p.). However, morphological and histological examination detected obvious differences in the composition of intratubular cells between the two genotypes (Fig. 1A–D). GC number was three times higher in testes of WT mice, whereas the number of SCs (and LCs) was not affected by the deletion of Cx43 (Figs 1, 2).
Fig. 1.
Representative histology of WT and SCCx43KO mouse testis at day 8 p.p. H&E staining of a WT mouse testis (A,C) shows SC and type A and B spermatogonia (arrows). By contrast, in seminiferous cords of an SCCx43KO mouse (B,D), only single GCs (arrows) are detectable. Scale bars: (A,B) 100 μm; (C,D) 50 μm.
Fig. 2.
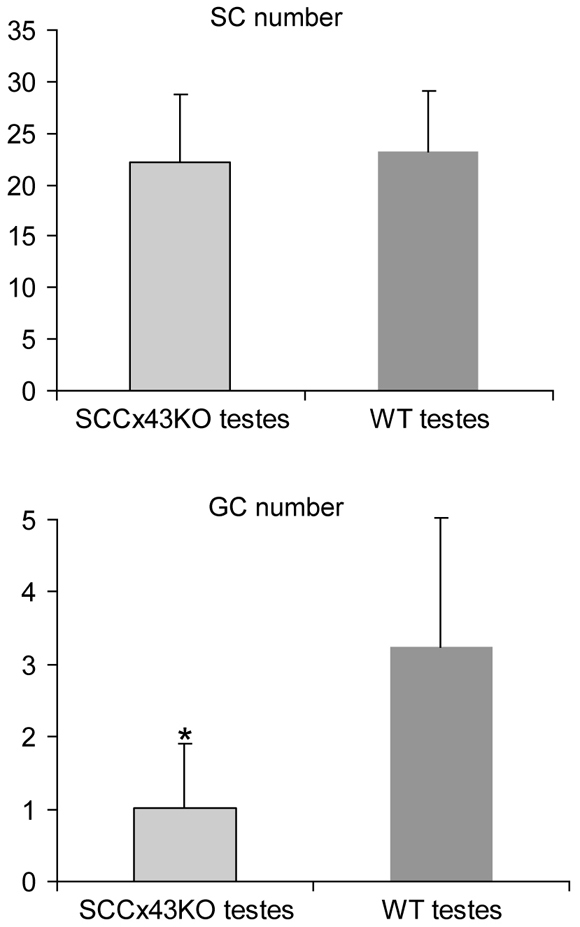
Comparison of SC and GC numbers of WT and SCCx43KO mouse testis at day 8 p.p. Average number of SCs and GCs per cord, comparing the two genotypes at day 8 p.p. GC number is significantly reduced in KO mice. Error bars represent standard deviation (s.d.). The asterisk indicates the statistical significant difference (P<0.05) between the WT and SCCx43KO mice. Seminiferous cords of WT mice contain 3.24±1.78 GCs and 23.17±5.92 SCs. Cords of KO mice have 1.02±0.89 GCs and 22.17±6.64 SCs.
Differential gene expression in WT and KO mice
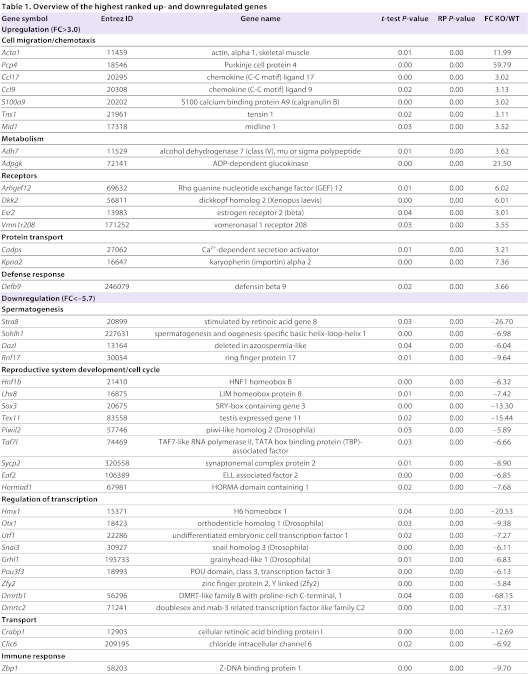
Microarray analysis revealed 658 significantly regulated genes [P≤0.05 and −2≥fold change (FC)≥2]. Of these, 135 genes showed higher gene expression values, whereas 523 displayed lower gene expression values in SCCx43KO mice compared with WT mice. Annotation was possible for 475 downregulated and 106 upregulated genes. Table 1 gives an overview of the highest ranked genes and the functional category they belong to. All significantly regulated genes can be found in supplementary material Table S1A,B.
Table 1.
Overview of the highest ranked up- and downregulated genes
Hierarchical clustering of the 658 significantly regulated genes shows two clusters clearly differing between WT and KO mice (Fig. 3). Within each cluster, the gene expression profiles of the biological replicates of WT1-3 and KO1-3 mice were homogenous, indicating reproducibility of the microarray results. Within the WT group, two subclusters are present, i.e. WT1 versus WT2/3. This small difference in the expression profile can be explained because WT2 and 3 were from the same litter, whereas WT1 was another litter from the same mouse.
Fig. 3.
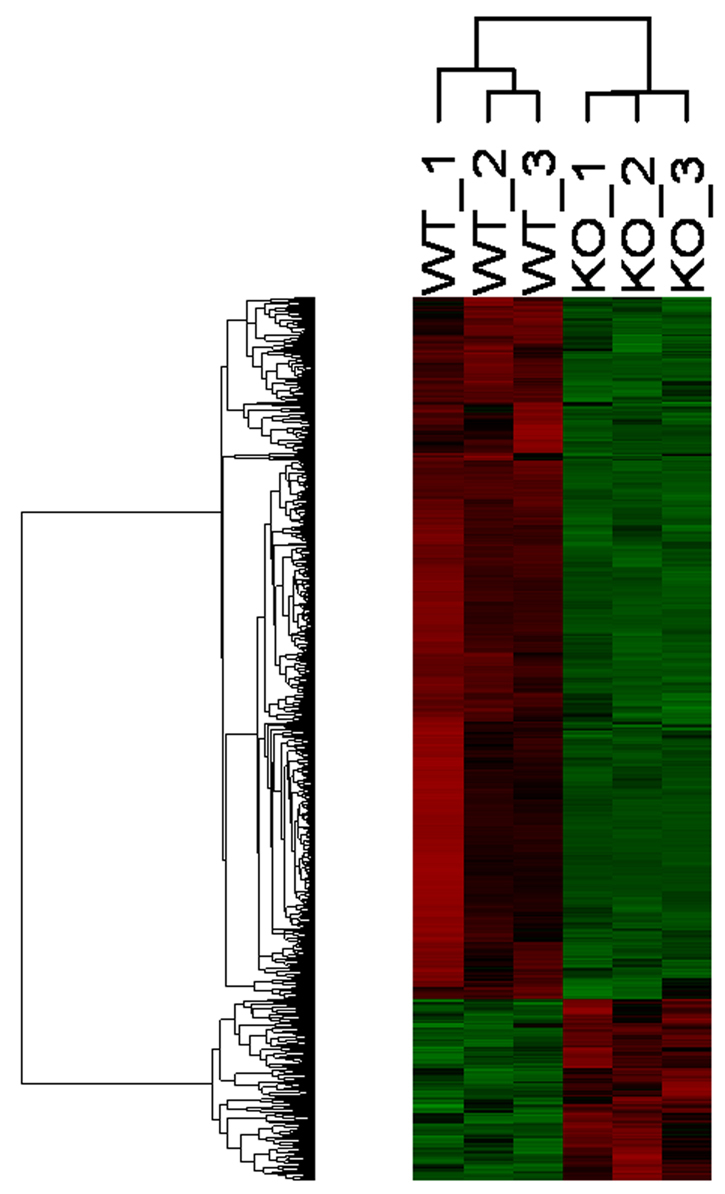
Hierarchical clustering of 658 significantly regulated genes. Genes are depicted in rows and samples in columns. Green indicates downregulation; red indicates upregulation.
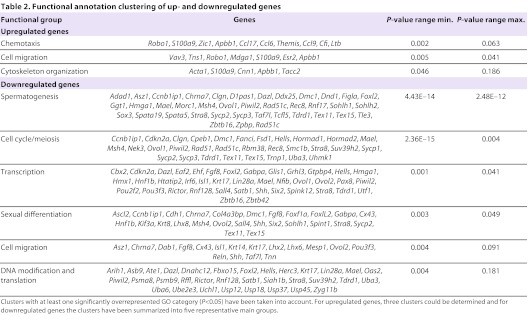
Gene ontology (GO) of the differentially regulated genes revealed clusters of biological categories representing functional annotation groups. The upregulated genes were assigned to three clusters, involved in chemotaxis, cell migration and cytoskeleton organization (Table 2). The downregulated genes could be assigned to 16 clusters, with the majority of these genes being involved in spermatogenesis, cell cycle/meiosis, transcription, sexual differentiation, cell migration, DNA modification and translation (Table 2). The detailed functional annotation clusters can be found in supplementary material Table S2A,B.
Table 2.
Functional annotation clustering of up- and downregulated genes
Pathway analysis for functions with the differentially regulated genes resulted in spermatogenesis as a top function. The 29 genes within spermatogenesis were mainly downregulated and involved in subfunctions such as meiosis of male GCs or expression of RNA, highlighting the influence of the KO gene Cx43 on the different aspects of spermatogenesis (Fig. 4).
Fig. 4.
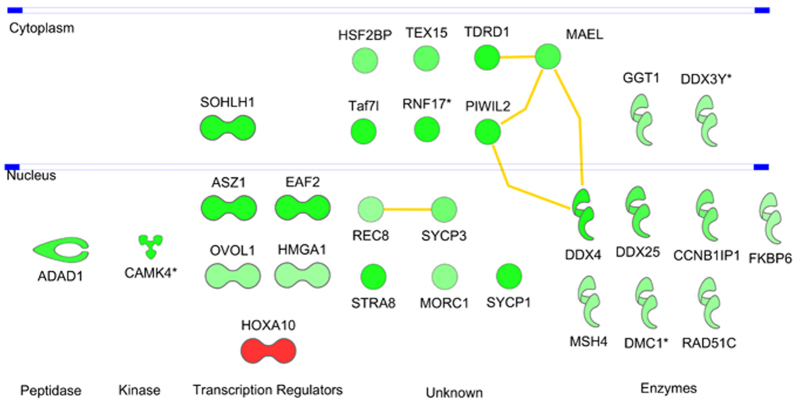
Interaction network of functions involved in spermatogenesis. Analysis of all regulated genes in IPA revealed spermatogenesis as a top function. Known protein-protein interactions for the involved genes are highlighted by yellow lines. Colors represent the FC in expression of the genes. Red, significant upregulation with FC>2; green, significant downregulation with FC<–2. Genes in dark green show higher FC compared with genes in light green (e.g. Sohlh1 with FC −7.0 and Rec8 with FC −2.4).
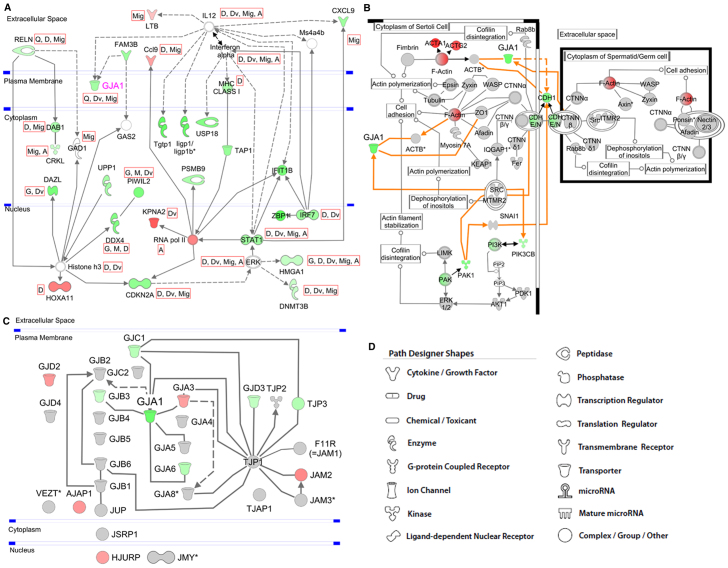
Additionally, pathway analysis revealed ten networks each containing interactions of nine or more genes from the list of differentially regulated genes (supplementary material Table S3). The top network contained the KO gene Cx43 and genes that were overall involved in infectious disease, gene expression and antigen presentation. The network is depicted in Fig. 5A, showing the interaction of Cx43 with these genes. Remarkably, most of the genes in the network involving Cx43 are downregulated and involved in gametogenesis, gene activation, quantity of gap junctions, cell division, cell differentiation and cell migration.
Fig. 5.
The interaction networks and canonical pathways were generated with IPA. A description of the used symbols is given in D. The symbols are colored according to the FC of gene expression in KO versus WT. A solid line represents a direct relationship, and a dashed line an indirect relationship between molecules. An asterisk denotes the presence of two or more probes on the chip for the respective gene. (A) Interaction network of Cx43 (Gja1) with genes deriving from the top network of differentially regulated genes. Genes and/or molecules are connected via indirect or direct connections. The gene symbols are colored according to their FC: red, significant upregulation with FC>2; green, significant downregulation with FC<–2. Some relevant functions have been selected and assigned to the respective genes symbols. These are: G, gametogenesis; A, activation of gene(s); Q, quantity of gap junctions; M, meiosis; D, differentiation; Dv, division; Mig, migration of cells. (B) Canonical GC-SC junction pathway. The predefined canonical pathway ‘GC-SC junction’ has been expanded by adding Cx43 (Gja1) and its relations to pathway-related genes (orange lines). Only the section in which GJA1 is involved is shown. Red, significant upregulation with FC>2; green, significant downregulation with FC<–2. (C) Network of members of the family of CX genes and other junction-related genes. Genes with an FC higher than 1.2 are colored in red, and genes with an FC lower than −1.2 are colored in green. (D) Symbols used for graphical presentation of pathways.
Further interactions of Cx43 are indicated in the predefined canonical ‘GC-SC junction pathway’ (Fig. 5B). It is shown that interaction between SCs and GCs can be supported by protein complexes and/or proteins such as CDHE- or CDHN-CTNNB1 (CDHE/N-CTNNB1), PONSIN-AFADIN-NECTIN2, ITGα6β1, TNFR and TGFβR.
When overlaying gene expression data onto the canonical pathway, only the interaction via CDHE/N-CTNNB1 is affected by downregulation of Cdh1. The putative connections of CX43 and the canonical pathway are given as orange arrows in Fig. 5B. CX43 can be related to this canonical pathway by interaction with ACTB, TJP1 and with a complex consisting of proto-oncogene products SRC and MTMR2.
The family of CX genes comprises 20 members in the mouse (in the groups Gja-Gje). To assess whether other junction genes, especially the members of the gap junction family, were also affected from the KO of Cx43, the gene expression of those junction genes available on the microarray was determined (supplementary material Table S4). Beside Cx43, none of the remaining junction genes reached statistical (P≤0.05) or biological (−2≥FC≥2) significance. Nevertheless, Gja6, Gjb3, Gjc1, Gjd3 and Tjp3 were biologically downregulated (FC ranging from −1.3 to −1.8), with Gjb3, Gjc1 and Gjd3 being statistically significant (P≤0.05). The interaction of the junction genes with Cx43 is depicted in Fig. 5C.
The majority of genes associated with spermatogenesis are GC-specific
The majority of the differentially expressed genes can be associated with spermatogenesis. The majority of these genes are exclusively expressed in GCs (Table 3).
Table 3.
List of differentially expressed GC-specific genes associated with spermatogenesis
Validation of the microarray data by quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was applied to validate the microarray data. In total, 19 candidate genes, showing different ranges of values (up, down, unchanged), were selected to be analyzed. Among the chosen genes, some are mainly associated with the BTB formed by SCs, such as Cdh2, Cld11, Ocln and Tjp1. Other genes were selected for their FC value or their importance in spermatogenesis. After applying the calculated correction factors, the qRT-PCR data were mostly consistent with the microarray results, displaying a significant correlation between the log ratios of the considered genes in the qRT-PCR and the corresponding log ratios in the microarray analysis with correlation coefficient of 0.86 and significance P-value <0.01. Relative mRNA levels of Sohlh1, Ddx25, Cdkn2a, Stra8, Dmrt6, Sox3, Dazl, Cx43, Piwil2, Tex11 and Sycp2 were lower in the KO mice compared with WT animals (Fig. 6).
Fig. 6.
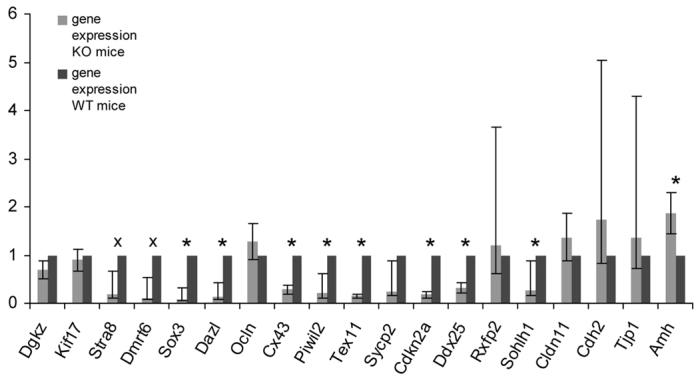
Relative gene expression of WT mice versus SCCx43KO mice after applying the calculated correction factors. Bars represent mean±s.d. mRNA levels of three independent qRT-PCR experiments. Calculations were realized determining Actb and Hsp90ab1 as housekeeping genes and applying the ΔΔC(t) method. The genes Sox3, Dazl, Cx43, Piwil2, Tex11, Ddx25, Cdkn2a and Sohlh1 were significantly downregulated (*P<0.05), whereas the downregulation of Stra8 and Dmrt6 was close to significant (x). The only significant upregulation was detected for Amh.
Confirmation of Cx43 deletion by immunohistochemistry
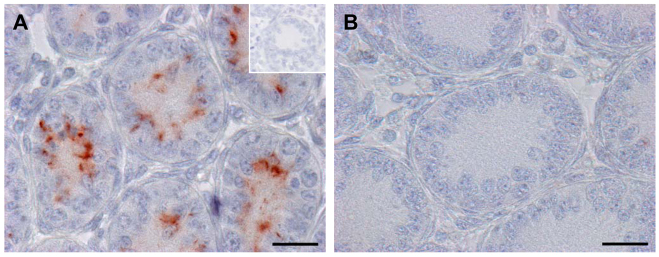
CX43 immunostaining at day 8 p.p. revealed no immunoreactivity in seminiferous cords of SCCx43KO mice (Fig. 7), whereas, in seminiferous cords of the prepubertal WT males, a typical immature and diffuse adluminal signal was detectable.
Fig. 7.
Confirmation of Cx43 deletion by IHC. In seminiferous cords of WT mice (A) a typical prepubertal diffuse adluminal staining pattern for CX43 can be seen. By contrast, cords of SCCx43KO mice show no CX43 immunoreactivity at all (B). Insert in A: negative control. Scale bars: 20 μm.
Immunohistochemistry confirmed qRT-PCR results at protein level
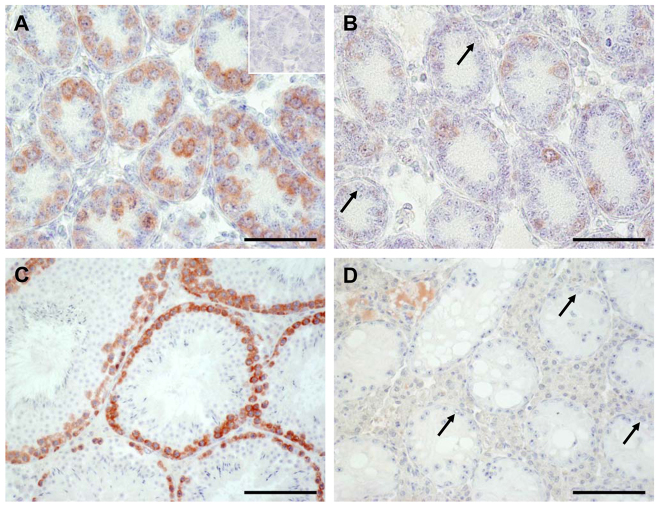
Selecting two highly downregulated GC-specific genes [Stra8 (Fig. 8) and Dazl (Fig. 9)] and one gene associated with the BTB and with no significant alteration at mRNA level [Ocln (Fig. 10)], we applied immunohistochemistry (IHC) to examine their level of protein expression.
Fig. 8.
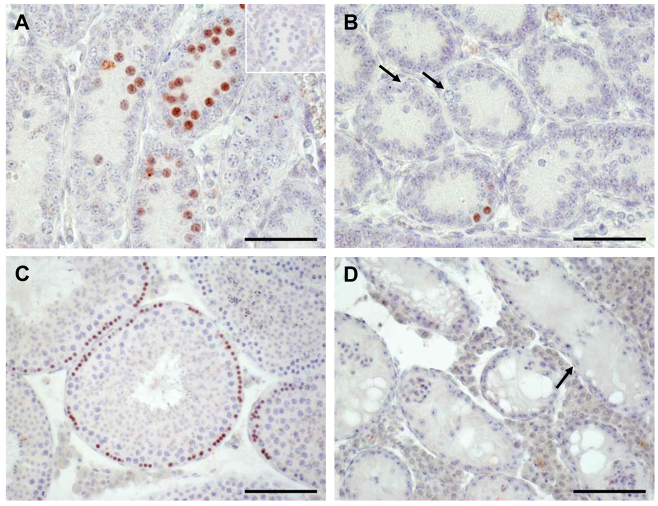
IHC confirmed the findings obtained by microarray analysis and qRT-PCR for Stra8 (GCs). The occurrence of STRA8 protein revealed obvious differences between WT (A,C) and SCCx43KO (B,D) mice. In seminiferous cords of the 8-day-old WT mice, many spermatogonia and first preleptotene spermatocytes show an intensive staining for STRA8 (A). By contrast, only single cells of the SCCx43KO mice revealed an immunoreactivity for STRA8 (B). Note the immunonegative spermatogonia in B (arrows). In adult WT mice, a stage-specific immunoreaction for STRA8 can be seen (C), whereas no immunoreaction at all can be detected in seminiferous tubules of adult mutants showing an arrest of spermatogenesis at the level of spermatogonia (arrow, D). Insert in A: representative negative control for STRA8 experiments. Scale bars: (A,B) 50 μm; (C,D) 100 μm.
Fig. 9.
IHC confirmed also the findings obtained by microarray analysis and qRT-PCR for Dazl (GCs). The occurrence of GC-specific DAZL protein revealed obvious differences between WT (A,C) and SCCx43KO (B,D) mice. The number of stained cells is drastically reduced in 8-day-old SCCx43KO mice (B), confirming results obtained by qRT-PCR. Note immunonegative spermatogonia in B (arrows). In adult mice, a clear immunoreaction for DAZL can be seen in spermatogonia and spermatocytes (C). By contrast, in adult SCCx43KO mice not even spermatogonia (arrows) show a DAZL immunostaining (D). Insert in A: representative negative control for DAZL experiments. Scale bars: (A,B) 50 μm; (C,D) 100 μm.
Fig. 10.
IHC (without counterstaining) also approved the findings obtained by microarray analysis and qRT-PCR for Ocln (SCs). No differences for OCLN are detectable comparing WT (A) and SCCx43KO (B) mice. Insert in A: representative negative control for OCLN experiments. Note faint unspecific binding in LCs. Scale bars: 50 μm.
In seminiferous cords of the 8-day-old WT mice, many spermatogonia and first preleptotene spermatocytes showed an intensive staining for STRA8 (Fig. 8A). By contrast, only single cells of the SCCx43KO mice revealed an immunoreactivity for STRA8 (Fig. 8B). Note the immunonegative spermatogonia in Fig. 8B (arrows). In adult WT mice, a stage-specific immunoreaction for STRA8 can be seen (Fig. 8C), whereas no immunoreaction at all can be detected in seminiferous tubules that show an arrest of spermatogenesis at the level of spermatogonia (arrow) in adult mutants (Fig. 8D).
Regarding the distribution pattern of DAZL, there is also an obvious difference between the two genotypes (Fig. 9A–D). The number of stained cells is drastically reduced in 8-day-old SCCx43KO mice, confirming results obtained by qRT-PCR. Note the immunonegative spermatogonia in Fig. 9B (arrows). In adult mice, a clear immunoreaction for DAZL can be seen in spermatogonia and spermatocytes (Fig. 9C). By contrast, in seminiferous tubules showing an arrest of spermatogenesis at the level of spermatogonia (arrows) in adult SCCx43KO mice, not even spermatogonia show a DAZL immunostaining (Fig. 9D, arrows).
OCLN represents an integral SC membrane protein and is involved in the formation of the BTB. Deletion of Cx43 in SCs results in no obvious changes in OCLN protein synthesis and localization when comparing 8-day-old WT (Fig. 10A) and mutant (Fig. 10B) animals.
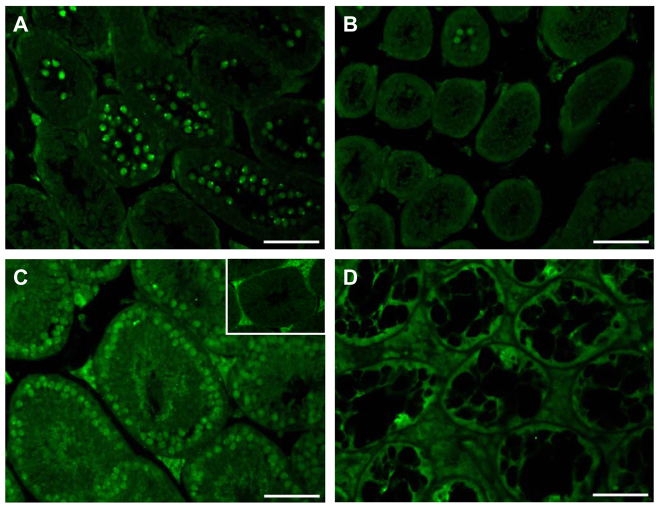
Evaluation of DMRT7 protein synthesis using immunofluorescence
Immunofluorescence (IF) revealed no immunoreactivity for DMRT7 in most seminiferous tubules in 15-day-old KO mice (Fig. 11B), whereas the WT mice showed a specific staining in most pachytene spermatocytes (Fig. 11A). In adult WT males, a specific staining can be detected in nearly all spermatocytes (Fig. 11C), whereas only paraffin autofluorescence is seen in KO tubules (Fig. 11D). The insert in Fig. 11C shows paraffin autofluorescence in interstitial LCs in negative controls.
Fig. 11.
IF for DMRT7. WT (A,C) and SCCx43KO (B,D) on day 15 p.p. (A,B) and in adulthood (C,D) were compared. Results showed a higher number of DMRT7 immunopositive cells in pubertal WT testis compared with SCCx43KO mice (A,B). In adult WT males, a specific staining can be detected in nearly all spermatocytes (C), whereas only paraffin autofluorescence is seen in KO tubules (D). Insert in C: note paraffin autofluorescence in interstitial LCs in negative controls. Scale bars: 50 μm.
DISCUSSION
The prevalence of human couple infertility is extremely high and in many countries affects one in seven couples (Sharpe, 2010). Although the nature and exact proportion of the predominant cause of the problem remains controversial, the World Health Organization (WHO) reports that, in nearly 40% of cases the cause can be attributed to the female, in 20% to the male and in 25% to both; however, in 15% the cause still remains unknown (WHO, 1987). On the basis of these figures, the incidence of human male factor infertility in the general population is approximately 7%, making it more prevalent than type 1 and 2 diabetes mellitus combined, and this proportion is expected to rise. With at least one third of infertile men being diagnosed as having idiopathic infertility associated with impaired spermatogenesis, there is an urgent need to better understand the status of male reproductive health, especially in Europe and industrialized countries (EU Strategic Research Plan, 2010).
Impaired spermatogenesis (with e.g. an arrest of spermatogenesis at different stages and/or SCO syndrome) can result from a variety of causes. For example, genetic defects like Klinefelter syndrome and cryptorchidism exhibit a fivefold increased risk for the development of testicular cancer, which represents the most common cancer among men (Møller and Skakkebaek, 1999; Skakkebaek et al., 2001; Asklund et al., 2004). In approximately 25% of these men, an aberrant spermatogenic appearance is also evident in the ‘assumed normal’ contralateral testis. In a series of prospective and well-standardized studies over the past 10 years, it has been found that the prevalence of an abnormally low sperm count in young men is as high as 15–20% (Jørgensen et al., 2006; Andersson et al., 2008). Testicular biopsies of these men often reveal mixed atrophy showing seminiferous tubules with qualitatively normal spermatogenesis directly adjacent to tubules with spermatogenic arrest and/or SCO syndrome (Steger et al., 1996; Steger et al., 1998).
Recent studies revealed that the gap junction protein CX43 seems to be involved in different forms of spermatogenic disorders (Steger et al., 1999; Brehm et al., 2002; Defamie et al., 2003; Steiner et al., 2011) and in the pathogenesis of human testicular germ cell tumors (Brehm et al., 2002; Steiner et al., 2011), so a transgenic mouse model (SCCx43KO) has been established to investigate cellular and molecular mechanisms that are essential for male GC differentiation and to identify aberrant signaling pathways in ‘male factor infertility due to impaired spermatogenesis’ (Brehm et al., 2007; Sridharan et al., 2007a).
In the present study, using the microarray technology and after applying the calculated correction factors, several candidate genes possibly involved in the observed reduction of GC number, in preventing the initiation of spermatogenesis and thus in the disturbed progression beyond mitosis of the remaining GCs in adult SCCx43KO mice have been detected. These genes (connected with a CX43 deficiency) could also represent helpful markers for investigators exploring the molecular mechanisms of human male sterility. GO analysis revealed chemotaxis, cell migration and cytoskeleton organization as the major functional clusters affected within the upregulated genes. Interestingly, genes associated to the group of cell migration are also highly present within the group of downregulated genes, indicating an important role for CX43-meditated communication in this functional gene class.
Within the seminiferous epithelium, CX43 is thought to be important for both the SC-SC functional synchronization and the SC-GC crosstalk (Pointis et al., 2010). Here, direct contact-dependent junctional pathways play a pivotal role in the regulation of GC and SC proliferation and differentiation, and the maintenance of the male phenotype (Mruk and Cheng, 2004). In addition to (intermingled) CX43 gap junctions, the other junctions are composed of specialized proteins implicated in cell adhesion, the regulation of paracellular diffusion, the establishment and maintenance of cell polarity, the regulation of cell attachment, and the regulation of actin proteins (Cheng and Mruk, 2002; Derangeron et al., 2009; Cheng et al., 2011). CX43 interactions with the cytoskeleton have been suggested by several previous studies. The C-terminus of CX43 has been shown to bind TJP1 (Giepmans et al., 2001a), facilitating the linkage of the membrane with the actin cytoskeleton (Itoh et al., 1997; Hartsock and Nelson, 2008). CX43 can also directly bind to tubulin (Giepmans et al., 2001b). In a very recent work, CX43 was shown to modulate cell migration by regulating microtubule dynamics associated with abnormal actin stress fiber organization (Francis et al., 2011). In this context, in the present study, Tuba3 [encoding for TUBA3 (FC −4.5)] and different Krt genes (Krt14, 17, 24 and 8; encoding for keratins) were significantly downregulated, implying alterations in cytoskeleton dynamics.
During embryonal development, human SCs contain both CK18 and vimentin; however, only the latter persists in adult testis (Franke et al., 1979) and CK18 expression nearly disappears after the 20th week of gestation. CK18 intermediate filaments are completely lost around birth, thereby signaling the earliest transition of SCs to a mature state. CK18 expression, therefore, indicates a state of undifferentiation (Rogatsch et al., 1996; Franke et al., 2004). Interestingly, co-expression of vimentin together with CK18 occurs in SCs of adult testes under pathological conditions (Steger et al., 1996; Kliesch et al., 1998) concomitant with a reduction or loss of CX43 (Brehm et al., 2002). Moreover, in a recent study, tubulin was identified as a 14-3-3β binding partner, and thus is essential for cell adhesion within the seminiferous epithelium, cell polarity of SCs and normal spermatogenesis (Graf et al., 2011).
Further GO groups include strongly downregulated genes involving spermatogenesis, cell cycle/meiosis, transcription, sexual differentiation, cell migration, and DNA modification and translation, confirming a requirement for Cx43 expression of these features.
Interestingly, among the group of downregulated genes that can be associated with spermatogenesis, a very high percentage of GC-specific genes were present (Ruggiu et al., 1997; Dai et al., 1998; Reber and Cereghini, 2001; Sapiro et al., 2002; Saunders et al., 2003; Li et al., 2005; Raverot et al., 2005; Ballow et al., 2006; Pangas et al., 2006; Cheng et al., 2007; Lin et al., 2007; Anderson et al., 2008; Zhou et al., 2008). This was surprising, because an SC-specific gene deletion would be expected first to lead to an altered expression pattern related to this cell population. However, our results can be explained by the juxtaposition and dependency of the two cell types, which is interrupted by the deletion of the Cx43 gene. As shown by dye transfer studies, the coupling between SCs and GCs is unidirectional from SCs to GCs (Decrouy et al., 2004). Together with the observation that the gene expression of mainly GCs is influenced, our results might lead to the assumption that the interruption of poorly characterized mediators of signals from SCs to GCs affect primarily the normal development of early GC populations, preventing the initiation of spermatogenesis. Examples for such GC-specific genes are depicted in Table 3. The physiological relevance of the genes identified by the microarray study is underscored by the finding that at least eight downregulated genes have already been shown to be essential for normal spermatogenesis. Using qRT-PCR, IHC and IF, we were able to verify the microarray results for selected genes of interest, such as Stra8, Dazl and Dmrt7.
Stra8 (FC −26.70) is a vertebrate-specific factor expressed in GCs in response to retinoic acid (RA) (Anderson et al., 2008). STRA8 protein expression at day 8 p.p. is restricted to the spermatogonia and first preleptotene spermatocytes (Zhou et al., 2008). RA is known to be essential for normal spermatogenesis. The actions of RA are mediated through six distinct ligand-dependent transcription factors, including three RA receptors (RARα, β and γ) and three retinoid X receptors (Zhou et al., 2008). It is well known that RA stimulates the expression of Cx43 (Batias et al., 2000; Tanmahasamut and Sidell, 2005; Vine and Bertram, 2005). A recent publication by Chung et al. further demonstrated that a deletion of RARα leads to an alteration of gap-junction-based cell coupling (Chung et al., 2010). KO studies of the Stra8 gene revealed its importance for functional spermatogenesis: its deletion leads to infertility of male and female mice. In male KO mice, infertility is caused by a failure in meiotic initiation, blocking the transition into the meiotic prophase (Anderson et al., 2008). The close relation between Cx43 and Stra8 is affirmed by their overlapping regulatory mechanisms, their common requirement during GC development and the dependency of Stra8 expression on Cx43 expression. Whether this interaction is causally related or the result of altered interactions between GCs and SCs leading to a loss of signaling molecules that are essential for Stra8 transcription and STRA8 protein synthesis in GCs requires further investigation. However, in a very recent study a close relationship between RA signaling, STRA8 expression and the control of meiotic entry in the human fetal gonad was shown (Childs et al., 2011).
Dazl (FC −6.04), also established as a GC-specific gene, can be detected in the cytoplasm of type B spermatogonia and less abundantly in pachytene, preleptotene and zygotene spermatocytes (Ruggiu et al., 1997). Like Stra8, KO studies revealed a pivotal role for this gene in spermatogenesis. Interestingly, the nature of the Dazl KO is very similar to the observed one in SCCx43KO mice. In both murine lines the males are infertile and exhibit a reduced number of GCs, and the remaining GCs show an arrest of spermatogenesis, precluding progression through meiotic prophase (Saunders et al., 2003). A comparison of significantly regulated genes in 5- and 7-day-old testes of WT and Dazl KO mice with results from the present study showed that just two genes were significantly regulated in both experiments, namely Ovol1 and Nfx2 (Maratou et al., 2004). These results could lead to the conclusion that there might be a synergistic action of Dazl and Cx43, rather than a shared pathway leading to the meiotic failure observed in the two mutant mouse lines. Moreover, Dazl has been demonstrated recently to have a significant contribution in the differentiation of embryonic stem cells into pre- and post-meiotic GCs (Kerr and Cheng, 2010), and humans with spermatogenic failures showed significantly lower levels of Dazl transcripts compared with men with normal spermatogenesis (Lin et al., 2001). Furthermore, these data provide evidence for an important role of Nxf2 and Ovol1 for GC development beyond mitosis.
The DM family of genes encode a widely conserved transcription factor family; these proteins are involved in sexual differentiation at least along three phyla (Murphy et al., 2007). Mammals express at least seven DM domain genes (Dmrt1-7) (Kim et al., 2003). Dmrt1 is known to be essential for sexual differentiation in mammals (Raymond et al., 2001), whereas little information is available for Dmrt6 [also known as Dmrtb1 (FC −68.15)]. Kim et al. detected a very high expression of Dmrt2, 5, 6 and 7 in adult testis (Kim et al., 2007). Because it is known that Dmrt7 [also known as Dmrtc2 (FC −7.31)] is essential for male meiosis and spermatogenesis, we explored its protein expression comparing WT and SCCx43KO mice. Because DMRT protein is restricted to pachytene spermatocytes (and sperm), 15-day-old mice were used for this experiment. In these SCCx43KO mice, only single spermatocytes were present and showed a weak signal for DMRT7 protein, suggesting that the reduction of gene expression of DM family members observed in 8-day-old KO males proceeds and is finally abrogated in older KO mice, particularly because Dmrt mRNA has also been detected in spermatogonia (Kawamata et al., 2007). This hypothesis was confirmed by DMRT7 IF in adult KO mice. Based on the fact that at least two members of the DM-domain family (Dmrt6 and Dmrt7) show an altered expression pattern, it is very probable that this gene family displays a contribution to the disturbed spermatogenesis and GC loss observed in adult SCCx43KO mice. Furthermore, Dmrt1 has been linked with human testicular germ cell tumor development (Looijenga et al., 2006) and susceptibility (Kanetsky et al., 2011).
Beyond the genes confirmed by qRT-PCR and IHC or IF, the expression of several more genes, for example Sohlh1 (FC −6.98), Sox3 (FC −13.30), Sycp2 (FC −8.90) and Piwil2 (FC −5.89), was significantly altered in our study and might also be interesting new candidate genes for comparative human investigations. Sohlh1 and Sox3 single KO mice show an arrest of spermatogenesis at the level of spermatogonia and, comparable to the present mouse model, their deletion leads to a disruption of GC differentiation beyond early developmental stages and to infertility (Raverot et al., 2005; Ballow et al., 2006). By contrast, the deletion of Sycp2 and Piwil2 leads to a spermatogenic arrest from zygotene to early pachytene stages (Kuramochi-Miyagawa et al., 2004; Yang et al., 2006). The Piwi family genes play essential roles in stem cell self-renewal and gametogenesis (Lee et al., 2006a). Thus, it might be possible that Cx43 acts as a molecular upstream promoter of Piwil2 that is in turn known to be able to modulate the expression of stem-cell-specific genes, including Stra8 or Cx45. These genes require further examination to elucidate their possible role in the phenotype of adult SCCx43KO mice and their connection to CX43.
CX43 gap junctions between SCs are further known to form an intercellular communication network within the seminiferous epithelium corresponding to an initial ‘syncytium-like organization’ that allows the tubular coordination of SC metabolism and, indirectly, via metabolic and signaling coupling, the synchronization of GC proliferation and differentiation. At present, it cannot totally be excluded that the failure to initiate spermatogenesis and the observed GC deficiency in adult SCCx43KO mice reflects an altered state of SC maturation and a sign for functional immaturity of the SC, as proposed by Sridharan et al. (Sridharan et al., 2007a). Thus, impaired GC development as early as day 8 p.p. might represent underlying abnormalities in SCs, because there exists a reciprocal regulation of SC and GC differentiation, and functional SCs are a prerequisite for normal spermatogenesis (Sharpe et al., 2003; Griswold, 1995). Also, seminiferous tubules of adult humans with different forms of spermatogenic impairment house immature SC populations (Steger et al., 1996). Thus, a disturbed maturation of nursing SCs might consequently result in an asynchronous and altered differentiation of SC-dependent GCs, leading to alterations of candidate gene expression in GCs of SCCx43KO mice (and infertile men).
Multiple genes that are essential for BTB formation, such as Ocln, Tjp1 or Cld11, were subjected to qRT-PCR analysis (Saitou et al., 2000). In agreement with the microarray data, examination of their mRNA expression on day 8 p.p. revealed no significant differences between WT and SCCx43KO mice, although most genes associated with this barrier showed a higher transcription rate in KO mice. Using IHC, localization of OCLN protein seemed to be similar in WT and SCCx43KO mice, implying no functional alterations.
Direct evidence for a possible relationship between CX43, junction dynamics and spermatogenesis derives from Carette et al. (Carette et al., 2010). By using SCCx43KO mice, specific anti-Cx43 siRNA and gap junction blockers in the SerW3 SC line, this study demonstrated that CX43-based gap junctions participate in the regulation of adherens and tight junction protein expression. Interestingly, increased protein levels of CDHN, β-CTNNB1 and OCLN, but decreased TJP1 levels, were observed in adult KO mice compared with their WT littermates (Carette et al., 2010). Because no significant alterations in Tjp1 and Ocln gene expression profiles have been detected in the present study at day 8 p.p., the observed alterations in TJP1 and OCLN levels in adult KO mice seem to occur after postnatal day 8, together with a functional BTB formation but possibly associated with (1) an impairment in the dynamic process of opening and closing of this barrier and/or (2) permanent BTB closure as suggested by the authors. The close relationship between gap and adherens junctions has been demonstrated in studies investigating CDHE, and it was shown that CDHE (Cdh1; FC −2.8 in the present study) was able to influence the intracellular trafficking and function of both CX26 and CX43 (Hernandez-Blazquez et al., 2001). In the human testis, an altered expression of TJP1 and TJP2, and a dysfunction of claudin-11, have been demonstrated in SCs associated with CIS (Fink et al., 2006; Fink et al., 2009).
Because it has been further demonstrated that CX43 immunolocalization shifts from the luminal to the basal region of the seminiferous epithelium concomitant with BTB formation (Bravo-Moreno et al., 2001), it may be speculated that CX43 has a functional role in the compartmental reorganization of the seminiferous epithelium during puberty. Thus, it is possible that loss of CX43 in SCs results in an altered organization of the seminiferous epithelium leading to (1) morphological, structural and/or metabolic disorganization, (2) altered synchronization of SC-GC metabolism and (3) altered maturation during the first waves of spermatogenesis. This again could result in impaired spermatogenesis in adult SCCx43KO mice.
In contrast to adult SCCx43KO mice, in which the number of SCs per tubule cross-section is significantly higher in comparison to WT mice (Brehm et al., 2007; Sridharan et al., 2007a), no significant difference concerning the SC number between 8-day-old KO and WT mice can be found. In rodents, SC proliferation takes place during the fetal period until 3 weeks p.p., with a maximum at around 2 days before birth (Orth, 1982). With puberty the mitotic activity stops, SCs mature and the final number of SCs is established. Owing to the Cre mice used in this study (Amh-Cre; Cre-expression under the control of the Amh promoter), possible alterations do mainly occur in our SCCx43KO mice during the time period with the highest mitotic activity and proliferation of SCs. This fact supports the assumption of a remaining proliferation potential (capacity) of SCs in adult SCCx43KO mice, as suggested by Sridharan et al. (Sridharan et al., 2007b).
By contrast, the GC number was significantly decreased as early as day 8 p.p. in SCCx43KO mice, similar to results seen in adult KO mice. These results are supported by other published data obtained by grafting the testes of Cx43-deficient mice under the kidney capsule of WT mice, showing that the GC population fails to expand postnatally in the absence of CX43 (Roscoe et al., 2001). In the last decade, different Cx43 KO mice have been generated and the importance of Cx43 to male gametogenesis has been shown: e.g. there is a 50% depletion of primordial GCs in fetal male mice lacking the Cx43 gene (Juneja et al., 1999) and a significant reduction of GCs in adult SCCx43KO mice (Brehm et al., 2007; Sridharan et al., 2007a) (and in the present study). It was further demonstrated that primordial GCs are already gap-junction intercellular-communication-competent cells and that GC deficiency in Cx43 KO embryos might arise from an increased apoptosis by abnormal p53 activation (Francis and Lo, 2006). A recent in vitro study confirms the involvement of CX43 gap junctions in the regulation of GC number by controlling spermatogonia survival rather than proliferation (Gilleron et al., 2009). To answer the question of whether the number of GCs is already altered before postnatal day 8 and whether the migration of GCs is altered, new breedings to obtain younger KO mice have been performed. Preliminary results comparing KO and WT mice at day 2 p.p. showed that already at this very early postnatal age the number of GCs per tubule cross-section is drastically reduced in KO mice compared with WT. To investigate even earlier (fetal) developmental ages, for the investigation of primordial GC migration, a study design similar to that of Francis and Lo (Francis and Lo, 2006) and new breeding strategies are planned. These data imply an important role for Cx43 in SCs on maintaining the number of GCs by regulating CX43 gap junctions. Whether CX43 from SCs is involved in GC growth by controlling primordial GC and/or spermatogonia survival rather than their proliferation, as shown by Gilleron et al. (Gilleron et al., 2009), remains to be elucidated for SCCx43KO mutants in future studies. In this context, it was shown that a low spermatogenic efficiency in infertile men showing an arrest of spermatogenesis at the level of spermatogonia was not only due to postmeiotic events, but also to a decrease in the mitotic activity of spermatogonia (Steger et al., 1998). And, finally, it was demonstrated that many infertile individuals face, besides meiotic defects, the problem of reduced numbers of spermatogonia and spermatogonial stem cells (Hentrich et al., 2011).
Our data reveal that (1) there are considerable differences in the gene expression patterns of SCCx43KO and WT mice at day 8 p.p. (and these differences have a negative impact on protein synthesis in young and adult KO mice), (2) SC-specific deletion of Cx43 seems to effect primarily GCs and (3) expression of important GC-specific genes seems to be Cx43 associated and/or regulated. Because several of the altered genes have been shown to be essential for normal (mitotic and meiotic) progression of GC development and initiation of spermatogenesis, it was additionally demonstrated that corresponding proteins are altered in adult mutants. Thus, these genes and their proteins could be involved in the developmental and histological disorder observed in adult SCCx43KO mice (and in similar human spermatogenic defects).
In conclusion, observed changes in gene expression in the mutant mice suggest a role for SC-GC crosstalk via CX43 gap junctions. However, in addition to its classical role in direct intercellular communication, observed alterations in KO gene expression might also be due to (1) a loss of CX43 hemichannel function in SCs and consequently within the seminiferous epithelium, (2) a loss of direct or indirect interaction with other junctional proteins and/or (3) a loss of interaction or binding of CX43 in SCs to corresponding cytoskeleton partners. Thus, loss of CX43 hemichannels in SCs, which might act as a paracrine conduit to spread factors that modulate the fate of SCs themselves, and GCs can lead to their maturational arrest.
Changes in candidate gene expression patterns could have also been caused by (4) a loss of direct Cx43 effects on gene transcription, because it was demonstrated in a study working with fetal Cx43-null mutants that the loss of the Cx43 gene is able to exert profound effects on the expression pattern of other testicular CX genes. In fetal testes of Cx43 KO mice, only four CX mRNAs were detectable (Cx26, Cx37, Cx40 and Cx45), compared with normal fetal WT testes exhibiting eight CX members (Juneja, 2003). In the present study, a ‘close to significant’ downregulation of Gja6 (coding for CX33) was detected, confirming its close association with CX43 (Fiorini et al., 2004). It is finally also possible that (5) a disturbed maturation of nursing SCs might consequently result in alterations in: differentiation of SC-dependent GCs, candidate gene expression in GCs of SCCx43KO mice and the observed GC numbers.
A time-course study comparing SCCx43KO and WT mice from day 6, 10, 12 and 14 p.p. would be useful in confirming the role of these candidate genes and could lead to the identification of altered pathways before and during the first waves of spermatogenesis. Moreover, current studies are comparing histological phenotypes of impaired spermatogenesis and significantly altered candidate genes in SCCx43KO mice with corresponding deficiencies in men by using human testicular biopsies. In addition, these biopsies will be investigated for possible mutations in the Cx43 gene in SCs or GCs using UV-laser-assisted cell picking (microdissection approach).
It is for all these reasons that the established transgenic SCCx43KO mouse model provides an interesting tool to study and understand the causes of impaired spermatogenesis because it is directly relevant to different corresponding cases of human subfertility and sterility.
METHODS
Mice
Breeding strategy, genotyping and confirmation of Cx43 gene loss by β-galactosidase IHC of WT and homozygous SCCx43KO mice were carried out as described previously (Brehm et al., 2007). For all experiments, 8-day-old mice were used (n=3 per genotype), except for IF where 15-day-old mice were utilized (n=3 per genotype), because DMRT7 protein is known to be first detectable from day 13.5 onwards. Additionally, for STRA8 and DAZL IHC and for DMRT7 IF, adult WT and SCCx43KO mice (n=3 per genotype) were investigated. Three KO mice and two WT mice were littermates, one WT mouse was from another litter but from the same parents. Genotyping showed that the used KO animals were all ‘homozygously floxed Cx43 and Cre positive’ mice, whereas the WT mice (littermates) were ‘Cx43 homoflox but Cre negative’. Within the WT group (microarray), two subclusters were present, i.e. WT1 versus WT2/3. This small difference in the expression profile can be explained because WT2 and 3 were from the same litter whereas WT1 was from another litter but from the same mouse (parents). Animal experiments were approved by the animal rights committee at the regional commission of Giessen, Germany (decision V54-19c 20/15 c GI 18/1) and the ethics commission at the University of Giessen, Germany (decision 56/05).
Tissue retrieval and treatment
Mice were anesthetized and sacrificed by intraperitoneal injection of an overdose cocktail of ketamine hydrochloride (Medistar, Holzwickede, Germany) and xylazine (Serumwerk Bernburg, Bernburg, Germany). The body and testis weights were defined. Both testes were removed immediately. One testis was snap-frozen in liquid nitrogen and then stored at −80°C until RNA extraction, the other was placed in Bouin’s solution overnight for IHC and hematoxylin and eosin staining (H&E), followed by paraffin embedding. For IF the second testis was placed in formalin for 2 hours before paraffin embedding.
Histochemical techniques
For histological evaluation and IHC, 5-μm slices from all KO and WT mice were sectioned. Sections 1, 10, 20, 30, 40 and 50 were stained with H&E according to standard techniques; sections 2, 11, 21, 31, 41 and 51 were used for β-galactosidase IHC. With the latter sections and method, the mean number of SCs (nuclei) per tubule cross-section was determined, avoiding in this way that single spermatogonia mimic and displace SCs, resulting in reproducible SC numbers per tubule cross-section. Then, the number of SCs and GCs per tubule cross-section was counted for at least 25 tubules per section and the average number was calculated to compare the real cell composition of WT and SCCx43KO mice. This quantification method was published previously (Brehm et al., 2007; Weider et al., 2011b). Quantitative microscopic analysis was performed using a Leica DM LB microscope (Leica, Wetzlar, Germany) at a magnification of 400×. Statistical evaluation was done using BMDP Statistical Software Package (Statistical Solutions Ltd, Cork, Ireland).
On the basis of this data, a correction factor of 1/(ØGC number) was calculated for GC-specific genes and a factor of 1/(ØGC+ØSC number) for genes expressed in GCs and SCs. This implied a factor of approximately 1/3 for WT and 1/1 for SCCx43KO mice for genes exclusively expressed in GCs and a factor of ca. 1/26 for WT and 1/24 for SCCx43KO mice for genes expressed in both GCs and SCs. The obtained factor was used to correct the data of the microarray analysis by the number of GCs for genes expressed only in GCs or in both cell compartments.
RNA extraction
To carry out microarray analysis, one testis of three mice of each genotype was used. Total RNA was prepared using RNeasy Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Microarray analysis
Gene expression profiling
RNA was subjected to transcription and hybridization as follows: target preparation was done using the MessageAmp II Kit (Ambion, Applied Biosystems) following the original protocol. Briefly: 1 μg total RNA was used in cDNA synthesis reaction with a poly-A binding primer containing the T7-polymerase promoter. Resulting cDNA was transcribed into cRNA in one round amplification in the presence of 11-Bio-UTP. Double-stranded cDNA and biotin-labeled cRNA were purified using the mini columns included in the kit. The eluted cRNA was quantified with the NanoDrop (NanoDrop Technologies, Rockland, DE) and a quality profile with the Agilent 2100 bioanalyzer (Agilent Technologies) was made. Portions of 20 μg cRNA were subjected to fragmentation in the presence of Mg2+. Subsequently, 10 μg fragmented cRNA (target) was loaded onto CodeLink Mouse Whole Genome Microarray glass slides containing 36.227 probe sets (Applied Microarrays, Tempe, AZ) and hybridized for 18 hours in a Minitron shaker incubator (Infors AG, Bottmingen, Germany) at 37°C/300 rpm (n=2 arrays per sample). Washing and dyeing with Cy-5-coupled streptavidin was done according to the original protocol for CodeLink arrays (Applied Microarrays, Tempe, AZ) and the arrays were scanned using a GenePix 4000 B scanner and GenePix Pro 4.0 Software (Axon Instruments, Arlington, TX).
Spot signals of CodeLink bioarrays were quantified using CodeLink System Software consisting of Batch Submission (V2.2.27) and Expression Analysis (V2.2.25) (GE Healthcare) as outlined in the user’s manual. CodeLink Expression Software 1.21 generated background corrected raw as well as median centered intra-slide normalized data. The intra-slide normalized data were used for further analysis. The software automatically calculated thresholds for intra-slide normalized intensities for each array and flagged genes as TRUE when the gene intensity was higher than the threshold or FALSE when the intensity was lower than the threshold. The present call of a microarray was given as the ratio of genes flagged as TRUE/total number of genes on microarray. Microarrays subjected to data analysis showed a mean present call of 83%, indicating a high number of genes above threshold, i.e. being flagged as TRUE. Furthermore, the software flagged each gene value as GOOD, EMPTY, POOR, NEG or MSR, defining different quality measures as outlined in the user’s manual. Only gene values flagged as GOOD or EMPTY were used in the following analysis workflow:
Definition of groups: Group 1: WT mice [testes of three WT mice at day 8 p.p. (three biological replicates), each on two microarrays (two technical replicates)]. Group 2: SCCx43KO mice [testes of three KO mice at day 8 p.p. (three biological replicates), each on two microarrays (two technical replicates)]. A total of 12 microarrays (six per group) were subjected to analyses.
Removal of genes with a high number of missing values or of values being flagged as FALSE: genes with missing values ≥50% of all arrays in a group were excluded from the dataset. Genes that were flagged as FALSE in >50% of arrays in each group were also excluded from the dataset. A total of 26,044 probe sets remained after quality control.
Gene expression values deviating more than three times from the group median were classified as outliers and have been removed.
Imputation of remaining missing values: remaining missing values were imputed using sequential K-nearest neighbor (SKNN) imputation (Kim et al., 2004) with k=5.
Normalization of imputed dataset: imputed dataset was normalized using quantile normalization in R (Bolstad et al., 2003) and logged to base 2.
Array outlier detection: dissimilarity matrices of the normalized dataset were generated in AVADIS-Pride (Gwadry et al., 2005) to determine outlier arrays within the dataset. No outlier arrays were identified in the dataset.
Correction for differing GC numbers: to exclude that the differentially gene expression was due to the differing numbers of GCs in the two genotypes, microarray data were normalized with the correcting factor obtained by the statistical evaluation of the morphometrical data. There was nearly no difference in the FC of gene expression either with or without adjustment for cell numbers.
Statistical analysis of microarrays: for each gene, the mean value of all technical replicates of a mouse and the FC thereof was calculated in MAYDAY (Battke et al., 2010). To identify differentially regulated genes between WT and SCCx43KO mice, the dataset was subjected to a two-class rank statistics [Rank products (RP)] as described below (Breitling et al., 2004; Breitling and Herzyk, 2005). Additionally, the Student’s t-test was applied. For each gene, a significance level of P-value<0.05 was determined for both tests combined with an FC of at least twofold. A differentially regulated gene was considered to be significant when the P-value in the t-test was less than 0.05, the P-value in RP was less than 0.05 and the FC was equal to/higher than 2 or equal to/less than 2.
Annotation of genes: significantly regulated genes were annotated using the web-based annotation tools SOURCE (Diehn et al., 2003) and the Database for Annotation, Visualization and Integrated Discovery (DAVID Bioinformatics Resources 2008) (Dennis et al., 2003) as described in the manual.
Hierarchical cluster analysis: hierarchical cluster analysis of the significantly genes was performed in dCHIP using Euclidean distance and centroid linkage.
Enriched functional categories: enriched functional categories within the differentially regulated genes were determined using DAVID (Dennis et al., 2003) version 2.0. Based on statistical methods, significance levels were calculated that describe the overrepresentation of functional categories by a list of genes – the differentially regulated genes in our case. If a GO category or biological process is significantly overrepresented, which means that a relatively high number of genes assigned to the respective function is present in the selection, this will result in a low P-value. In functional annotation clustering, GO categories are grouped according to the genes from the list of regulated genes that have been assigned to them. An enrichment score is calculated for each cluster, based on the proportion of significantly overrepresented biological processes. Clusters with an enrichment score above 1.2 have been taken into account. Those clusters represented at least one significantly enriched biological process (i.e. with a P-value of less than 0.05).
Pathway analysis: a relationship between genes and their connection to existing regulatory pathways was examined using Ingenuity Pathway Analysis (IPA), a web-based entry tool developed by Ingenuity Systems, Inc. (http://www.ingenuity.com).
Complete data are available at the Gene Expression Omnibus (GEO) database under the accession number GSE23431. This study adhered to the MIAME standards (Brazma et al., 2001).
cDNA synthesis and qRT-PCR
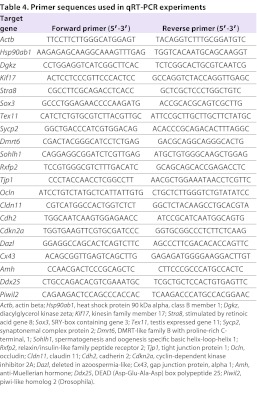
Changes in mRNA levels of altered genes identified by the CodeLink Mouse Whole Genome Bioarray were confirmed using qRT-PCR. Three biological replicates per genotype were processed. DNAse treatment was performed according to manufacturer’s instructions using the RNase-Free DNase Set (Qiagen, Hilden, Germany). cDNA was synthesized from 15 μl total RNA (adjusted to 200 nmol/30 μl) using MultiScribe Reverse Transcriptase (Applied Biosystems Inc., Darmstadt, Germany). Purification of cDNA was realized by QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). Specific primer pairs were created using Beacon Designer Software (Bio-Rad, München, Germany). Before use in the main experiment, primers were first tested in normal PCR to confirm that only one specific band could be produced. Afterwards, a qRT-PCR with a dilution series was performed to detect the efficiency of the qRT-PCR reaction and to prove that only one product can be detected within the melting curve. A representative number of PCR products were sequenced additionally. Only primers passing the multiple testing without interceptions finally were used. qRT-PCR was performed using IQ SYBR Green Supermix and CFX96 Real-Time System (Bio-Rad, München, Germany). Per sample, 1 μl cDNA was used for amplification. Specific forward and reverse primers (Table 4) were added to the Mastermix. Cycling conditions were 95°C for 3 minutes, followed by 40 cycles of 95°C for 10 seconds and 60°C for 1 minute. After 95°C for 10 seconds melt curve was produced with 65°C to 95°C and an increment of 0.5°C every 5 seconds. Actb and Hsp90ab1 were used as housekeeping genes. All experiments included negative controls lacking cDNA and were carried out as triplicates. Based on the ΔΔC(t) method, evaluation of the qRT-PCR was realized using the CFX Manager Software (Bio-Rad, Germany).
Table 4.
Primer sequences used in qRT-PCR experiments
TRANSLATIONAL IMPACT.
Clinical issue
Although a significant decline in human male reproductive function has been reported for the past 20 years, the molecular mechanisms responsible remain poorly understood. Recent studies indicate that the gap junction protein connexin-43 (CX43) – the predominant testicular connexin in most species, including humans – might be involved. For example, many individuals with impaired spermatogenesis exhibit a reduction or loss of CX43 expression in germ cells and Sertoli cells (SCs; part of the seminiferous tubule). It has been shown that adult male transgenic mice with a conditional knockout of the Gja1 gene (also referred to as Cx43; encoding CX43) in SCs (SCCx43KO) show a testicular phenotype similar to such individuals and are infertile.
Results
Here, the authors sought to identify possible signaling pathways and molecular mechanisms underlying the testicular phenotype and failure to initiate spermatogenesis in SCCx43KO mice. They used microarray analysis to compare testicular gene expression of 8-day-old SCCx43KO and wild-type (WT) mice. This analysis revealed that 658 genes were significantly regulated in testes of SCCx43KO mice. Of these genes, 135 were upregulated and 523 genes were downregulated. Most downregulated genes, including Stra8, Dazl and members of the DM (dsx and map-3) gene family, are germ-cell-specific and essential for mitotic and meiotic progression of spermatogenesis. Other genes altered are associated with transcription, metabolism, cell migration and cytoskeletal organization. These data show that deletion of Cx43 in SCs leads to changes in the expression of multiple genes in prepubertal mice, and affects primarily germ cells.
Implications and future directions
These data indicate that, among the different transgenic mouse models that are used to elucidate the multiple mechanisms underlying human male infertility, SCCx43KO mice provide a unique model for identifying candidate genes in germ cells. The candidate genes identified in this study could represent useful markers in investigations of human testicular biopsies from patients with spermatogenic deficiencies, and for studying the molecular mechanisms of human male infertility.
IHC and IF
In order to confirm whether the alterations at RNA level are reflected in protein synthesis, IHC and IF was applied. Slides were dewaxed, rehydrated and microwaved in sodium citrate buffer, pH 6.0. Tissue sections were treated with 3% H2O2, blocked with 5% bovine serum albumin to prevent nonspecific binding, followed by incubation with primary antibody at 4°C overnight. Primary antibodies were: (1) rabbit anti-STRA8 (stimulated by retinoic acid gene 8; 1:250; kindly provided by Michael Griswold, Pullman, Washington, DC), (2) rabbit anti-CX43 (1:100; New England Biolabs GmbH, Frankfurt, Germany), (3) rabbit anti-OCLN (1:500; Invitrogen, Karlsruhe, Germany) and (4) mouse anti-DAZL antiserum (deleted in azoospermia-like Dazla; 1:250; kindly provided by Howard Cooke, Edinburgh, UK). The slides were covered with a corresponding (compatible) biotinylated secondary antibody (goat anti-rabbit, goat anti-mouse or rabbit anti-goat, from DAKO, Hamburg, Germany) at room temperature (RT) for 1 hour, followed by the application of peroxidase-conjugated streptavidine (VECTASTAIN Elite ABC Standard Kit; Peroxidase, Biologo, Kronshagen, Germany) for 1 hour at RT. Peroxidase activity was visualized by the Peroxidase Substrat Kit AEC (Biologo, Kronshagen, Germany). For IF, formalin-fixed testes were used. After removal of the paraffin wax, rehydration and cooking in sodium citrate buffer with pH 6.0, slices were blocked with 5% goat serum for 1 hour and incubated overnight with the primary rabbit anti-DMRT7 antibody (1:200; kindly provided by David Zarkower, Minneapolis, MN). Exposure of the slices to a biotinylated secondary antibody (goat anti-rabbit; DAKO, Hamburg, Germany) at RT for 1 hour was followed by a 1 hour incubation with NeutrAvidin, DyLight 488 Conjugated (Fisher Scientific GmbH, Schwerte, Germany) and covering with Aqua Polymouth (Polysciences, Inc., Warrington, PA). Negative controls for both IHC and IF were performed by omitting the primary antibody.
Supplementary Material
Acknowledgments
We thank the German Research Foundation (DFG; BR3365/2-1 and KFO181) for their financial support, Juri Schklarenko for assistance in the microarray experiments, Prof. Klaus Willecke for the Cx43 floxed lacZ mice, Prof. Griswold for kindly providing the STRA8 antibody, Prof. Zarkower for kindly providing the DMRT7 antibody, and Prof. Cooke for kindly providing the DAZL antibody.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
S.G., H.H., T.C., M.B. and R.B. were involved in conception and design of the experiments and co-wrote the manuscript; S.G. and H.H. analyzed the data; S.G. performed most of the experiments, e.g. breeding of SCCx43KO mice and corresponding littermates, H&E stainings, RNA extraction, cDNA synthesis, qRT-PCR and IHC or IF; S.T. performed the microarray analysis; M.M. and K.F. performed statistical analysis and determined the used correction factors; F.G. provided the AMH-Cre mice for the breeding experiments; K.W. prepared figures, tables and supplemental data for publication; all authors read and commented on the manuscript.
FUNDING
This work was supported by the German Research Foundation (DFG; BR 3365/2-1 and KFO 181).
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.008649/-/DC1
REFERENCES
- Anderson E. L., Baltus A. E., Roepers-Gajadien H. L., Hassold T. J., de Rooij D. G., van Pelt A. M., Page D. C. (2008). Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA. 105, 14976–14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A. M., Jørgensen N., Main K. M., Toppari J., Rajpert-De Meyts E., Leffers H., Juul A., Jensen T. K., Skakkebaek N. E. (2008). Adverse trends in male reproductive health: we may have reached a crucial ‘tipping point’. Int. J. Androl. 31, 74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asklund C., Jørgensen N., Kold Jensen T., Skakkebaek N. E. (2004). Biology and epidemiology of testicular dysgenesis syndrome. BJU Int. 3, 6–11 [DOI] [PubMed] [Google Scholar]
- Ballow D., Meistrich M. L., Matzuk M., Rajkovic A. (2006). Sohlh1 is essential for spermatogonial differentiation. Dev. Biol. 294, 161–167 [DOI] [PubMed] [Google Scholar]
- Batias C., Siffroi J. P., Fenichel P., Pointis G., Segretain D. (2000). Connexin43 gene expression and regulation in the rodent seminiferous epithelium. J. Histochem. Cytochem. 48, 793–805 [DOI] [PubMed] [Google Scholar]
- Battke F., Symons S., Nieselt K. (2010). Mayday – integrative analytics for expression data. BMC Bioinformatics 11, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellvé A. R., Cavicchia J. C., Millette C. F., O’Brien D. A., Bhatnagar Y. M., Dym M. (1977). Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 74, 68–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M., Kliesch S. (1994). The distribution pattern of cytokeratin and vimentin immunoreactivity in testicular biopsies of infertile men. Anat. Embryol. 190, 515–520 [DOI] [PubMed] [Google Scholar]
- Beyret E., Lin H. (2011). Pinpointing the expression of piRNAs and function of the PIWI protein subfamily during spermatogenesis in the mouse. Dev. Biol. 355, 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigliardi E., Vegni-Talluri M. (1977). Gap junctions between Sertoli cells in the infertile human testis. Fertil. Steril. 28, 755–758 [DOI] [PubMed] [Google Scholar]
- Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 [DOI] [PubMed] [Google Scholar]
- Bravo-Moreno J. F., Diaz-Sanchez V., Montoya-Flores J. G., Lamoyi E., Saez J. C., Perez-Armendariz E. M. (2001). Expression of connexin43 in mouse Leydig, Sertoli, and germinal cells at different stages of postnatal development. Anat. Rec. 264, 13–24 [DOI] [PubMed] [Google Scholar]
- Brazma A., Hingamp P., Quackenbush J., Sherlock G., Spellman P., Stoeckert C., Aach J., Ansorge W., Ball C. A., Causton H. C., et al. (2001). Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat. Genet. 29, 365–371 [DOI] [PubMed] [Google Scholar]
- Brehm R., Marks A., Rey R., Kliesch S., Bergmann M., Steger K. (2002). Altered expression of connexins 26 and 43 in Sertoli cells in seminiferous tubules infiltrated with carcinoma-in-situ or seminoma. J. Pathol. 197, 647–653 [DOI] [PubMed] [Google Scholar]
- Brehm R., Rüttinger C., Fischer P., Gashaw I., Winterhager E., Kliesch S., Bohle R. M., Steger K., Bergmann M. (2006). Transition from preinvasive carcinoma in situ to seminoma is accompanied by a reduction of connexin 43 expression in Sertoli cells and germ cells. Neoplasia 8, 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm R., Zeiler M., Ruttinger C., Herde K., Kibschull M., Winterhager E., Willecke K., Guillou F., Lecureuil C., Steger K., et al. (2007). A Sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am. J. Pathol. 171, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling R., Herzyk P. (2005). Rank-based methods as a non-parametric alternative of the T-statistic for the analysis of biological microarray data. J. Bioinform. Comput. Biol. 3, 1171–1189 [DOI] [PubMed] [Google Scholar]
- Breitling R., Armengaud P., Amtmann A., Herzyk P. (2004). Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS. Lett. 573, 83–92 [DOI] [PubMed] [Google Scholar]
- Bruzzone R., White T. W., Paul D. L. (1996). Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 238, 1–27 [DOI] [PubMed] [Google Scholar]
- Carette D., Weider K., Gilleron J., Giese S., Dompierre J., Bergmann M., Brehm R., Denizot J. P., Segretain D., Pointis G. (2010). Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev. Biol. 346, 54–67 [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Mruk D. D. (2002). Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Wong E. W., Lie P. P., Mruk D. D., Xiao X., Li M. W., Lui W. Y., Lee W. M. (2011). Polarity proteins and actin regulatory proteins are unlikely partners that regulate cell adhesion in the seminiferous epithelium during spermatogenesis. Histol. Histopathol. 26, 1465–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Buffone M. G., Kouadio M., Goodheart M., Page D. C., Gerton G. L., Davidson I., Wang P. J. (2007). Abnormal sperm in mice lacking the Taf7l gene. Mol. Cell. Biol. 27, 2582–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs A. J., Cowan G., Kinnell H. L., Anderson R. A., Saunders P. T. (2011). Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE 6, e20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. S., Choi C., Wang X., Hallock L., Wolgemuth D. J. (2010). Aberrant distribution of junctional complex components in retinoic acid receptor alpha-deficient mice. Microsc. Res. Tech. 73, 583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Schonbaum C., Degenstein L., Bai W., Mahowald A., Fuchs E. (1998). The ovo gene required for cuticle formation and oogenesis in flies is involved in hair formation and spermatogenesis in mice. Genes Dev. 12, 3452–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrouy X., Gasc J. M., Pointis G., Segretain D. (2004). Functional characterization of Cx43 based gap junctions during spermatogenesis. J. Cell. Physiol. 200, 146–154 [DOI] [PubMed] [Google Scholar]
- Defamie N., Berthaut I., Mograbi B., Chevallier D., Dadoune J. P., Fénichel P., Segretain D., Pointis G. (2003). Impaired gap junction connexin43 in Sertoli cells of patients with secretory azoospermia: a marker of undifferentiated Sertoli cells. Lab. Invest. 83, 449–456 [DOI] [PubMed] [Google Scholar]
- Dennis G., Jr, Sherman B. T., Hosack D. A., Yang J., Gao W., Lane H. C., Lempicki R. A. (2003). DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 4, P3. [PubMed] [Google Scholar]
- Derangeon M., Spray D. C., Bourmeyster N., Sarrouilhe D., Hervé J. C. (2009). Reciprocal influence of connexins and apical junction proteins on their expressions and functions. Biochim. Biophys. Acta 1788, 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn M., Sherlock G., Binkley G., Jin H., Matese J. C., Hernandez-Boussard T., Rees C. A., Cherry J. M., Botstein D., Brown P. O., et al. (2003). SOURCE: a unified genomic resource of functional annotations, ontologies, and gene expression data. Nucleic Acids Res. 31, 219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EU Strategic Research Plan (2010). Male reproductive health: its impact in relation to general well-being and low European fertility rates. ESF Science Policy Briefing 40, 1–12 [Google Scholar]
- Fink C., Weigel R., Hembes T., Lauke-Wettwer H., Kliesch S., Bergmann M., Brehm R. H. (2006). Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood-testis barrier integrity in testicular carcinoma in situ. Neoplasia 8, 1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C., Weigel R., Fink L., Wilhelm J., Kliesch S., Zeiler M., Bergmann M., Brehm R. (2009). Claudin-11 is over-expressed and dislocated from the blood-testis barrier in Sertoli cells associated with testicular intraepithelial neoplasia in men. Histochem. Cell Biol. 131, 755–764 [DOI] [PubMed] [Google Scholar]
- Fiorini C., Tilloy-Ellul A., Chevalier S., Charuel C., Pointis G. (2004). Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod. Toxicol. 18, 413–421 [DOI] [PubMed] [Google Scholar]
- Francis R., Xu X., Park H., Wei C. J., Chang S., Chatterjee B., Lo C. (2011). Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS ONE 6, e26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R. J., Lo C. W. (2006). Primordial germ cell deficiency in the connexin 43 knockout mouse arises from apoptosis associated with abnormal p53 activation. Development 133, 3451–3460 [DOI] [PubMed] [Google Scholar]
- Franke F. E., Pauls K., Rey R., Marks A., Bergmann M., Steger K. (2004). Differentiation markers of Sertoli cells and germ cells in fetal and early postnatal human testis. Anat. Embryol. (Berl.) 209, 169–177 [DOI] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Schmid E. (1979). Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur. J. Cell Biol. 19, 269–275 [PubMed] [Google Scholar]
- Giepmans B. N., Verlaan I., Moolenaar W. H. (2001a). Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Commun. Adhes. 8, 219–223 [DOI] [PubMed] [Google Scholar]
- Giepmans B. N., Verlaan I., Hengeveld T., Janssen H., Calafat J., Falk M. M., Moolenaar W. H. (2001b). Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 11, 1364–1368 [DOI] [PubMed] [Google Scholar]
- Gilleron J., Carette D., Durand P., Pointis G., Segretain D. (2009). Connexin 43 a potential regulator of cell proliferation and apoptosis within the seminiferous epithelium. Int. J. Biochem. Cell Biol. 41, 1381–1390 [DOI] [PubMed] [Google Scholar]
- Gilleron J., Malassiné A., Carette D., Segretain D., Pointis G. (2011). Chemical connexin impairment in the developing gonad associated with offspring infertility. Curr. Med. Chem. 18, 5145–5158 [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Goliger J. A., Paul D. L. (1996). Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 65, 475–502 [DOI] [PubMed] [Google Scholar]
- Graf M., Brobeil A., Sturm K., Steger K., Wimmer M. (2011). 14-3-3 beta in the healthy and diseased male reproductive system. Hum. Reprod. 26, 59–66 [DOI] [PubMed] [Google Scholar]
- Gregory M., Kahiri C. N., Barr K. J., Smith C. E., Hermo L., Cyr D. G., Kidder G. M. (2011). Male reproductive system defects and subfertility in a mutant mouse model of oculodentodigital dysplasia. Int. J. Androl. 34, e630–e641 [DOI] [PubMed] [Google Scholar]
- Griswold M. D. (1995). Interactions between germ cells and Sertoli cells in the testis. Biol. Reprod. 52, 211–216 [DOI] [PubMed] [Google Scholar]
- Gutti R. K., Tsai-Morris C. H., Dufau M. L. (2008). Gonadotropin-regulated testicular helicase (DDX25), an essential regulator of spermatogenesis, prevents testicular germ cell apoptosis. J. Biol. Chem. 283, 17055–17064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwadry F. G., Sequeira A., Hoke G., Ffrench-Mullen J. M., Turecki G. (2005). Molecular characterization of suicide by microarray analysis. Am. J. Med. Genet. C. Semin. Med. Genet. 133C, 48–56 [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. (2008). Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentrich A., Wolter M., Szardening-Kirchner C., Lüers G. H., Bergmann M., Kliesch S., Konrad L. (2011). Reduced numbers of Sertoli, germ, and spermatogonial stem cells in impaired spermatogenesis. Mod. Pathol. 24, 1380–1389 [DOI] [PubMed] [Google Scholar]
- Hernandez-Blazquez F. J., Joazeiro P. P., Omori Y., Yamasaki H. (2001). Control of intracellular movement of connexins by E-cadherin in murine skin papilloma cells. Exp. Cell. Res. 270, 235–247 [DOI] [PubMed] [Google Scholar]
- Huang G. Y., Cooper E. S., Waldo K., Kirby M. L., Gilula N. B., Lo C. W. (1998). Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J. Cell Biol. 143, 1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Moroi S., Tsukita S. (1997). Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 138, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen N., Asklund C., Carlsen E., Skakkebaek N. E. (2006). Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int. J. Androl. 29, 54–61 [DOI] [PubMed] [Google Scholar]
- Juneja S. C. (2003). mRNA expression pattern of multiple members of connexin gene family in normal and abnormal fetal gonads in mouse. Indian J. Physiol. Pharmacol. 47, 147–156 [PubMed] [Google Scholar]
- Juneja S. C., Barr K. J., Enders G. C., Kidder G. M. (1999). Defects in the germ line and gonads of mice lacking connexin43. Biol. Reprod. 60, 1263–1270 [DOI] [PubMed] [Google Scholar]
- Kameritsch P., Pogoda K., Pohl U. (2011). Channel-independent influence of connexin 43 on cell migration. Biochim. Biophys. Acta doi: 10.1016/j.bbamem.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Kanetsky P. A., Mitra N., Vardhanabhuti S., Vaughn D. J., Li M., Ciosek S. L., Letrero R., D’Andrea K., Vaddi M., Doody D. R., et al. (2011). A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum. Mol. Genet. 20, 3109–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardami E., Dang X., Iacobas D. A., Nickel B. E., Jeyaraman M., Srisakuldee W., Makazan J., Tanguy S., Spray D. C. (2007). The role of connexins in controlling cell growth and gene expression. Prog. Biophys. Mol. Biol. 94, 245–264 [DOI] [PubMed] [Google Scholar]
- Kawamata M., Inoue H., Nishimori K. (2007). Male-specific function of Dmrt7 by sexually dimorphic translation in mouse testis. Sex. Dev. 1, 297–304 [DOI] [PubMed] [Google Scholar]
- Kerr C. L., Cheng L. (2010). The dazzle in germ cell differentiation. J. Mol. Cell. Biol. 2, 26–29 [DOI] [PubMed] [Google Scholar]
- Kim S., Kettlewell J. R., Anderson R. C., Bardwell V. J., Zarkower D. (2003). Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad. Gene. Expr. Patterns 3, 77–82 [DOI] [PubMed] [Google Scholar]
- Kim S., Namekawa S. H., Niswander L. M., Ward J. O., Lee J. T., Bardwell V. J., Zarkower D. (2007). A mammal-specific Doublesex homolog associates with male sex chromatin and is required for male meiosis. PLoS Genet. 3, e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. Y., Kim B. J., Yi G. S. (2004). Reuse of imputed data in microarray analysis increases imputation efficiency. BMC Bioinformatics 5, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliesch S., Behre H. M., Hertle L., Bergmann M. (1998). Alteration of Sertoli cell differentiation in the presence of carcinoma in situ in human testes. J. Urol. 160, 1894–1898 [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S., Kimura T., Ijiri T. W., Isobe T., Asada N., Fujita Y., Ikawa M., Iwai N., Okabe M., Deng W., et al. (2004). Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131, 839–849 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Engel W., Nayernia K. (2006a). Stem cell protein piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol. Reprod. Dev. 73, 173–179 [DOI] [PubMed] [Google Scholar]
- Lee N. P., Leung K. W., Wo J. Y., Tam P. C., Yeung W. S., Luk J. M. (2006b). Blockage of testicular connexins induced apoptosis in rat seminiferous epithelium. Apoptosis 11, 1215–1229 [DOI] [PubMed] [Google Scholar]
- Li B., Nair M., Mackay D. R., Bilanchone V., Hu M., Fallahi M., Song H., Dai Q., Cohen P. E., Dai X. (2005). Ovol1 regulates meiotic pachytene progression during spermatogenesis by repressing Id2 expression. Development 132, 1463–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. M., Chen C. W., Sun H. S., Tsai S. J., Hsu C. C., Teng Y. N., Lin J. S., Kuo P. L. (2001). Expression patterns and transcript concentrations of the autosomal DAZL gene in testes of azoospermic men. Mol. Hum. Reprod. 7, 1015–1022 [DOI] [PubMed] [Google Scholar]
- Lin Y. N., Roy A., Yan W., Burns K. H., Matzuk M. M. (2007). Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol. Cell. Biol. 27, 6794–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looijenga L. H., Hersmus R., Gillis A. J., Pfundt R., Stoop H. J., van Gurp R. J., Veltman J., Beverloo H. B., van Drunen E., van Kessel A. G., et al. (2006). Genomic and expression profiling of human spermatocytic seminomas: primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res. 66, 290–302 [DOI] [PubMed] [Google Scholar]
- Maratou K., Forster T., Costa Y., Taggart M., Speed R. M., Ireland J., Teague P., Roy D., Cooke H. J. (2004). Expression profiling of the developing testis in wild-type and Dazl knockout mice. Mol. Reprod. Dev. 67, 26–54 [DOI] [PubMed] [Google Scholar]
- Møller H., Skakkebaek N. E. (1999). Risk of testicular cancer in subfertile men: case-control study. BMJ 318, 559–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y. (2004). Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 [DOI] [PubMed] [Google Scholar]
- Murphy M. W., Zarkower D., Bardwell V. J. (2007). Vertebrate DM domain proteins bind similar DNA sequences and can heterodimerize on DNA. BMC Mol. Biol. 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T., Suzuki F. (1976). Freeze-fracture observations on the intercellular junctions of Sertoli cells and of Leydig cells in the human testis. Cell Tissue Res. 166, 37–48 [DOI] [PubMed] [Google Scholar]
- Orth J. M. (1982). Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat. Rec. 203, 485–492 [DOI] [PubMed] [Google Scholar]
- Pangas S. A., Choi Y., Ballow D. J., Zhao Y., Westphal H., Matzuk M. M., Rajkovic A. (2006). Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc. Natl. Acad. Sci. USA 103, 8090–8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R. M. (1995). The distribution of connexin 43 is associated with the germ cell differentiation and with the modulation of the Sertoli cell junctional barrier in continual (guinea pig) and seasonal breeders’ (mink) testes. J. Androl. 16, 400–409 [PubMed] [Google Scholar]
- Pérez-Armendariz E. M., Romano M. C., Luna J., Miranda C., Bennett M. V., Moreno A. P. (1994). Characterization of gap junctions between pairs of Leydig cells from mouse testis. Am. J. Physiol. 267, C570–C580 [DOI] [PubMed] [Google Scholar]
- Pointis G., Segretain D. (2005). Role of connexin-based gap junction channels in testis. Trends Endocrinol. Metab. 16, 300–306 [DOI] [PubMed] [Google Scholar]
- Pointis G., Fiorini C., Defamie N., Segretain D. (2005). Gap junctional communication in the male reproductive system. Biochim. Biophys. Acta 1719, 102–116 [DOI] [PubMed] [Google Scholar]
- Pointis G., Gilleron J., Carette D., Segretain D. (2010). Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1607–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointis G., Gilleron J., Carette D., Segretain D. (2011). Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis 1, 303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povey A. C., Stocks S. J. (2010). Epidemiology and trends in male subfertility. Hum. Fertil. (Camb.) 13, 182–188 [DOI] [PubMed] [Google Scholar]
- Raverot G., Weiss J., Park S. Y., Hurley L., Jameson J. L. (2005). Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Dev. Biol. 283, 215–225 [DOI] [PubMed] [Google Scholar]
- Raymond C. S., Murphy M. W., O’Sullivan M. G., Bardwell V. J., Zarkower D. (2001). Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 14, 2587–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume A. G., de Sousa P. A., Kulkarni S., Langille B. L., Zhu D., Davies T. C., Juneja S. C., Kidder G. M., Rossant J. (1995). Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831–1834 [DOI] [PubMed] [Google Scholar]
- Reber M., Cereghini S. (2001). Variant hepatocyte nuclear factor 1 expression in the mouse genital tract. Mech. Dev. 100, 75–78 [DOI] [PubMed] [Google Scholar]
- Risley M. S., Tan I. P., Roy C., Saez J. C. (1992). Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J. Cell Sci. 103, 81–96 [DOI] [PubMed] [Google Scholar]
- Rodriguez I., Ody C., Araki K., Garcia I., Vassalli P. (1997). An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 16, 2262–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsch H., Jezek D., Hittmair A., Mikuz G., Feichtinger H. (1996). Expression of vimentin, cytokeratin, and desmin in Sertoli cells of human fetal, cryptorchid, and tumour-adjacent testicular tissue. Virchows Arch. 427, 497–502 [DOI] [PubMed] [Google Scholar]
- Roger C., Mograbi B., Chevallier D., Michiels J. F., Tanaka H., Segretain D., Pointis G., Fenichel P. (2004). Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J. Pathol. 202, 241–246 [DOI] [PubMed] [Google Scholar]
- Roscoe W. A., Barr K. J., Mhawi A. A., Pomerantz D. K., Kidder G. M. (2001). Failure of spermatogenesis in mice lacking connexin43. Biol. Reprod. 65, 829–838 [DOI] [PubMed] [Google Scholar]
- Ruggiu M., Speed R., Taggart M., McKay S. J., Kilanowski F., Saunders P., Dorin J., Cooke H. J. (1997). The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389, 73–77 [DOI] [PubMed] [Google Scholar]
- Saitou M., Furuse M., Sasaki H., Schulzke J. D., Fromm M., Takano H., Noda T., Tsukita S. (2000). Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiro R., Kostetskii I., Olds-Clarke P., Gerton G. L., Radice G. L., Strauss I. J. (2002). Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol. Cell. Biol. 22, 6298–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders P. T., Turner J. M., Ruggiu M., Taggart M., Burgoyne P. S., Elliott D., Cooke H. J. (2003). Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction 126, 589–597 [DOI] [PubMed] [Google Scholar]
- Schleiermacher E. (1980). Ultrastructural changes of the intercellular relationship in impaired human spermatogenesis. Hum. Genet. 54, 391–404 [DOI] [PubMed] [Google Scholar]
- Sharpe R. M. (2010). Environmental/lifestyle effects on spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1697–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M., McKinnell C., Kivlin C., Fisher J. S. (2003). Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125, 769–784 [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E., Rajpert-De Meyts E., Main K. M. (2001). Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 [DOI] [PubMed] [Google Scholar]
- Sohl G., Willecke K. (2004). Gap junctions and the connexin protein family. Cardiovasc. Res. 62, 228–232 [DOI] [PubMed] [Google Scholar]
- Spray D. C., Ye Z. C., Ransom B. R. (2006). Functional connexin “hemichannels”: a critical appraisal. Glia 54, 758–773 [DOI] [PubMed] [Google Scholar]
- Sridharan S., Simon L., Meling D. D., Cyr D. G., Gutstein D. E., Fishman G. I., Guillou F., Cooke P. S. (2007a). Proliferation of adult Sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol. Reprod. 76, 804–812 [DOI] [PubMed] [Google Scholar]
- Sridharan S., Brehm R., Bergmann M., Cooke P. S. (2007b). Role of connexin 43 in Sertoli cells of testis. Ann. N. Y. Acad. Sci. 1120, 131–143 [DOI] [PubMed] [Google Scholar]
- Steger K., Rey R., Kliesch S., Louis F., Schleicher G., Bergmann M. (1996). Immunohistochemical detection of immature Sertoli cell markers in testicular tissue of infertile adult men: a preliminary study. Int. J. Androl. 19, 122–128 [DOI] [PubMed] [Google Scholar]
- Steger K., Aleithe I., Behre H., Bergmann M. (1998). The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: an immunohistochemical study using monoclonal antibodies against Ki–67 protein and proliferating cell nuclear antigen. Mol. Hum. Reprod. 4, 227–233 [DOI] [PubMed] [Google Scholar]
- Steger K., Tetens F., Bergmann M. (1999). Expression of connexin 43 in human testis. Histochem. Cell Biol. 112, 215–220 [DOI] [PubMed] [Google Scholar]
- Steiner M., Weipoltshammer K., Viehberger G., Meixner E. M., Lunglmayr G., Schöfer C. (2011). Immunohistochemical expression analysis of Cx43, Cx26, c-KIT and PlAP in contralateral testis biopsies of patients with non-seminomatous testicular germ cell tumor. Histochem. Cell Biol. 135, 73–81 [DOI] [PubMed] [Google Scholar]
- Stout C., Goodenough D. A., Paul D. L. (2004). Connexins: functions without junctions. Curr. Opin. Cell Biol. 16, 507–512 [DOI] [PubMed] [Google Scholar]
- Tanmahasamut P., Sidell N. (2005). Up-regulation of gap junctional intercellular communication and connexin43 expression by retinoic acid in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 90, 4151–4156 [DOI] [PubMed] [Google Scholar]
- Tsai-Morris C. H., Sheng Y., Lee E., Lei K. J., Dufau M. L. (2004). Gonadotropin-regulated testicular RNA helicase (GRTH Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc. Natl. Acad. Sci. USA 101, 6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine A. L., Bertram J. S. (2005). Upregulation of connexin 43 by retinoids but not by non-provitamin A carotenoids requires RARs. Nutr. Cancer 52, 105–113 [DOI] [PubMed] [Google Scholar]
- Wang P. J., McCarreay J. R., Yang F., Page D. C. (2001). An abundance of X-linked genes expressed in spermatogonia. Nat. Genet. 27, 422–426 [DOI] [PubMed] [Google Scholar]
- Weider K., Bergmann M., Brehm R. (2011a). Connexin 43, its regulatory role in testicular junction dynamics and spermatogenesis. Histol. Histopathol. 26, 1343–1352 [DOI] [PubMed] [Google Scholar]
- Weider K., Bergmann M., Giese S., Guillou F., Failing K., Brehm R. (2011b). Altered differentiation and clustering of Sertoli cells in transgenic mice showing a Sertoli cell specific knockout of the connexin 43 gene. Differentiation 82, 38–49 [DOI] [PubMed] [Google Scholar]
- WHO (1987). Towards more objectivity in diagnosis and management of male fertility. Int. J. Androl. 7, 1–53 [Google Scholar]
- Yang F., De La Fuente R., Leu N. A., Baumann C., McLaughlin K. J., Wang P. J. (2006). Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J. Cell Biol. 173, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Gell K., van der Heijden G. W., Eckardt S., Leu A. N., Page D. C., Benavente R., Her C., Höög C., McLaughlin K. J., et al. (2008). Meiotic failure in male mice lacking an X-linked factor. Genes Dev. 22, 682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Nie R., Li Y., Friel P., Mitchellm D., Hess R. A., Small C., Griswold M. D. (2008). Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol. Reprod. 79, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.