SUMMARY
Tp53 mutations are common in human prostate cancer (CaP), occurring with a frequency of ∼30% and ∼70% in localized and metastatic disease, respectively. In vitro studies have determined several common mutations of Tp53 that have specific gain-of-function properties in addition to loss of function, including the ability to promote castration-resistant (CR) growth of CaP cells in some contexts. To date, a lack of suitable mouse models has prohibited investigation of the role played by Tp53 mutations in mediating CaP progression in vivo. Here, we describe the effects of conditional expression of a mutant Tp53 (Tp53R270H; equivalent to the human hotspot mutant R273H) in the prostate epithelium of mice. Heterozygous “Tp53LSL-R270H/+” [129S4(Trp53tm3Tyj)] and “Nkx3.1-Cre” [129S(Nkx3-1tm3(cre)Mms)] mice with prostate-specific expression of the Tp53R270H mutation (p53R270H/+ Nkx3.1-Cre mice) were bred onto an FVB/N background via speed congenesis to produce strain FVB.129S4(Trp53tm3Tyj/wt); FVB.129S(Nkx3-1tm3(cre)Mms/wt) and littermate genotype negative control mice. These mutant mice had significantly increased incidences of prostatic intraepithelial neoplasia (PIN) lesions, and these appeared earlier, compared with the Nkx3.1 haploinsufficient (Nkx3.1-Cre het) littermate mice, which did not express the Tp53 mutation. PIN lesions in these mice showed consistent progression and some developed into invasive adenocarcinoma with a high grade, sarcomatoid or epithelial-mesenchymal transition (EMT) phenotype. PIN lesions were similar to those seen in PTEN conditional knockout mice, with evidence of AKT activation concomitant with neoplastic proliferation. However, the invasive tumor phenotype is rarely seen in previously described mouse models of prostatic neoplasia. These data indicate that the Tp53R270H mutation plays a role in CaP initiation. This finding has not previously been reported. Further characterization of this model, particularly in a setting of androgen deprivation, should allow further insight into the mechanisms by which the Tp53R270H mutation mediates CaP progression.
INTRODUCTION
Prostate cancer (CaP) is the leading cancer diagnosis in men in the United States, with new cases for 2012 estimated at 241,740 and over 28,000 estimated annual deaths from the disease (http://www.cancer.gov/cancertopics/types/prostate). Clinical cures are achieved in approximately 80% of patients presenting with localized disease; however, once metastasis occurs, response to second-line therapy, usually treatment with an androgen receptor agonist such as bicalutamide, is only 25%, with the response duration lasting for only a few months. Although multiple studies have documented a link between mutations in Tp53 and disease progression in individuals with CaP, the exact mechanism by which these Tp53 mutations, in particular gain-of-function (GOF) Tp53 mutations, mediate disease progression remains to be fully elucidated (Tomkova et al., 2008).
Tp53-null mice do not develop prostatic intraepithelial neoplasia (PIN) or CaP; however, acceleration and advancement of tumorigenesis is observed in Tp53-null compound models (combining additional oncogenic genetic manipulations), supporting the current dogma that mutations in Tp53 drive late-stage CaP progression (Navone et al., 1993). Somewhat surprisingly, very few genetically engineered mouse models (GEM) that address the contribution of mutant Tp53 to CaP progression exist. Transgenic mice expressing Tp53R270H (equivalent to the human hotspot mutant R273H) under control of the probasin promoter did develop PIN at 1 year of age, but targeted ‘knock in’ of Tp53R270H did not result in any observed prostate pathology (Elgavish et al., 2004; Olive et al., 2004).
Owing to the high frequency with which Tp53 is mutated in human CaP, we aimed to develop and characterize GEM with prostate-specific expression of mutant Tp53. Our mouse model challenges the current dogma that mutation in Tp53 is only important in promoting disease progression in late-stage CaP by demonstrating that Tp53 can act as an initiating factor. Here we describe the generation and characterization of the Tp53R270H/+ Nkx3.1-Cre mouse model. This model, which has been bred onto a fully congenic FVB/NJ strain background, conditionally expresses the R270H Tp53 GOF mutation in the prostate epithelium of mice, resulting in the development of both PIN and CaP lesions.
RESULTS
Validation, congenesis and characterization of Tp53R270H/+ Nkx3.1-Cre mice
Fig. 1A shows a schematic representation of the targeting vector and the floxed allele following excision of the STOP cassette by Cre recombinase. Nkx3.1-Cre mice were used to induce Cre-mediated deletion of the floxed STOP cassette specifically in the prostate gland. Nkx3.1 is a transcription factor that is expressed in the prostate epithelium and is one of the earliest markers for prostate development (Bhatia-Gaur et al., 1999). Speed congenic backcross with medium density single-nucleotide polymorphism (SNP) analysis of strain background contribution with FVB/NJ target was performed to ensure a uniform strain background (Fig. 1B) resulting in the fully congenic strain FVB.129S4(Trp53tm3Tyj);FVB.129S(Nkx3-1tm3(cre)Mms) (hereafter abbreviated to Tp53R270H/+ Nkx3.1-Cre). Laser-capture microdissection followed by RNA extraction, reverse-transcriptase PCR (RT-PCR) and sequencing confirmed the expression of the Tp53R270H mutation in PIN lesions (Fig. 1C).
Fig. 1.
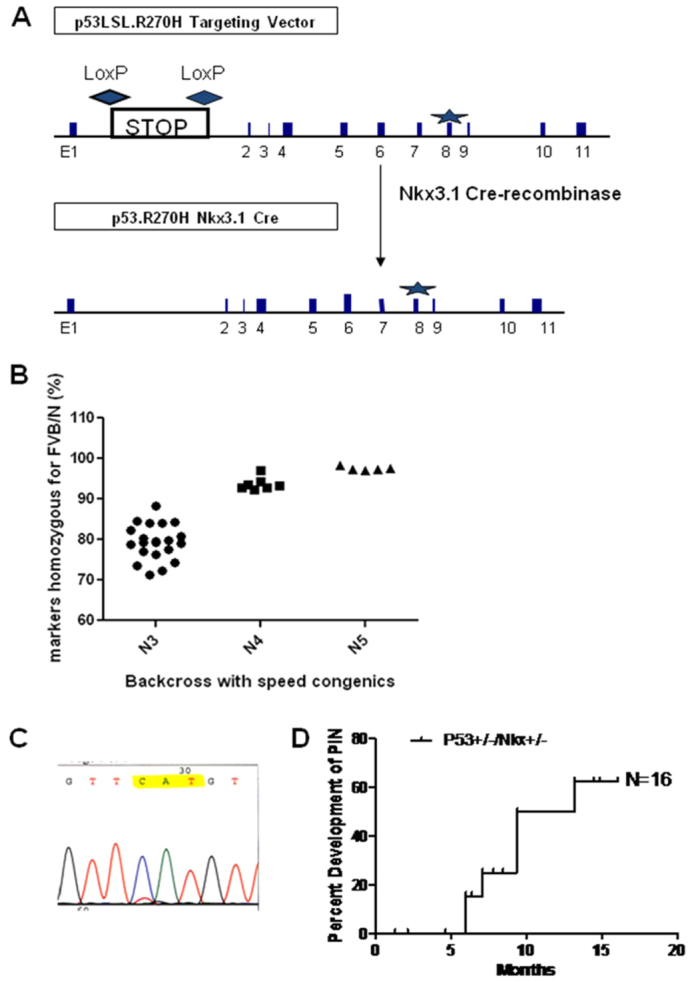
Validation and characterization of the Tp53R270H/+Nkx3.1-Cre mouse. (A) Schematic representation of the targeting vector and the floxed allele following excision of the STOP cassette by Nkx3.1 Cre recombinase. The R270H mutation is located in exon 8 (marked as a star). (B) Congenic backcross onto FVB/NJ was performed to ensure a uniform strain background. Speed congenics (N3 through N5) produced a close to 100% FVB/NJ congenic mouse. (C) RT-PCR and sequencing analysis of laser capture microdissected PIN lesions confirmed that Nkx3.1-Cre-mediated deletion of the floxed STOP cassette resulted in prostate-specific expression of the p53 R270H mutation. (D) Well-developed PIN lesions were observed in Tp53R270H/+ Nkx3.1-Cre mice as early as 4 months and more than 60% of mice examined between 5 and 60 weeks developed grade 2 or higher PIN (n=16). Nkx3.1 haploinsufficient littermate mice (Nkx3.1-Cre; n=6), which did not express the Tp53 mutation, did not develop PIN even at 60 weeks (see Fig. 2).
Prostate-specific expression of the Tp53 mutant initiates early PIN lesions in Nkx3.1-Cre heterozygous mice
Heterozygous Tp53R270H/+ Nkx3.1-Cre mice with prostate-specific expression of the Tp53R270H mutation (n=16) had a high incidence of atypia or PIN grade 1 (PIN 1) with progression to PIN 2 and PIN 3 as described (Park et al., 2002), which, in homozygous Tp53R270H/R270H Nkx3.1-Cre+/– mice, could be observed as early as 5 weeks of age (Fig. 1D, Fig. 2D–F) and, in heterozygous Tp53R270/+ Nkx3.1-Cre+/– mice, was seen by 6 months of age. Most lesions were in the dorsolateral prostate and coagulating gland and less so in the ventral prostate. No changes were observed in the seminal vesicles or the periurethral glands.
Fig. 2.
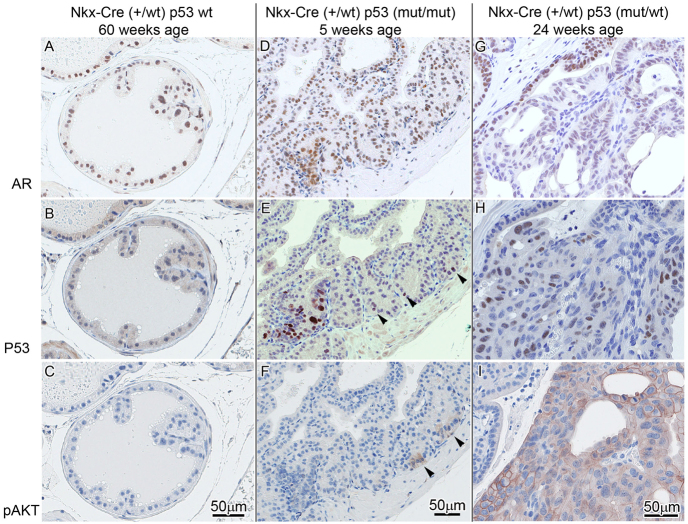
Lesion progression in Tp53R270H/+ Nkx3.1-Cre mouse prostate. (A–C) In Tp53 wild-type mice with Nkx3.1 haploinsufficiency, foci of atypia were seen in older mice (60 weeks) but are not associated with well-developed PIN. p53 (B) and pAKT (C) are negative. (D–F) Early pre-PIN atypia/PIN 1 lesion in a 5-week-old mouse with p53 mutation/stabilization as detected by IHC (bottom left and arrowheads in E) seems to precede pAKT (focally seen arrowheads in F) and AR expression in normal and atypical areas (D). (G–I) At 24 weeks, areas with p53 mutation/stabilization in PIN 3–4 are accompanied by pAKT expression (I) and decreased AR nuclear intensity and percentage (G).
Nkx3.1 haploinsufficient littermate mice (Nkx3.1-Cre+/–; n=6) that did not express the Tp53 mutation did not develop PIN of grade 2 or higher but did have some focal atypia after 12 months of age (Fig. 2A–C). Tp53 mutant mice (Tp53R270H/+ Nkx3.1-Cre+/–) younger than 6 months did not have PIN lesions of grade 2 or higher (Fig. 3A,B) but some had focal atypia seen as enlarged and hyperchromatic nuclei in small clusters with loss of cell polarity and foci of PIN grade 1, although the distinction of focal atypia and PIN 1 was subjective. Mice with homozygous Tp53R270H/R270H Nkx3.1-Cre+/– had PIN 2 lesions as early as 4 months of age (Fig. 1D) and animals with PIN lesions of higher grades almost always had areas of lower grades of PIN as well. In one mouse, there was a single lobe/gland that demonstrated grades 1–4 of PIN (Fig. 3B). Qualitatively, Ki67 staining confirmed higher proliferation in the PIN lesions, with 40% of PIN lesion cells being positive for Ki67 (data not shown). Non-atypical adjacent or control prostate had less than 1% Ki67 positivity. The combined data infer that the Tp53R270H mutation (equivalent to the human Tp53R273H hotspot mutation) can mediate initiation of CaP, at least in the context of Nkx3.1 haploinsufficiency and on a congenic FVB/NJ strain background. This has not previously been reported.
Fig. 3.
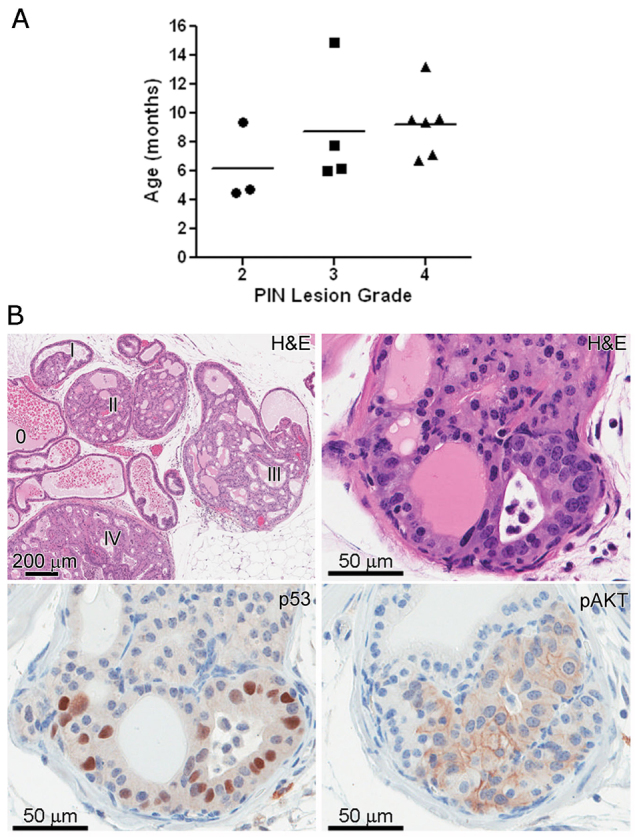
Distribution of PIN lesion grade among Tp53R270H/+ Nkx3.1-Cre mice. The scatterplot represents the highest grade lesion for a particular mouse (A), though often areas of lower grade were also seen (B). The horizontal line within each grade represents the mean age. Grading was performed as described previously (Park et al., 2002). Phenotypically, PIN lesions in Tp53R270H/+ Nkx3.1-Cre mice are similar to those seen in PTEN conditional knockout mice (B, upper right panel and lower 2 panels). Immunohistochemical analysis determined that p53 is stabilized in 10–70% of the cells within PIN lesions (B, lower left), further validating the expression of the p53 R270H mutation in these lesions. Levels of pAKT were elevated in almost all PIN lesions (B, lower right panel).
Determination of Tp53 stabilization and expression of AR and pAKT in PIN lesions
Immunohistochemical analysis determined that Tp53 is stabilized in a variable percent of cells (ranging from 10–70%) in the PIN lesions, indicating that mutant p53 is expressed by these cells (Fig. 3B, lower left panel). Occasional atypical cells with p53 stabilization, as determined by immunohistochemistry (IHC) detection, were seen in areas not recognized as PIN on hematoxylin and eosin (H&E) stains, probably representing small neoplastic initiation foci. These data indicate that the expression of the Tp53R270H mutant is clonal. Future studies will focus on elucidating why this is. Rare non-atypical nuclei also had p53 positivity, but diffuse stabilization of p53 in all or most prostate epithelial cells was not seen. Androgen receptor (AR) expression was present throughout the prostate epithelium and included areas of CaP (Fig. 4A, Fig. 5B,C) and PIN (Fig. 5A). The intensity of the AR expression was decreased in many of the PIN lesions, and was undetectable in some (Fig. 2G, Fig. 5A). Levels of phosphorylated AKT (pAKT) were elevated in PIN lesions, indicating possible direct activation of the AKT pathway by expression of the Tp53R270H allele (Fig. 2F,I, Fig. 3B, lower right panel). In careful analyses of the earliest lesions (as illustrated in Fig. 2D–F), p53 mutation, as detected by IHC positivity, consistent with protein stabilization, seemed to precede AKT activation/phosphorylation, and AR expression was maintained in early lesions. Reduced AR expression was seen later in more well developed PIN lesions (Fig. 2I).
Fig. 4.
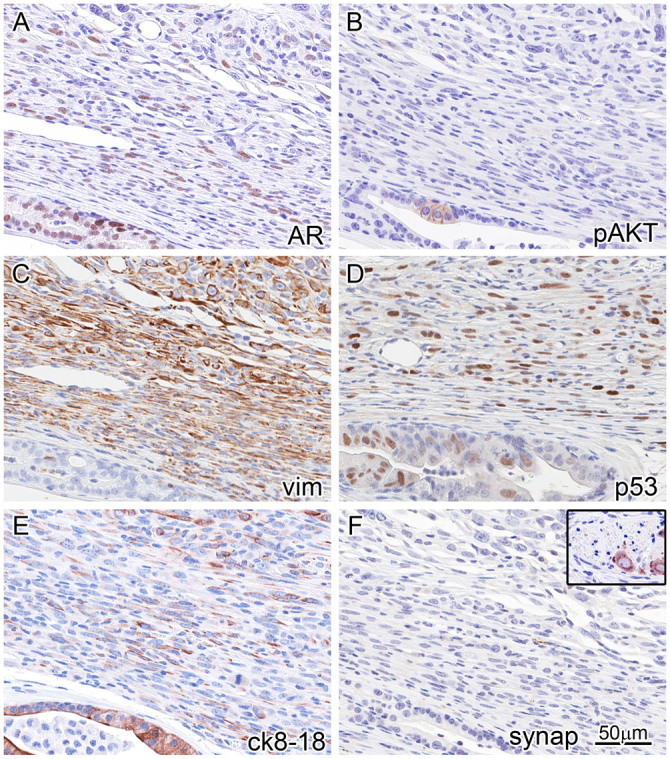
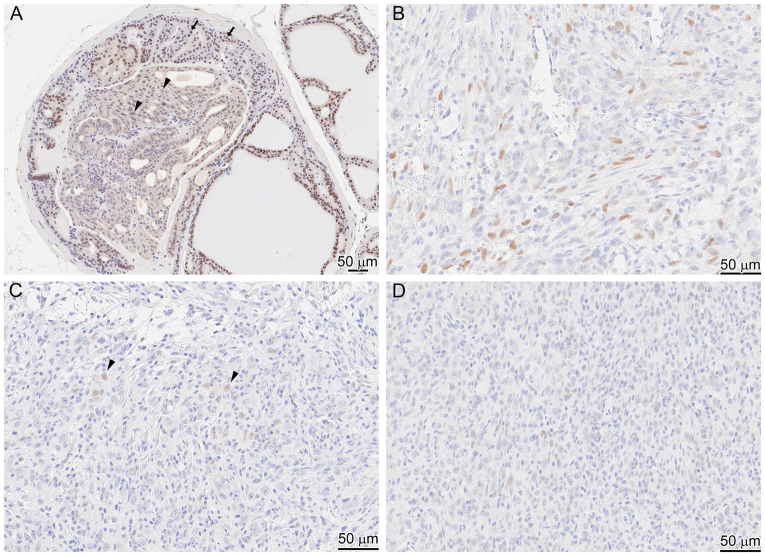
Immunophenotyping of invasive carcinoma reveals an EMT phenotype and AKT ‘de-addiction’. IHC (markers indicated lower right of each panel) on the same area (near-serial sections) of the periphery of an invasive tumor with an adjacent duct (bottom and lower left of each panel). This reveals strong AR expression in the duct, and weaker and lower percentage AR expression in the tumor cell nuclei (A). Focal pAKT is seen in the in situ atypia (B), corresponding to Tp53 stabilization/mutation (D). The tumor co-expresses vimentin (C) and luminal CK8/18 (E), and loses expression of pAKT (B). Although AKT activation is likely to be a driver of the PIN lesions, the invasive tumor seems to be independent of AKT (is ‘de-addicted’). The in situ areas are vimentin negative and cytokeratin positive (C,E). The tumor is negative for neuroendocrine differentiation as detected by a negative synaptophysin (F); inset is from the same tissue section showing a neural ganglion as internal positive control.
Fig. 5.
Heterogeneity of staining for AR in a PIN lesion and invasive carcinoma. In some PIN lesions (A), AR staining of the nuclei has become faint or is even lost (arrowheads) compared with nuclei of normal prostate epithelium (arrows). In invasive carcinoma, progressive loss of AR is seen with areas of strong nuclear staining in less than 50% of tumor cells (B), weak nuclear staining in less than 20% of cells (C; arrowheads) and areas of negative nuclear AR staining (D).
Prostate-specific expression of the Tp53 mutant can mediate progression to CaP
Heterozygous Tp53R270H/+ Nkx3.1-Cre mice developed invasive CaP by 30 weeks of age, with incomplete penetrance. Although the observed PIN lesions were typical of GEM prostate models, including the PTEN conditional models, the invasive tumor phenotype was not (Fig. 4).
The invasive CaP showed a distinct sarcomatoid or epithelial-mesenchymal transition (EMT) phenotype. A similar EMT phenotype has been observed with an inducible FGFR1 mouse model; however, the involvement of p53 in that model was not reported (Acevedo et al., 2007). Tumor cells were highly atypical, and mitoses were numerous. Invasive tumor grew in solid nests and sheets, with only focal evidence of glandular or microacinar differentiation. Gleason grade for such a tumor in the human prostate would be high, numerical pattern ‘5’ or a score of ‘5+5’. Cells comprising the tumor were both epithelioid and spindled, and invaded around normal glands. IHC of the CaP lesions showed p53 staining but AR expression was lost in an increasing percentage of cells and decreased in intensity even in positive cells (Fig. 5B–D). In addition, markers of EMT, including coexpression of cytokeratins 8/18 and vimentin, verify the impression that the tumor has both epithelial (CK8/18) and mesenchymal (vimentin) differentiation (Fig. 4C,E). Although p53 expression and pAKT were seen in the adjacent PIN or atypia (Fig. 2B,C, bottom left) no vimentin expression was seen in the in situ lesions. Neuroendocrine differentiation, a common feature in some mouse models with Tp53 inactivation through SV40 large T antigen expression (Masumori et al., 2001; Chiaverotti et al., 2008), was not observed in the in situ or invasive carcinomas in these mice (Fig. 2F). No metastases to other sites were observed.
DISCUSSION
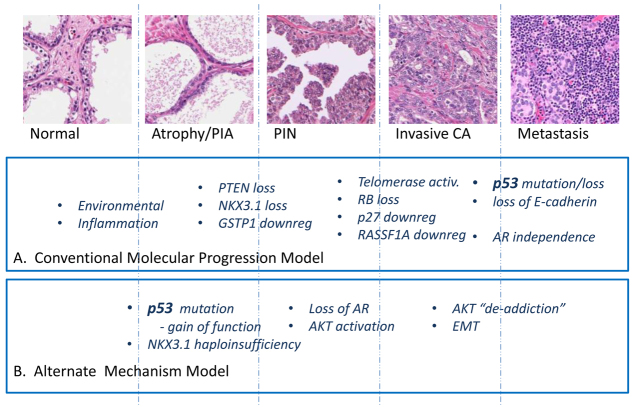
Tp53R270H/+ Nkx3.1-Cre mice develop foci of atypia or PIN 1 as early as 5 weeks, then well-developed neoplastic foci (PIN 2 or higher) as early as 4 months of age. In early lesions, Tp53 mutation, as detected by IHC positivity and confirmed by laser capture microdissection sequencing, precedes the earliest foci of AKT hyperphosphorylation, and these foci seem to give rise to the larger PIN lesions with progression to invasive carcinomas. This alternative progression model (Fig. 6) therefore challenges the conventional concept that loss of Tp53 function is exclusively a late event in CaP progression and that aberrations in the PTEN-AKT pathway occur prior to Tp53 mutation (Gonzalgo and Isaacs, 2003; Shen and Abate-Shen, 2010). The data suggest that Tp53 mutation can be an initiating event in prostate neoplasia, and suggest that early Tp53 mutation might be associated with progression towards higher grade carcinomas with tumor virulence features including EMT.
Fig. 6.
Schematic diagram of alternative molecular progression in prostate cancer compared with conventional model. CA, carcinoma; PIA, proliferative inflammatory atrophy.
High Tp53 mutation frequencies have indeed been detected in metastatic and CR CaP, with 52–89% of tumors expressing Tp53 protein by IHC (Olivier et al., 2009). However, in support of our finding, our group and others have documented the occurrence of Tp53 mutations in ∼30% of localized human CaP (Chi et al., 1994; Heidenberg et al., 1995; Hughes et al., 1995; Bauer et al., 1996; Moul et al., 1996; Prendergast et al., 1996; Byrne et al., 1997; Meyers et al., 1998; Schlechte et al., 1998; Shi et al., 2002; Downing et al., 2003). The difference in rates of Tp53 mutation reported by these studies is likely to be due in part to the different methodologies used to assess the presence of p53 mutations (for a review, see Robles and Harris, 2010). The presence of Tp53 mutations has also been documented in human PIN lesions (Downing et al., 2001).
Although the role of mutant Tp53 in promoting the initiation of CaP remains controversial, mutant Tp53 has been shown to facilitate initiation in other cancer types, for example breast, lung and esophageal cancers (Olivier et al., 2009). Several models have been proposed for defining how Tp53 mutations infer oncogenicity (Soussi, 2007). Current data suggest that genomic instability is the most likely mechanism of action that mediates initiation of Tp53R270H-driven breast carcinomas (Wijnhoven et al., 2005), whereas GOF activity is important in the initiation of Tp53R270H-driven lung carcinomas (Olive et al., 2004). Further analysis and manipulation of our Tp53R270H/+ Nkx3.1-Cre model will allow for elucidation of the mechanism(s) by which Tp53R270H drives initiation and progression of CaP.
Similar to Tp53 mutation, inactivation of PTEN is also a frequent occurrence in individuals with CaP (Shen and Abate-Shen, 2010). Increased AKT activity suggests that PTEN inactivation might occur in our model, although PTEN status remains to be determined. The current dogma is that p53 acts as a ‘failsafe’ protein after loss of PTEN function. Although it is well known that combined inactivation of PTEN and Tp53 in compound models of CaP greatly accelerates tumor development (Jeet et al., 2010), in terms of disease progression the importance of whether p53 or PTEN loss of function occurs first remains to be determined.
It should be noted that, although Nkx3.1-null mice do not develop PIN until 1–2 years of age, it is possible that Nkx3.1 haploinsufficiency is required for the phenotype observed in our Tp53R270H/+ Nkx3.1-Cre mice. CaP lesions are detected in Tp53R270H/+ Nkx3.1-Cre mice at ∼7–8 months and phenotypic markers of EMT are detected in these lesions. This finding indicates that future studies of metastatic potential are warranted in the Tp53R270H/+ Nkx3.1-Cre mouse model. Our ongoing studies are focused on determining whether CR growth occurs in the Tp53R270H/+ Nkx3.1-Cre mouse. In human CaP cell lines, the Tp53R273H mutation can mediate CR growth (Takahashi et al., 2002; Nesslinger et al., 2003; Vinall et al., 2006). This model will also be used to further elucidate the mechanisms by which mutant Tp53 promotes CaP initiation and progression.
METHODS
Generation of conditionally inactive mutant Tp53 mice
Mice containing a Cre-activatable Tp53R270H knock-in allele were obtained from the Tyler Jacks Lab (Olive et al., 2004). The loxP-flanked conditional STOP cassette (Tuveson et al., 2004) was cloned into the XhoI site in intron 1 of the murine Tp53 locus. The R270H missense mutation was generated as described previously (de Vries et al., 2002). In our colony, the presence of the loxP–STOP-cassette–loxP (LSL) cassette in intron 1 of Tp53 genomic DNA is detected using the following primers to generate a wild-type band of 170 bp and a mutant band of 270 bp: wt F- 5′-TTACACATCCAGCCTCTGTGG-3′; mutant F- 5′-AGCTAG-CCACCATGGCTTGAGTAAGTCTGCA-3′; R- 3′ -CTTGGA-GACATAGCCACACTG-3′. To determine the presence of the recombined alleles of mutant Tp53, genomic DNA was amplified using the following primers flanking the integration site of the remaining loxP site in Tp53 intron 1: F- 5′-AGCCTGCC-TAGCTTCCTCAGG-3′; R- 5′ -CTTGGAGACATAGCCA-CACTG-3′. The Tp53R270H (amino acid 270 in mice Tp53 corresponds to 273 in human Tp53) mutant has a wild-type Tp53 conformation but is defective in DNA binding, because R270H is in the DNA-binding domain of Tp53. The Nkx3.1-Cre mouse is a knock-out/knock-in mouse in which the Nkx3.1 coding region is replaced with the Cre recombinase coding sequence to result in prostate-specific expression of Cre, but also an Nkx3.1-null allele (Lin et al., 2007; Thomsen et al., 2008). The presence of wild-type and/or Cre alleles at Nkx3.1 were determined using primers: F- 5′-CAGATGGGCGCGGCAACACC-3′; R- 5′ -GCGCGGTCTGGC-AGTAAAAAC-3′. In a speed congenics backcross strategy, using the Illumina medium density mouse SNP panel service through the UC Davis Mouse Biology Program Genetic Analysis Laboratory, these mice have been bred onto a fully congenic FVB/NJ strain background. All animal procedures were reviewed and approved by the UC Davis Institutional Animal Care and Use Committee (IACUC). The generation of the CaP Tp53R270H mouse is outlined in Fig. 1.
TRANSLATIONAL IMPACT.
Clinical issue
Tp53 mutations are common in prostate cancer (CaP), occurring with a frequency of ∼30% in localized disease and ∼70% in metastatic disease. Although multiple studies have documented a link between mutations in Tp53 and disease progression in individuals with CaP, Tp53 mutation is generally believed to be a late event in tumor evolution. In mouse models of germline Tp53 mutations, prostatic intraepithelial neoplasia (PIN) is not reported; however, because sarcomas and lymphomas develop rapidly and frequently in these mice, it has been unclear whether Tp53 mutations simply do not have time to impact the prostate. Here, the authors sought to address this issue by engineering mice carrying a prostate-conditional Tp53 knock-in mutation. They chose to model the Tp53R270H mutation (which is directly analogous to the common human ‘hotspot’ mutation Tp53R273H) because it is frequency observed in human CaP, and because it is associated with an androgen-insensitive gain of function.
Results
Heterozygous Tp53LSL3R270H/+;Nkx3.1-Cre mice with prostate-specific expression of the Tp53R270H mutation were generated and bred onto a fully congenic FVB/NJ background. The effect of prostate-specific expression of the Tp53R270H mutation on disease progression was characterized over a 16-month period. Compared with Nkx3.1 haploinsufficient mice (Nkx3.1-Cre), which did not express the Tp53 mutation, Tp53R270H/+ Nkx3.1-Cre littermate mice had a significantly increased incidence of PIN lesions and these appeared at earlier time points. PIN lesions showed consistent progression and invasive carcinoma with a high-grade sarcomatoid or epithelial-mesenchymal transition phenotype. These PIN lesions were similar to those observed in PTEN conditional knockout mice, with evidence of AKT activation concomitant with neoplastic proliferation.
Implications and future directions
These data demonstrate that the Tp53R270H mutation plays a role in both the initiation and development of CaP in mice. In the late stages of progression, the mutation can confer an aggressive cancer phenotype. Interestingly, however, the engineered mutation also seems to contribute to initiation of early PIN lesions in the prostate. This has not been previously reported; even in studies in which the identical knock-in mutation was expressed in all tissues, prostates were found to be normal. Future studies will focus on determining how the Tp53R270H mutation impacts the effectiveness of agents frequently used to treat patients with CaP, particularly androgen ablation and radiation therapy.
Congenesis to an FVB/NJ strain background
Mixed strain background (C57B6/129svj) mice bearing the Nkx3.1-Cre knock-in allele and mice bearing the Flox-STOP R270H Tp53 conditional allele were bred with wild-type FVB/NJ mice (Jackson Laboratory, Bar Harbor, ME), screened for target allele positivity by tail DNA PCR (as above), and then ‘best males’ for speed congenesis were selected by medium density SNP analysis (Illumina, San Diego, CA) in the UC Davis Mouse Biology Program Murine Genetic Analysis Laboratory. Five generations of speed selected backcrossing were performed after two initial generations of non-speed backcross to FVB/NJ.
Microdissection, PCR and sequencing
RNA isolated from microdissected PIN lesion was amplified with the following primers to validate expression of Tp53R270H mRNA: Forward primer 5′ -TTCTGGGACGGGACAGCTTTG-3′, Reverse primer 5′-GAAATACGCTCCATCAAGTG-3′. PCR products were purified using Takara recochips (Clontech, Madison, WI) and sequenced in both directions at the UC Davis Department of Biological Sciences DNA sequencing core facility.
Histopathology and immunohistochemistry
Standard histopathology and IHC was performed as described previously (Park et al., 2002). The presence and number of PIN lesions was assessed by pathological analysis of H&E-stained sections. PIN lesion grades for GEM are described elsewhere (Park et al., 2002). Primary antibodies used were anti-Ki67 Ab-4 (1:500; Neomarker, Fremont, CA), anti-pAKT (1:2000; Cell Signaling, Danvers, MA), anti-CK8/18 (1:2000; Fitzgerald, Acton, MA), anti-AR (Millipore, Billerica, MA), anti-vimentin (Epitomics, Burlingame, CA), anti-p53 (Santa Cruz Biotech, Santa Cruz, CA) and anti-synaptophysin (Invitrogen, Carlsbad, CA).
Acknowledgments
We wish to thank Tyler Jacks, Kenneth Olive and David Tuveson for providing us with the mice containing a Cre-activatable Tp53R270H knock-in allele, and Katie Bell for histotechnology. We also thank Brandon Willis for medium density SNP analysis used in the speed congenesis.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
A.D.B. and R.W.D.W. conceived the idea for the mouse model, S.S.S., M.M.S. and A.D.B. generated the mouse model, R.L.V. and J.Q.C. characterized the mouse model, J.Q.C. genotyped the mice and dissected the tissues, and R.L.V., N.E.H. and A.D.B. prepared the manuscript.
FUNDING
This work was supported by the National Institutes of Health, including the National Cancer Institute [U01 CA141582 (to A.D.B.); 1P01CA154293-01A1 (to M.M.S.); 3P30CA093373-09S3 (to R.D.W.)] and the National Center for Research Resources [K26 RR024037 (to A.D.B.)]; and by a grant from the University of California Davis, School of Medicine, Department of Pathology, Advisory Research Committee (to A.D.B.).
REFERENCES
- Acevedo V. D., Gangula R. D., Freeman K. W., Li R., Zhang Y., Wang F., Ayala G. E., Peterson L. E., Ittmann M., Spencer D. M. (2007). Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell 12, 559–571 [DOI] [PubMed] [Google Scholar]
- Bauer J. J., Sesterhenn I. A., Mostofi F. K., McLeod D. G., Srivastava S., Moul J. W. (1996). Elevated levels of apoptosis regulator proteins p53 and bcl-2 are independent prognostic biomarkers in surgically treated clinically localized prostate cancer. J. Urol. 156, 1511–1516 [PubMed] [Google Scholar]
- Bhatia-Gaur R., Donjacour A. A., Sciavolino P. J., Kim M., Desai N., Young P., Norton C. R., Gridley T., Cardiff R. D., Cunha G. R., et al. (1999). Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 13, 966–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R. L., Horne C. H., Robinson M. C., Autzen P., Apakama I., Bishop R. I., Neal D. E., Hamdy F. C. (1997). The expression of waf-1, p53 and bcl-2 in prostatic adenocarcinoma. Br. J. Urol. 79, 190–195 [DOI] [PubMed] [Google Scholar]
- Chi S. G., deVere White R. W., Meyers F. J., Siders D. B., Lee F., Gumerlock P. H. (1994). p53 in prostate cancer: frequent expressed transition mutations. J. Natl. Cancer Inst. 86, 926–933 [DOI] [PubMed] [Google Scholar]
- Chiaverotti T., Couto S. S., Donjacour A., Mao J. H., Nagase H., Cardiff R. D., Cunha G. R., Balmain A. (2008). Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am. J. Pathol. 172, 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries A., Flores E. R., Miranda B., Hsieh H. M., van Oostrom C. T., Sage J., Jacks T. (2002). Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. Proc. Natl. Acad. Sci. USA 99, 2948–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing S. R., Jackson P., Russell P. J. (2001). Mutations within the tumour suppressor gene p53 are not confined to a late event in prostate cancer progression. a review of the evidence. Urol. Oncol. 6, 103–110 [DOI] [PubMed] [Google Scholar]
- Downing S. R., Russell P. J., Jackson P. (2003). Alterations of p53 are common in early stage prostate cancer. Can. J. Urol. 10, 1924–1933 [PubMed] [Google Scholar]
- Elgavish A., Wood P. A., Pinkert C. A., Eltoum I. E., Cartee T., Wilbanks J., Mentor-Marcel R., Tian L., Scroggins S. E. (2004). Transgenic mouse with human mutant p53 expression in the prostate epithelium. Prostate 61, 26–34 [DOI] [PubMed] [Google Scholar]
- Gonzalgo M. L., Isaacs W. B. (2003). Molecular pathways to prostate cancer. J. Urol. 170, 2444–2452 [DOI] [PubMed] [Google Scholar]
- Heidenberg H. B., Sesterhenn I. A., Gaddipati J. P., Weghorst C. M., Buzard G. S., Moul J. W., Srivastava S. (1995). Alteration of the tumor suppressor gene p53 in a high fraction of hormone refractory prostate cancer. J. Urol. 154, 414–421 [DOI] [PubMed] [Google Scholar]
- Hughes J. H., Cohen M. B., Robinson R. A. (1995). p53 immunoreactivity in primary and metastatic prostatic adenocarcinoma. Mod. Pathol. 8, 462–466 [PubMed] [Google Scholar]
- Jeet V., Russell P. J., Khatri A. (2010). Modeling prostate cancer: a perspective on transgenic mouse models. Cancer Metastasis Rev. 29, 123–142 [DOI] [PubMed] [Google Scholar]
- Lin Y., Liu G., Zhang Y., Hu Y. P., Yu K., Lin C., McKeehan K., Xuan J. W., Ornitz D. M., Shen M. M., et al. (2007). Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development 134, 723–734 [DOI] [PubMed] [Google Scholar]
- Masumori N., Thomas T. Z., Chaurand P., Case T., Paul M., Kasper S., Caprioli R. M., Tsukamoto T., Shappell S. B., Matusik R. J. (2001). A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res. 61, 2239–2249 [PubMed] [Google Scholar]
- Meyers F. J., Gumerlock P. H., Chi S. G., Borchers H., Deitch A. D., deVere White R. W. (1998). Very frequent p53 mutations in metastatic prostate carcinoma and in matched primary tumors. Cancer 83, 2534–2539 [PubMed] [Google Scholar]
- Moul J. W., Bettencourt M. C., Sesterhenn I. A., Mostofi F. K., McLeod D. G., Srivastava S., Bauer J. J. (1996). Protein expression of p53, bcl-2, and KI-67 (MIB-1) as prognostic biomarkers in patients with surgically treated, clinically localized prostate cancer. Surgery 120, 159–166; discussion 166–167. [DOI] [PubMed] [Google Scholar]
- Navone N. M., Troncoso P., Pisters L. L., Goodrow T. L., Palmer J. L., Nichols W. W., von Eschenbach A. C., Conti C. J. (1993). p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J. Natl. Cancer Inst. 85, 1657–1669 [DOI] [PubMed] [Google Scholar]
- Nesslinger N. J., Shi X. B., deVere White R. W. (2003). Androgen-independent growth of LNCaP prostate cancer cells is mediated by gain-of-function mutant p53. Cancer Res. 63, 2228–2233 [PubMed] [Google Scholar]
- Olive K. P., Tuveson D. A., Ruhe Z. C., Yin B., Willis N. A., Bronson R. T., Crowley D., Jacks T. (2004). Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 [DOI] [PubMed] [Google Scholar]
- Olivier M., Petitjean A., Marcel V., Petre A., Mounawar M., Plymoth A., de Fromentel C. C., Hainaut P. (2009). Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 16, 1–12 [DOI] [PubMed] [Google Scholar]
- Park J. H., Walls J. E., Galvez J. J., Kim M., Abate-Shen C., Shen M. M., Cardiff R. D. (2002). Prostatic intraepithelial neoplasia in genetically engineered mice. Am. J. Pathol. 161, 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast N. J., Atkins M. R., Schatte E. C., Paulson D. F., Walther P. J. (1996). p53 immunohistochemical and genetic alterations are associated at high incidence with post-irradiated locally persistent prostate carcinoma. J. Urol. 155, 1685–1692 [PubMed] [Google Scholar]
- Robles A. I., Harris C. C. (2010). Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb. Perspect. Biol. 2, a001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechte H., Lenk S. V., Loning T., Schnorr D., Rudolph B. D., Ditscherlein G., Loening S. A. (1998). p53 tumour suppressor gene mutations in benign prostatic hyperplasia and prostate cancer. Eur. Urol. 34, 433–440 [DOI] [PubMed] [Google Scholar]
- Shen M. M., Abate-Shen C. (2010). Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. B., Di Mauro S. M., Highshaw R., Deitch A. D., Evans C. P., Gumerlock P. H., deVere White R. W. (2002). Application of a yeast assay to detect functional p53 mutations in archival prostate cancer tissue. Cancer Biother. Radiopharm. 17, 657–664 [DOI] [PubMed] [Google Scholar]
- Soussi T. (2007). p53 alterations in human cancer: more questions than answers. Oncogene 26, 2145–2156 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Munakata M., Ohtsuka Y., Nisihara H., Nasuhara Y., Kamachi-Satoh A., Dosaka-Akita H., Homma Y., Kawakami Y. (2002). Expression and alteration of ras and p53 proteins in patients with lung carcinoma accompanied by idiopathic pulmonary fibrosis. Cancer 95, 624–633 [DOI] [PubMed] [Google Scholar]
- Thomsen M. K., Butler C. M., Shen M. M., Swain A. (2008). Sox9 is required for prostate development. Dev. Biol. 316, 302–311 [DOI] [PubMed] [Google Scholar]
- Tomkova K., Tomka M., Zajac V. (2008). Contribution of p53, p63, and p73 to the developmental diseases and cancer. Neoplasma 55, 177–181 [PubMed] [Google Scholar]
- Tuveson D. A., Shaw A. T., Willis N. A., Silver D. P., Jackson E. L., Chang S., Mercer K. L., Grochow R., Hock H., Crowley D., et al. (2004). Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 [DOI] [PubMed] [Google Scholar]
- Vinall R. L., Tepper C. G., Shi X. B., Xue L. A., Gandour-Edwards R., deVere White R. W. (2006). The R273H p53 mutation can facilitate the androgen-independent growth of LNCaP by a mechanism that involves H2 relaxin and its cognate receptor LGR7. Oncogene 25, 2082–2093 [DOI] [PubMed] [Google Scholar]
- Wijnhoven S. W., Zwart E., Speksnijder E. N., Beems R. B., Olive K. P., Tuveson D. A., Jonkers J., Schaap M. M., van den Berg J., Jacks T., et al. (2005). Mice expressing a mammary gland-specific R270H mutation in the p53 tumor suppressor gene mimic human breast cancer development. Cancer Res. 65, 8166–8173 [DOI] [PubMed] [Google Scholar]