Abstract
Although protein kinase C-θ (PKC-θ)-deficient mice are resistant to the induction of Th17-dependent experimental autoimmune encephalomyelitis, the function of PKC-θ in Th17 differentiation remains unknown. Here we show that purified, naive CD4 PKC-θ−/− T cells were defective in Th17 differentiation, whereas Th1 and Th2 differentiation appeared normal. Activation of PKC-θ with PMA promoted Th17 differentiation in wild type (WT) but not PKC-θ−/− T cells. Furthermore, PKC-θ−/− T cells had notably lower levels of Stat3, a transcription factor required for Th17 differentiation, and PMA markedly stimulated the expression of Stat3 in WT but not PKC-θ−/− T cells. In contrast, activation of Stat4 and Stat6, which are critical for Th1 and Th2 differentiation, was normal in PKC-θ−/− T cells. Forced expression of Stat3 significantly increased Th17 differentiation in PKC-θ−/− T cells, suggesting that reduced Stat3 levels were responsible for impaired Th17 differentiation and that Stat3 lies downstream of PKC-θ. Constitutively active PKC-θ or WT PKC-θ activated by either PMA or TCR cross-linking, stimulated expression of a luciferase reporter gene driven by the Stat3 promoter. PKC-θ-mediated activation of the Stat3 promoter was inhibited by dominant negative AP-1 and IκB kinase-β, but stimulated by WT AP-1 and IκB kinase-β, suggesting that PKC-θ stimulates Stat3 transcription via the AP-1 and NF-κB pathways. Lastly, conditions favoring Th17 differentiation induced the highest activation level of PKC-θ. Altogether the data indicate that PKC-θ integrates the signals from TCR signaling and Th17 priming cytokines to up-regulate Stat3 via NF-κB and AP-1, resulting in the stimulation of Th17 differentiation.
Introduction
Differentiation of naïve T cells into specific T helper lineages is a critical checkpoint for controlling immune responses. Altered regulation of this checkpoint may lead to aggravated autoimmunity by overproduction of Th cells that cause pathogenic inflammation. Historically, T helper cells have been classified as either Th1 cells, which produce IFNγ or Th2 cells, which produce IL-4 (1). In 2003 however, Cua et al. made the seminal observation that IL-23, rather than the Th1 cytokine IL-12, was critical for the development of experimental autoimmune encephalomyelitis (EAE), a condition long believed to be a Th1-dependent autoimmune disease (2). Langrish et al. then showed that IL-23 is required for the expansion of a population of IL-17-producing, pathogenic T cells that induce EAE when adoptively transferred to naïve mice (3). This observation shifted the traditional Th1/Th2 paradigm by addition of a new lineage of Th17 cells to the Th1/Th2 model. Th17 cells produce IL-17, IL-21, IL-22 and GM-CSF cytokines (4–6), that can induce massive tissue inflammation due to the broad distribution of their receptors on both immune and non-immune cells. It should be noted that IL-17 is not absolutely required for the development of EAE but it is able to induce and promote EAE development (7). In addition to EAE, Th17 cells are also the pathogenic T cell in animal models of collagen-induced arthritis (8) and inflammatory bowel disease (9). Circumstantial evidence has accumulated to suggest a pathoimmunological role for Th17 cells in multiple human autoimmune disorders, including multiple sclerosis (10), rheumatoid arthritis (11), asthma (12), inflammatory bowel disease (13) and psoriasis (14). A greater understanding of the mechanisms responsible for the regulation of Th17 differentiation will facilitate the development of treatments targeting Th17-mediated autoimmunity.
To differentiate into Th17 cells, naïve T cells must be activated via the TCR in the presence of TGF-β and IL-6 (15, 16). TGF-β is required for the expression of retinoic acid-related orphan receptor γt (RORγt), a master transcription factor (17), and IL-6 is needed to activate Stat3 (18, 19). Both factors are critical for Th17 differentiation (20). Upon binding to IL-6, the IL-6 receptor component gp130 dimerizes and activates JAK, which physically associates with gp130 and phosphorylates its cytoplasmic domain. This creates a docking site for the Stat3 Src Homology 2 domain and recruited Stat3 molecules are phosphorylated at tyrosine 705. This results in protein dimerization, which is required for translocation of Stat3 into the nucleus and its ability to bind to target DNA. The absolute requirement for Stat3 in Th17 differentiation was demonstrated by the failure to form Th17 cells in the absence of Stat3, as well as the increased production of IL-17 by T cells expressing a constitutively active form of Stat3 (20, 21). Chen et al. demonstrated that Stat3 directly binds to and activates the IL-17 promoter (22). Although it is clear that IL-6-induced Stat3 activation is critical for Th17 differentiation, little is known about how the expression of Stat3 is regulated during the differentiation process.
PKC-θ is a critical molecule that mediates TCR signals during T cell activation and differentiation (23). Our studies have contributed to understanding the role of PKC-θ in vivo by the creation of PKC-θ−/− mice (23–29). Purified PKC-θ−/− T cells exhibit significantly diminished T cell proliferation and IL-2 production due to defects in the activation of the AP-1, NF-κB and NFAT pathways (24, 27, 30), and this result has been confirmed by other investigators using a strain of independently generated PKC-θ deficient mice (31). PKC-θ was initially thought to be required for Th1 responses, based on studies using the EAE model (32). With the recent discovery that Th17 cells are responsible for the EAE phenotype (33), this raises the possibility that PKC-θ is required for the differentiation or function of Th17 cells (34).
In this study, we demonstrate that PKC-θ mediates the signals required to stimulate Stat3 expression which is critical for Th17 differentiation (20, 21). Th17 differentiation was greatly inhibited in PKC-θ−/− T cells and this was accompanied by reduced levels of Stat3. However, forced expression of Stat3 rescued the Th17 differentiation phenotype in PKC-θθ T cells. Reduced Stat3 was observed at both the protein and mRNA levels in PKC-θ−/− T cells, suggesting PKC-θ-mediates signals that are required to stimulate Stat3 transcription. Indeed, Stat3 mRNA was up-regulated by the PKC-θ activator PMA, to a much greater degree in wild type (WT) than in PKC-θ−/− T cells. Furthermore, PMA also increased Th17 differentiation in naive WT, but not naive PKC-θ−/− T cells, both results indicating PMA exerts its effects through the activation of PKC-θ. We also showed that PKC-θ-mediated activation of the AP-1 and NF-κB pathways stimulated the Stat3 promoter. Taken together, our results indicate that PKC-θ stimulates Th17 differentiation, at least in part, via AP-1 and NF-κB-dependent up-regulation of Stat3 expression.
MATERIALS AND METHODS
Animal care and purification of CD4
WT and PKC-θ−/− C57BL/6 mice have been described previously (29). Mouse care and experimental procedures were performed under pathogen-free conditions following institutional guidance and approved protocols from the Research Animal Care Committee of the City of Hope Medical Center. CD4+ T cells were purified from spleen by negative selection using AutoMax (Miltenyi Biotec, Bergisch-Gladbach, Germany). Purified T cells were cultured in RPMI 1640 media with 10% FBS, 100 μl/ml streptomycin, and 100 units/ml penicillin.
T helper cell differentiation
Naïve T cells were stimulated (3 days) with plate bound anti-CD3, soluble anti-CD28 (BD Pharmingen, San Diego, CA), TGF-β (R&D System, Minneapolis, MN), IL-6 (Peprotech, London, UK), and IL-23 (R&D System), including anti-mouse IL-4 and anti-mouse IFNγ (BioLegend, San Diego, CA). For detection of IL-21, IL-22 and GM-CSF, naïve T cells were cultured under Th17 priming conditions for three days, with anti-CD3 and anti-CD28 in presence of IL-6 (20 ng/ml) and TGF-β1 (1 ng/ml) in IMDM with 10% FBS, 2 mM L-glutamine, 50 μM β-mercaptoethanol, 100 U/ml penicillin and 100 ug/ml streptomycin at 37°C with 5% CO2. Then cells were rested for 2 days with IL-2 (2 ng/ml). Cells were further restimulated with Th17 priming conditions for three more days. For Th1 differentiation, naïve T cells were cultured with anti-CD3, anti-CD28, IL-12 (20 ng/ml) and anti-IL-4 (11B11, 10 ng/ml, eBioscience) in RMPI1640 with 10% FBS, 2 mM L-glutamine, 50μM β-mercaptoethanol, 100 U/ml penicillin and 100 ug/ml streptomycin at 37°C with 5% CO2 for three days. For Th2 differentiation, naïve T cells were cultured with anti-CD3, anti-CD28, IL-4 (10 ng/ml), anti-IFN-γ (XMG1.2, 10 ng/ml, eBioscience) and anti-IL-12 (C17.8, 10 ng/ml) in RMPI1640 for three days.
Retrovirus expression of Stat3 to rescue Th17 differentiation
Retroviral MSCV-IRES-GFP (MIG) or Stat3 expressing (MIG-Stat3) plasmids were transfected into Phoenix-Eco cells using lipofectamine 2000 (Invitrogen). After 48 hours, viral supernatants were collected, passed through 0.4 μM filters and stored at −80°C until use. For transduction, naïve T cells were first activated with anti-CD3 and anti-CD28 antibodies for 24 hours, then spin infected with viral supernatants (2500 rpm, 30°C for 2 hours) in the presence of 8 μg/ml of polybrene (Sigma-Aldrich). After spin infection, IL-6 and TGF-β1 were added to the culture media to induce Th17 differentiation.
Plasmid and Transfection And Luciferase assay
Constitutively active PKC-θ (CA) and dominant negative PKC-θ (DN) constructs were described previously (23). Anti-Stat3 and phospho-Stat3 (Y705F) antibodies were obtained from Cell Signaling (Cell Signaling, Danvers, MA). The Stat3 promoter reporter and Stat3 promoter reporters with mutant Stat3-binding element (SBE) and mutant cAmp response element (CRE), were obtained from Dr. Toshio Hirano (Osaka University Medical school, Japan). Jurkat cells were transfected with plasmid DNA by electroporation as described previously (28) and stimulated 24 h later with PMA or anti-CD3 and CD28 antibodies. Luciferase assays were performed using a dual luciferase assay kit (Promega, Milwaukee, WI).
Western blot analysis
Cells were treated with either PMA or Th17 priming cocktails then lysed. Western blot analyses were performed with antibodies recognizing Stat3 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-STAT3, phospho-PKC-θ and phospho-ERK (Cell Signaling), PKC-θ (BD Pharmingen) or β-actin (Sigma, St. Louis, MO).
RT-PCR and Quantitative PCR (q-PCR)
Total RNA was prepared using TRIzol and cDNA was prepared according to the manufacturers' instructions (Invitrogen Life Technologies, Carlsbad, CA). Primers for RT-PCR (5' to 3') were mouse IL-17A: forward, GATATCCCTCTGTGATCTGG; reverse, TTCTGAATCTGCCTCTGAAT; mouse Stat3: forward, CTGGACACA CGCTACCTGAA; reverse, ACTCTTGCAGGAATCGGCTA; mouse SOCS3: forward, TCCTAT GTGGGGCTAGGAGA; reverse, GCCAATGTCTTCCCAGTGTT; mouse HPRT: forward, CAA TGGATCCCATTACAGAT; reverse, GTGGGTAAATCATTTGGAGA. Primers used for qRT-PCR (5' to 3') were mouse Stat3: forward, CGGAGAAGCATTGTGAGTGA; reverse, CTTCCAGTCAGCCAGCTCTT; GAPDH: primer: forward, ACCACAGTCCATGCCATCAC; reverse, TCCACCACCCTGTTGCTGTA. PCR was performed using an iCycler (Bio-Rad Laboratories, Hercules, CA). All samples were normalized to GAPDH expression.
Chromatin immunoprecipiation (ChIP) assay
ChIP assays were performed as previously described (35). Briefly, chromatin samples were immunoprecipitated with antibodies recognizing c-fos, p65, p50 and acetylated histone H4 (AcH4). Immune complexes were captured using protein A-Sepharose beads and then eluted. After reverse cross-linking and digestion of proteins with proteinase K, DNA was purified by phenol/chloroform extraction. PCR was performed using primers designed to amplify a 368 bp fragment that contained the −478 to −110 region of the Stat3 promoter (5' to 3'): forward, GTGACACCTGGGGACCGCC; reverse, CGGGCGAGCGCTGTACTTCC. Primers designed to amplify the control β-actin promoter (5' to 3') were: forward, CAGCCAACTTTACGCCTAGC; reverse, TTT GGACAAAGACCCAGAGG.
FACS analysis
Th17 primed cells were activated (4 h) with PMA, ionomycin (Sigma) and Golgistop (BD Bioscience PharMingen). Cell surface staining using a FITC- conjugated anti-CD4 antibody and intracellular staining for IL-17 were performed as per manufacturer's instructions (BD Pharmingen). APC-conjugated antibodies specific to IL-17 (eBioscience, San Diego, CA) were used for FACS analysis using FACS Canto II (BD Bioscience) and Flowjo software.
Results
PKC-θ promotes Th17 differentiation
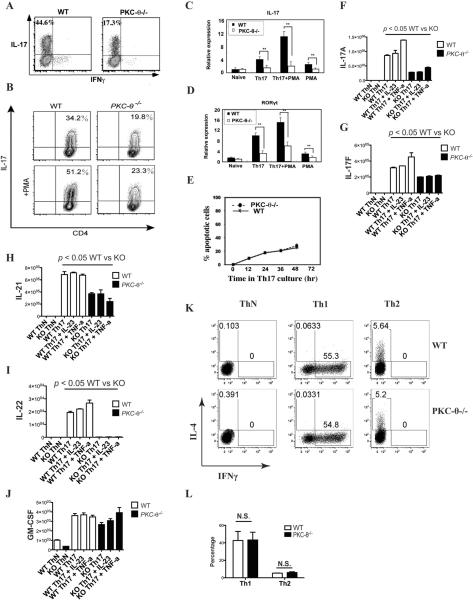
To investigate the role of PKC-θ in Th17 differentiation, purified naïve CD4 WT and PKC-θ−/− mouse T cells were stimulated with anti-CD3/28 antibodies in IMDM medium containing TGFβ and IL-6, a condition which has been reported to be optimal for Th17 differentiation (36). Under these conditions, WT T cells differentiated into greatly more IL-17 producing cells (44.6%) than PKC-θ−/− T cells (17.3%) (Fig. 1A). A similar experiment was performed using RPMI medium, which generally produces fewer Th17 cells than stimulation in IMDM medium (36). Consistent with the IMDM result, a greater proportion of Th17 cells were detected in the WT T cell (34.2%) than in PKC-θ−/− T cell population (19.8%) (Fig. 1B, top two panels). Therefore, the loss of PKC-θ inhibited Th17 differentiation. Conversely, the activation of PKC-θ in WT cells by PMA during differentiation dramatically increased the proportion of Th17 cells from 34.2% to 51.2% (Fig. 1B, left two panels). In contrast, PMA had almost no effect on the differentiation of Th17 cells in PKC-θ−/− T cells (19.8% without PMA vs 23.3% with PMA) (Fig. 1B, right two panels), suggesting that PKC-θ plays a specific role in the induction of Th17 differentiation. We used quantitative PCR (q-PCR), to detect the mRNA levels of IL-17 (Fig. 1C) and RORγt (Fig. 1D), which is the master transcription factor instructing Th17 differentiation. Under Th17 priming conditions, the expression of IL-17 and RORγt mRNA was significantly reduced in PKC-θ−/− T cells compared to WT T cells. Furthermore, the addition of PMA dramatically increased IL-17 and RORγt mRNA expression in WT T cells compared to PMA treated PKC-θ−/− T cells, indicating a specific and positive role for PKC-θ in RORγt-dependent Th17 differentiation. Apoptotic cells were monitored in Th17 differentiation, and no obvious difference in apoptosis was observed between WT and PKC-θ−/− T cells (Fig. 1 E). This result excludes the possibility that the reduced numbers of differentiated Th17 cells in PKC-θ−/− T cells is due to apoptosis. Th17 cells also produce IL-21, IL-22 and GM-CSF (4–6) but the differentiation method we used in the above studies produces little of these cytokines, so we adopted an alternative differentiation protocol (5) in order to augment their expression. Initially differentiated cells, as described in figure 1B, were expanded in IL-2 then re-stimulated in the presence of TGFβ and IL-6 alone or together with either IL-23 or TNFα. In the absence of TGFβ and IL-6 (condition “ThN”), IL-17A, IL-17F, IL-21, IL-22, and GM-CSF were either undetectable or at very low levels both in WT and PKC-θ−/− T cells (Fig. 1F–J), suggesting absence of Th17 cells. In contrast, the cytokines typically produced by Th17 cells were readily detectable following TGFβ– and IL-6-stimulation. The levels of most Th17 cytokines in stimulated WT cells (Fig. 1F–J, open bars) were significantly higher than those in the stimulated PKC-θ−/− T cells (Fig. 1F–J, black bars), except for GM-CSF which was nearly equivalent (Fig. 1J). These results further confirm an essential role for PKC-θ in the differentiation of Th17 cells, but indicate that PKC-θ is dispensable for GM-CSF production. As an additional control, we stimulated naive WT and PKC-θ−/− T cells under conditions that promote Th1 and Th2 differentiation, and did not detect obvious differences between the WT and PKC-θ−/− T cell populations for Th1 and Th2 cells (Fig. 1K–L), suggesting PKC-θ is not required for Th1 or Th2 differentiation under these conditions. Taken together, our results indicate that PKC-θ is specifically required to promote the differentiation of Th17 cells.
Figure 1.
PKC-θ promotes Th17 differentiation. A) Impaired Th17 differentiation is observed in PKC-θ−/− T cells. Purified naïve CD4+ WT and PKC-θ−/− T cells were stimulated by anti-CD3/28 antibodies in IMDM medium containing TGF-β and IL-6 for Th17 differentiation, the Th17 priming conditions; B) Activation of PKC-θ by PMA stimulates Th17 differentiation in WT but not PKC-θ−/− T cells. WT and PKC-θ−/− T cells were differentiated into Th17 in RPMI medium in the absence (top two panels) or presence of 10 nM PMA (bottom two panels); C–D) PKC-θ regulates the expression of IL-17 and RORγt. WT T cells (black bars) and PKC-θ−/− T cells (open bars) were stimulated as indicated in B, and expression of IL-17 (C) and RORγt (D) was detected by q-PCR. The expression levels are presented as the fold induction relative to the signals detected in naïve T cells; E) There are no obvious differences in apoptosis between WT and PKC-θ−/− T cells. WT and PKC-θ−/− T cells were stimulated by Th17 differentiation conditions for the indicated times, and apoptotic cells were monitored by annexin V and 7-AAD; F–J). Impaired production of Th17 cytokines in PKC-θ−/− T cells. WT T cells (open bars) and PKC-θ−/− T cells (black bars) were differentiated under Th17-inducing conditions then expanded in IL-2, and then restimulated using either neutral conditions without cytokines (ThN) or Th17 conditions with TGFβ and IL-6. q-PCR was then used to quantify the relative expression of IL-17F (F), IL-17A (G), IL-21 (H), IL-22 (I) and GM-CSF (J) in WT and PKC-θ−/− T cells; K) Normal Th1 and Th2 differentiation by PKC-θ−/− T cells. WT (top panels) and PKC-θ−/− (bottom panels) T cells were differentiated under ThN (neutral, left two panels), Th1 (middle two panels) or Th2 (right two panels) conditions, and the production of IFNγ or IL-4 was monitored by flow cytometric analysis of intracellularly stained cells; L) Results for Th1 and Th2 differentiation described in K were averaged from three independent experiments.
Reduced expression of Stat3 is responsible for impaired Th17 differentiation in PKC-θ−/− T cells
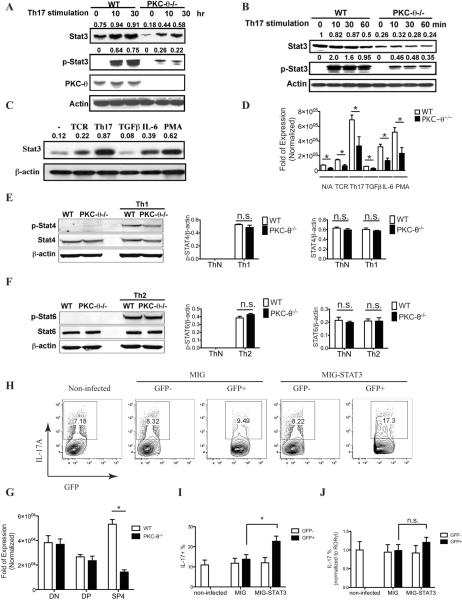
To identify molecules downstream of PKC-θ in the Th17 differentiation pathway, we looked in PKC-θ−/− T cells for altered expression of proteins that were known to regulate Th17 differentiation. We found that levels of Stat3, a transcription factor essential for Th17 differentiation (20, 21), were significantly lower in PKC-θ−/− T cells on western blots (Fig. 2A, top panel). The lower levels of Stat3 were observed even in untreated naïve PKC-θ−/− T cells (Fig. 2A, top panel, 0 time point), suggesting that PKC-θ is required for maintaining Stat3 expression in naïve peripheral T cells. In response to TCR stimulation, which greatly stimulates PKC-θ activity (26, 37), Stat3 levels increased with the stimulation time (10 h and 30 h) to a much greater degree in WT T cells than in PKC-θ−/− T cells, confirming the requirement of PKC-θ for Stat3 expression. Phosphorylation of tyrosine 705 (Y705) is essential for activation of Stat3 (19), therefore an antibody specifically recognizing the Y705-phosphorylated form of Stat3 (p-Stat3) was used to detect activated Stat3 on western blots (Fig. 2A, second panel). Phosphorylated Stat3 was not detected in untreated naïve T cells, and TCR stimulation led to phosphorylation of Stat3 as expected. Although phosphorylated Stat3 was readily detected in WT T cells after stimulation, phosphorylated Stat3 levels were markedly lower in PKC-θ−/− T cells, probably reflecting the overall lower levels of Stat3 in these cells (Fig. 2A). Shorter stimulation times (10 to 60 min) were also tested (Fig. 2B), and Stat3 levels were consistently lower in PKC-θ−/− T cells compared to WT T cells (Fig. 2B, top panel). This was also reflected by lower levels of phosphorylated Stat3 in the PKC-θ−/− T cells (Fig. 2B, second panel). However, in contrast to longer stimulation times (Fig. 2A), no obvious increase in Stat3 protein levels was observed following stimulation of either cell type (Fig. 2B), possibly because longer time periods are needed for transcription and protein synthesis.
Figure 2.
PKC-θ is required to up-regulate Stat3. A–B) Reduced Stat3 levels in PKC-θ−/− T cells. Purified WT and PKC-θ−/− T cells were stimulated with Th17 conditions for the indicated times in (A) and (B). Cells were then lysed for Western blot analysis that used antibodies specific for Stat3, tyrosine (705) phosphorylated Stat3 (p-Stat3), and PKC-θ. β-actin served as the loading control. The number above each Stat3 and p-Stat3 band is the ratio of intensity between the band and the corresponding load control; C) Th17 priming cytokines and PMA stimulate Stat3 expression. T cells were stimulated by indicated conditions, and Stat3 expression was analyzed by Western blot. The number above each band is the ratio of intensity between the band and the corresponding load control; D) Reduced Stat3 messengers in the absence of PKC-θ. WT and PKC-θ−/− T cells were stimulated as indicated and Stat3 messengers were detected by q-PCR; E) Normal activation of Stat4 in PKC-θ−/− T cells. WT and PKC-θ−/− T cells were differentiated under neutral or Th1 conditions, and the phosphorylated form of Stat4 (p-Stat4, top on left panel) and total Stat4 (Stat4, middle on left panel) were analyzed by western blots. Ratios of p-Stat4 to loading control β-actin (middle panel) and Stat4 to β-actin (right panel) were averaged from three independent experiments; F) Normal activation of Stat6 in PKC-θ−/− T cells. WT and PKC-θ−/− T cells were differentiated under neutral or Th2 conditions, and the phosphorylated form of Stat6 (p-Stat6, top on left panel) and total Stat6 (Stat6, middle on left panel) were analyzed by western blots. Ratios of p-Stat6 to loading control β-actin (middle panel) and Stat6 to β-actin (right panel) were averaged from three independent experiments; G) Reduced Stat3 levels in CD4 single positive thymocytes (SP4) cells but not in CD4−CD8− (DN) and CD4+CD8+ (DP) thymocytes. Stat3 levels in sorted DN, DP or SP4 thymocytes from WT and PKC-θ−/− mice were detected by q-PCR; H) Forced expression of Stat3 enhances Th17 differentiation in PKC-θ−/− T cells. PKC-θ−/− T cells were either untreated (left panel), transduced with empty retrovirus (MIG) or transduced with a retrovirus expressing Stat3 (MIG-Stat3). The cells were then stimulated using Th17 priming conditions, and intracellular IL-17 was detected by flow cytometry. Numbers indicate the percentage of IL-17 positive cells in untreated cells (left panel), in cells successfully transduced (GFP+) and in cells not transduced (GFP−) with retrovirus; I) Results were averaged from three independent experiments described in I; J) RORγt levels detected in sorted GFP+ and GFP− cells by q-PCR were used to normalize the percentage of IL-17+ cells in I. Data shown are representative of at least three independent experiments.
Because Th17 priming conditions increased Stat3 expression (Fig. 2A), we examined whether TCR signaling or Th17 differentiation cytokines were responsible for the stimulation of Stat3 expression (Fig. 2C). TCR stimulation only slightly increased Stat3 levels, whereas Th17 priming conditions, including both TCR and TGFβ/IL-6 stimulation, increased Stat3 expression to maximum levels, suggesting that TCR and cytokine signals cooperate to stimulate Stat3 expression. Furthermore, we showed that IL-6 but not TGFβ treatment increased Stat3 expression (Fig. 2C), suggesting that IL-6-mediated signals cooperate with TCR signals to maximally stimulate Stat3 expression. In addition, PMA, the PKC-θ activator, greatly increased Stat3 levels (Fig. 2C). To determine the role of PKC-θ in the observed up-regulation of Stat3, both WT and PKC-θ−/− T cells were stimulated by TCR or Th17 cytokines (Fig. 2D). Stat3 messengers were much lower in PKC-θ−/− T cells compared to WT T cells, indicating that PKC-θ is a critical regulator for Stat3 expression. As controls, we examined the expression of Stat4 (Fig. 2E), which is critical for Th1 differentiation, and Stat6 (Fig.2F), which is critical for Th2 differentiation. No obvious difference in the levels of the phosphorylated active form or total Stat4 were detected between WT and PKC-θ−/− T cells during Th1 differentiation (Fig. 2E, left panel), which was also confirmed by the ratio of phosphorylated Stat4 to the β-actin loading control (Fig. 2E, middle panel) and total Stat4 to β-actin (Fig. 2E, right panel). Similarly, no differences were detected in the phosphorylated active form of Stat6 or total Stat6 between WT and PKC-θ−/− T cells during Th2 differentiation (Fig. 2F). These results are consistent with our observation that the differentiation of PKC-θ-deficient cells into Th1 and Th2 is not affected in vitro (Fig. 1K–L), and suggest the selective requirement for PKC-θ in the expression of Stat3 is essential for Th17 differentiation. To determine if the lower Stat3 levels in PKC-θ−/− T cells begin early during T cell development, we looked for Stat3 in sorted CD4−CD8− and CD4+CD8+ immature thymocytes (Fig. 2G). As a control, CD4+ single positive T cells were also used. Stat3 was reduced only in PKC-θ−/− CD4+ T cells but not in immature CD4−CD8− and CD4+CD8+ thymocytes compared to the corresponding WT cells, suggesting that the reduced expression of Stat3 does not begin early in T cell development. Finally, we determined if lower Stat3 levels in PKC-θ−/− T cells were responsible for impaired Th17 differentiation by retrovirus-mediated expression of Stat3 (Fig. 2H–I). Consistent with previous results (Fig. 1A–B), the proportion of Th17 cells was low in PKC-θ−/− T cells (7.18%) (Fig. 2H, left panel). PKC-θ−/− T cells that were either nontransduced (GFP−) or transduced with empty virus MIG (GFP+) had low numbers of IL-17 cells (8.32% for GFP− cells and 9.49% for GFP+ cells) similar to the non-infected cells. In contrast, PKC-θ−/− T cells transduced with the retrovirus expressing Stat3 (MIG-Stat3) had increased numbers of Th17 cells (17.3% for GFP+, Fig. 2H right panel), whereas GFP− nontranduced cells retained the phenotype of lower IL-17 cells (8.22%, Fig. 2H second panel to the right). This result suggests that the forced expression of Stat3 increased the ability of PKC-θ−/− T cells to differentiate into Th17 cells. The levels of RORγt detected in sorted GFP− and GFP+ cells shown in figure 2H–I were used to normalize the percentage of Th17 cells in figure 2I (Fig. 2J). Although forced expression of Stat3 significantly increased Th17 cells in PKC-θ−/− T cells (Fig. 2I), after normalization to RORγt levels, there were no significant difference in the percentages of IL17 producing cells (Fig.2J), suggesting that rescued Th17 is proportional to increased RORγt levels. Therefore, it appears that Stat3 rescued Th17 cells via a RORγt-dependent pathway, and overall our data indicates that PKC-θ promotes Th17 differentiation via stimulation of Stat3 expression.
Critical role of PKC-θ in the regulation of Stat3 expression
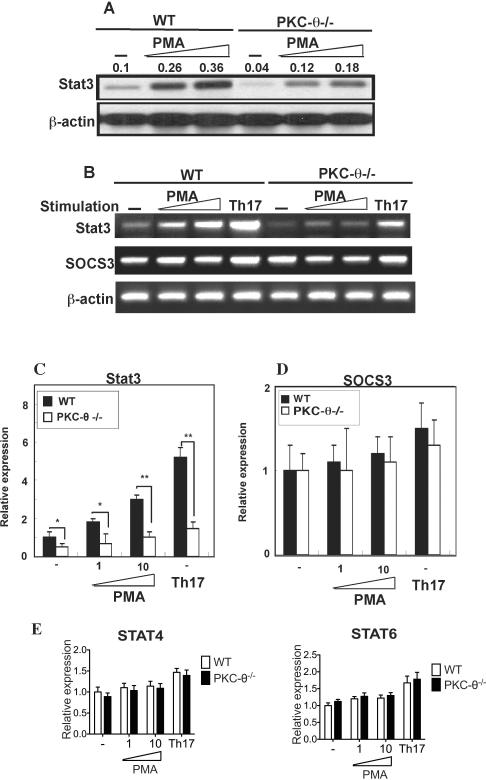
Our studies showed that PMA treatment stimulated Stat3 protein levels in T cells (Fig. 2C). However, because PMA activates multiple isoforms of PKC including PKC-θ, we therefore determined if PKC-θ is responsible for PMA-stimulated Stat3 levels using the PKC-θ−/− T cells (Fig. 3A). PMA treatment resulted in the up-regulation of Stat3 protein detected by Western blot in WT T cells, suggesting that activation of the PKC pathway alone is sufficient to stimulate Stat3 expression. In response to PMA stimulation, PKC-θ−/− T cells failed to increase Stat3 levels similar to those observed in WT T cells, suggesting it is likely that PKC-θ mediated the signals responsible for Stat3 up-regulation. To determine if increased Stat3 protein levels are due to increased transcription, Stat3 mRNA was analyzed by semi-quantitative RTPCR (Fig. 3B, top panel) and q-PCR (Fig. 2C). Consistent with the Stat3 protein data, PMA stimulated the expression of Stat3 mRNA to much higher levels in WT T cells than in PKC-θ−/− T cells, suggesting that PKC-θ mediates Stat3 expression via regulation of transcription. As a control we determined that PMA did not affect the mRNA levels of SOCS, a critical regulator of the Stat3 pathway, in either cell type using RT-PCR (Fig. 3B, second panel) or q-PCR (Fig. 3D). In addition, we detected similar levels of Stat4 and Stat6 in WT and PKC-θ−/− T cells stimulated with PMA or Th17 priming conditions (Fig. 3E), suggesting the requirement for PKC-θ in the regulation of Stat3 expression is specific. Taken together our results indicate that activation of PKC-θ by PMA specifically promotes Th17 differentiation (Fig. 1B) and leads to increased Stat3 transcription.
Figure 3.
PKC-θ regulates Stat3 transcription. A) PKC-θ activated by PMA stimulation increased Stat3 protein to much higher levels in WT cells than in PKC-θ−/− T cells. WT and PKC-θ−/− T cells were either untreated (−) or stimulated with increasing amounts of PMA. Total Stat3 protein was detected on Western blots using β-actin as a loading control. The number above each band is the ratio of intensity between the band and the corresponding load control; B–D) PKC-θ activated by PMA or Th17 differentiation conditions increased Stat3 mRNA to much higher levels in WT cells than that of PKC-θ−/− T cells. WT and PKC-θ−/− T cells were either untreated (−), stimulated with increasing amounts of PMA or stimulated using Th17 priming conditions. Stat3 and SOCS3 mRNA was detected by RT-PCR (B) or q-PCR (C and D); E) Loss of PKC-θ does not affect Stat4 and Stat6 levels. WT and PKC-θ−/− T cells were either untreated (−) or stimulated with increasing amounts of PMA or Th17 priming conditions, and Stat4 and Stat6 messengers were then detected by q-PCR. Data shown are representative of at least three independent experiments.
Activation of Stat3 promoter by PKC-θ-mediated signals
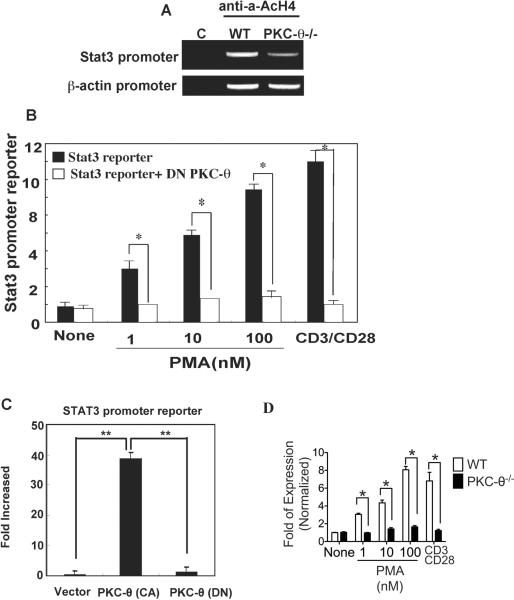
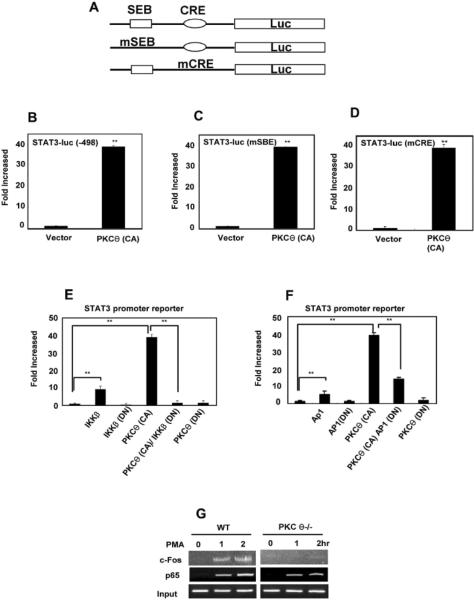
Reduced levels of Stat3 mRNA in PKC-θ-deficient cells suggest the possibility that PKC-θ-mediated signals activate Stat3 transcription via stimulation of its promoter. To test this possibility, we looked for binding of acetylated histone H4 (AcH4), an epigenetic marker for active promoters, to the Stat3 promoter region in vivo using ChIP (Fig. 4A) (38). No signal was detected with an isotype control antibody (C), but the anti-AcH4 immunoprecipitated DNA contained the Stat3 promoter from WT T cells, suggesting that AcH4 was associated with the Stat3 promoter region. A weaker signal was detected in PKC-θ−/− T cells, suggesting there is much lower Stat3 promoter activity in the absence of PKC-θ. No differences were observed between association of AcH4 with the β-actin control promoters from WT and PKC-θ−/− T cells, indicating specificity of PKC-θ in the regulation of endogenous Stat3 promoter activity. We next investigated PKC-θ-mediated regulation of Stat3 promoter activity by transfecting Jurkat cells with a luciferase reporter gene driven by a 498 bp region that contains the Stat3 proximal promoter (39). Stat3-promoter reporter activity (Fig. 4B, black bars) was determined in Jurkat cells stimulated with increasing concentrations of PMA, as well as CD3/28 TCR stimulation, both of which are known to activate PKC-θ (24, 26). To determine the contribution of PKC-θ to Stat3 reporter activity, a dominant negative PKC-θ [PKCθ(DN)] was also introduced (open bars). Stat3- promoter activity was greatly increased by PMA and CD3/28 stimulation, but almost completely inhibited by the PKCθ (DN), strongly suggesting that PKC-θ is responsible for the stimulation of the Stat3 promoter. Finally, we tested effects of a constitutively activate PKC-θ [PKCθ(CA)] on Stat3 promoter activity. The Stat3-promoter reporter was activated by PKC-θ(CA), but not by PKCθ(DN) (Fig. 4C), indicating that activation of PKC-θ alone is sufficient to stimulate the Stat3 promoter, which is consistent with our observation that activation of PKC-θ by PMA alone is sufficient to stimulate Stat3 expression. To determine the role of endogenous PKC-θ, the Stat3-promoter reporter construct was transfected into primary WT and PKC-θ−/− T cells that were then stimulated by PMA or TCR crosslinking (Fig. 4D). Stat3 promoter activity was consistently lower in the absence of PKC-θ. Altogether, these results indicate that PKC-θ plays a critical role in the stimulation of the Stat3 promoter both in vitro and in vivo.
Figure 4.
PKC-θ stimulates the Stat3 promoter. A) Impaired Stat3 promoter activity in PKC-θ−/− T cells. ChIP analysis of WT and PKC-θ−/− T cells was carried out using either anti-acetylated histone H4 as an epigenetic marker for promoter activity or a control antibody (C). Target sequences in the immunoprecipitated complexes were amplified by PCR reactions using primers specific for the Stat3 and β-actin promoter regions; B) Activation of PKC-θ by PMA or TCR cross-linking stimulates the Stat3 promoter. Jurkat cells were transfected with a Stat3 promoter-luciferase reporter construct either alone (black bars) or together with the plasmid expressing a dominant negative PKC-θ ([PKC-θ(DN)],open bars). After transfection (24 h), cells were stimulated with indicated amounts of PMA or anti-CD3/28 antibodies. Luciferase activity was measured 24 h after stimulation; C) Constitutively active PKC-θ is sufficient to activate Stat3 promoter. Jurkat cells were transfected with the Stat3 reporter construct alone or with constitutively active PKC-θ [PKC-θ (CA)] or the PKC-θ(DN). Luciferase activity was measured 24 h after transfection. Reporter activity is indicated as the fold of stimulation relative to the activity obtained from cells transfected with reporter alone; D) PKC-θ is required to stimulate Stat3 promoter activity in primary T cells. CD4+ T cells obtained from WT and PKC-θ−/− mice were transfected with a Stat3 promoter-luciferase reporter construct. Cells were then stimulated with indicated amounts of PMA or anti-CD3/28 antibodies. Luciferase activity was measured 24 h after stimulation. Values, average ±SD; n=3 independent experiments. Data shown are representative of at least three independent experiments.
PKC-θ-mediated activation of AP-1 and NF-κB for stimulation of the Stat3 promoter
To determine the mechanisms responsible for PKC-θ-mediated activation of Stat3, we examined two DNA elements known to work cooperatively to regulate Stat3 expression: SBE and CRE (40). The WT Stat3-promoter reporter or Stat3-promoter reporters containing mutations in the SBE binding site [Stat3(mSBE) reporter] or the CRE binding site [Stat3(mCRE) reporter] (Fig. 5A) were introduced to Jurkat cells alone or together with the activated form of PKC-θ [PKCθ(CA)] (Fig. 4A). There was a 40 fold stimulation of both mutant reporters by PKC-θ (CA) (Fig. 5C–D) that was equivalent to the fold stimulation observed for the WT Stat3 reporter (Fig. 5B), strongly suggesting that PKC-θ-mediated stimulation occurs independently of SBE and CRE. Because previous studies have demonstrated that PKC-θ activates the NF-κB, AP-1 and NFAT pathways in T cells (24, 26, 27, 41), we therefore determined if the Stat3 reporter gene was regulated by these pathways in Jurkat cells using WT and dominant negative constructs of critical proteins that either activate or block each pathway. The Stat3-promoter reporter gene was stimulated by WT IκB kinase-β (IKKβ), but not by a dominant negative IKKβ [IKKβ (DN)] (Fig. 5E), suggesting the NF-κB pathway was able to activate the Stat3 reporter. More importantly, the dominant negative IKKβ inhibited stimulation of the Stat3 reporter by PKC-θ (CA). Similarly, WT AP-1 stimulated Stat3 reporter activity, whereas the dominant negative AP-1 [AP-1(DN)] inhibited PKC-θ (CA) stimulation of the Stat3 reporter (Fig. 5F). However, dominant negative NFAT failed to inhibit PKC-θ (CA)-mediated Stat3 reporter activity (data not shown). These results suggest that PKC-θ-mediated activation of the Stat3 promoter is dependent on the NF-κB and AP-1 pathways but not the NFAT pathway.
Figure 5.
PKC-θ stimulates the Stat3 promoter via AP-1 and NF-κB. A) Schematic representation of WT Stat3, mSEB Stat3 and mCRE Stat3 reporter constructs; B–D) PKC-θ-mediated activation of the Stat3 promoter is independent of SBE and CRE. The WT Stat3 reporter (B) and the reporters containing mutations in SBE [Stat3(mSBE)] (B) or in CRE [Stat3(mCRE)] (D) were transfected into Jurkat cells alone (vector) or together with an expression plasmid for constitutively active PKC-θ [PKC-θ (CA)]. Reporter activity was measured 24 h after transfection; E) NF-κB is required for PKC-θ-mediated activation of the Stat3 promoter. The Stat3 reporter construct was co-transfected into Jurkat cells with expression plasmids that enhanced (IKKβ) or inhibited [dominant negative IKKβ (IKKβ DN)] the NF-κB activity. Luciferase activity was measured 24 h after transfection. Reporter activity is indicated as the fold of stimulation relative to the activity obtained from cells transfected with reporter alone; F) AP-1 is required for PKC-θ-mediated activation of the Stat3 promoter. The Stat3 reporter construct was co-transfected into Jurkat cells with expression plasmids that enhanced (AP-1) or inhibited [dominant negative AP-1 (AP-1 DN)] AP-1. Luciferase activity was measured 24 h after transfection. Reporter activity is indicated as the fold of stimulation relative to the activity obtained from cells transfected with reporter alone; G) c-Fos, a component of AP-1, and p65, a component of NF-κB bind to Stat3 promoter in vivo. T cells were stimulated with PMA for increasing times (0, 2 and 4 h) then subjected to ChIP analysis using antibodies specific for c-Fos and p65. Target sequences in the immunoprecipitated complexes were amplified by PCR using primers specific for the Stat3 promoter region. Prior to immunoprecipitation, a portion of the templates was used for PCR with the same primers as input controls. n=3 independent experiments.
ChIP assays were performed to determine if there were any direct interactions between the Stat3 promoter region and AP-1 (Fig. 5G, top panel). An anti-c-Fos antibody was used because c-Fos is an essential component of AP-1 (24). Strong signals were detected in WT cells after PMA treatment (Fig. 5G, top panel on left) suggesting PMA promotes binding of c-Fos to the Stat3 promoter in vivo. In contrast, the signals in PKC-θ−/− T cells were extremely weak (Fig. 5G, top panel on right), indicating that it is PKC-θ that promotes the binding of c-Fos to the Stat3 promoter after PMA stimulation. We also investigated the interaction between Stat3 promoter and NF-κB which consists of p50 and p65 subunits. Although no signals were detected with an anti-p50 antibody (data not shown), very strong signals were detected with an anti-p65 antibody in WT T cells, following PMA stimulation (Fig. 5G, second panel on left). The signals were weaker in PKC-θ−/− T cells (Fig. 5G, second panel on right). Furthermore, the signal strength increased with increasing PMA stimulation time. As an input control, DNA templates that were not subjected to immunoprecipitation (input) were used in the PCR reaction, and a band of expected size was amplified (bottom panels). These results suggested that AP-1 and NF-κB directly interact with the Stat3 promoter in vivo, and that activated PKC-θ regulates or promotes these interactions.
Enhancement of PKC-θ activation by Th17 priming conditions
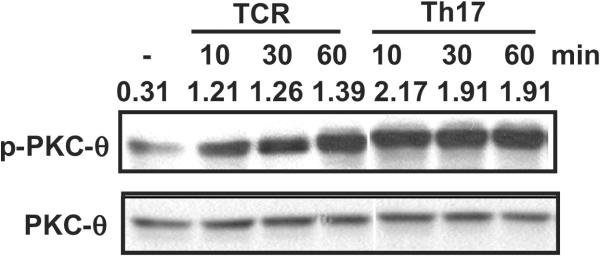
Our data indicates that the Th17 priming cytokines TGFβ and IL-6, combined with TCR stimulation, induced maximal Stat3 expression (Fig. 2C). We therefore monitored the activation of PKC-θ using an antibody that recognized threonine 538 phosphorylated PKC-θ (Fig. 6), because the phosphorylation of this site is thought to be critical for PKC-θ activation(42). Purified naïve WT T cells were stimulated by TCR cross-linking (TCR) or Th17 priming conditions (TCR stimulation + TGF-β + IL-6) (Th17). Consistent with our previous results (43), phosphorylation of PKC-θ at threonine 538 increased with increasing TCR stimulation time, suggesting PKC-θ is activated by TCR signaling. Phosphorylation of PKC-θ was greatly enhanced by the Th17 priming conditions compared to TCR cross-linking alone, indicating that the TGF-β and IL-6 cytokines enhance the activation of PKC-θ. Therefore, Th17 priming conditions which induced maximum levels of Stat3 expression was correlated with the highest PKC-θ activation status.
Figure 6.
Th17 priming cytokines cooperate with TCR signals to promote PKC-θ activation. CD4+ T cells were stimulated with anti-CD3 and anti-CD28 antibodies alone or in the presence of TGF-β and IL-6 (Th17 priming conditions) for the indicated times in minutes. Cell lysates were prepared and analyzed by Western blot using an antibody that recognizes T538 phospho-PKC-θ the activated form of PKC-θ (top panel) and an anti-PKC-θ antibody (bottom panel). The number above each band is the ratio of intensity between the phosphorylated band and the total PKC-θ band. Data shown are representative of at least three independent experiments.
Discussion
Studies using PKC-θ−/− mice have demonstrated that PKC-θ is required for the development of Th17-dependent EAE (32, 34). Consistent with this, we have shown that naïve PKC-θ−/− T cells were defective in their differentiation into Th17 cells in vitro. Given that (1) PKC-θ-deficient cells showed impaired Th17 differentiation and reduced levels of Stat3; (2) activation of PKC-θ by PMA significantly promoted Th17 differentiation and dramatically increased Stat3 levels in WT T cells compared to PKC-θ−/− T cells; and (3) forced expression of Stat3 increased Th17 differentiation in PKC-θ−/− T cells, our data support a critical role for PKC-θ in the regulation of Th17 differentiation, at least in part, via the up-regulation of Stat3 transcription. In addition, we have shown that conditions that induced Th17 differentiation also greatly facilitated the activation of PKC-θ, implicating PKC-θ as a critical checkpoint for integrating signals from the TCR, TGFβ and IL-6 for Th17 differentiation. Previously, we and others have shown that PKC-θ regulates the survival of activated T cells (23, 44), which raised the possibility that defective Th17 differentiation was due to apoptosis of PKC-θ−/− T cells. However, we did not observe any obvious difference in apoptosis between WT and PKC-θ−/− T cells during Th17 differentiation, which is likely to be due to presence of Th17 priming cytokines. Furthermore, exogenous IL-2, which inhibits the apoptosis of PKC-θ−/− T cells (23, 44), did not affect Th17 differentiation (data not shown). In contrast to impaired differentiation of Th17 cells, we did not observe any obvious defects in Th1 and Th2 cell differentiation in PKC-θ−/− T cells in vitro (Fig. 1K), and we observed normal activation of Stat4 and Stat6, which are critical for Th1 and Th2 differentiation, in both WT and PKC-θ−/− T cells (Fig. 2E–F). However, PKC-θ was previously shown to be essential for the development of effective Th2 responses induced by Helminth infection (45) and model allergens, but was dispensable for Th1 responses against viruses (45, 46). This discrepancy with our results may reflect the differences between in vitro differentiation and in vivo immune responses. Multiple PKC-θ-regulated functions may contribute to the defects observed in PKC-θ−/− mice including T cell activation (24), survival (23), activation-induced cell death (28) and T cell differentiation. Therefore, the defective Th2 responses in PKC-θ−/− mice could result from one or more other defects, rather than defective Th2 differentiation. Our in vitro differentiation experiments using purified T cells suggest a selective function for PKC-θ in Th17, but not Th1 or Th2 differentiation, and this is supported by a specific defect in Stat3 activation, but not Stat4 or Stat6 activation, observed in PKC-θ−/− T cells
In this study, we also addressed the mechanisms responsible for PKC-θ-regulated Stat3 expression. PMA-induced stimulation of Stat3 transcription to greater levels in WT compared to PKC-θ−/− T cells, strongly suggesting that PKC-θ activation is necessary and sufficient to activate the Stat3 gene. Indeed, expression of a Stat3 promoter reporter gene construct was stimulated by co-expression of a constitutively active PKC-θ, and completely suppressed by co-expression of a dominant-negative PKC-θ. When we asked how PKC-θ activates the Stat3 promoter, we found that PKC-θ-mediated stimulation of the Stat3 promoter, was inhibited by dominant negative IKKβ and AP-1, indicating that AP-1 and NF-κB are required. The direct interaction between c-Fos (AP-1) or p65 (NF-κB) and the Stat3 promoter was then confirmed by ChIP analysis. Although both anti-c-jun and anti- NF-κB p50 antibodies worked well in our eletromobility shift assay, the fact we could not detect Stat3 promoter binding to c-jun nor NF-κB p50 is probably due to these antibodies not working well in ChIP assays (24). We showed that Stat3 levels were much lower in naïve PKC-θ−/− T cells than naïve WT cells, suggesting that PKC-θ is required to maintain a high basal level of Stat3 in naïve T cells. The fact that reduced Stat3 was detected only in naïve T cells but not in immature thymocytes in the absence of PKC-θ does not support an argument that lower Stat3 expression results from defective T cell development. TCR signals, that are significantly weaker than the signals mediating T cell activation, are required to maintain the homeostasis of T cells (47). It seems likely that PKC-θ mediates weak TCR signals to maintain Stat3 levels in naïve cells. Although our results do not exclude other pathways except NFAT, they do suggest that PKC-θ-regulated signals up-regulate Stat3 transcription via the AP-1 and NF-κB pathways. The fact that Stat3 could be up-regulated by PMA or Th17 stimulation even in PKC-θ−/− T cells suggests there are other factors regulating Stat3 expression in a PKC-θ-independent manner. Therefore, it is likely that PKC-θ stimulates Stat3 expression via cooperation with other signaling pathways. Although previous studies have shown that the highly conserved SBE and CRE regulate Stat3 expression (39, 40), we have excluded these two sites as the response elements for PKC-θ-mediated activation of Stat3 expression (Fig. 5).
The highest Stat3 levels were detected when WT cells were stimulated using Th17 priming conditions, rather than TCR stimulation alone. These conditions also produced the highest levels of phosphorylated PKC-θ in WT cells, suggesting that TGF-β and IL-6 facilitate PKC-θ activation. To date, the mechanisms responsible for cytokine-enhanced activation of PKC-θ have not been fully elucidated. Traditionally, PKC-θ is thought to be activated by diacylglycerol, which is the product of the enzymatic activity of phospholipase Cγ1 (48). However, activation of PKC-θ is also determined by its appropriate translocation to lipid rafts, where clusters of both upstream and downstream TCR signaling molecules are localized (49). PDK1 has been shown to be critical for PKC-θ recruitment to lipid rafts (43). Consistent with this, PI3K, which regulates the activation of PDK1, is also essential for the recruitment of PKC-θ to lipid rafts (50). Interestingly, IL-6 mediates the activation of PI3K via its receptor gp130 (51), which suggests IL-6 may promote PKC-θ activation via PI3K-regulated recruitment of PKC-θ. Our results indicate that PKC-θ is activated by TCR stimulation and greatly enhanced by co-stimulation with TGF-β and IL-6. Under these conditions, Stat3 expression is up-regulated and this, in turn, promotes Th17 differentiation. We observed some up-regulation of expression and phosphorylation of Stat3 in PKC-θ-deficient cells (Fig. 2), suggesting that expression and activation of Stat3 is not absolutely dependent on PKC-θ. IL-6 is sufficient to induce Stat3 activation via the Jak/Stat pathway which is likely to be independent of PKC-θ (52). However, maximal Stat3 activity during Th17 differentiation is likely to be dependent both on IL-6-induced Stat3 phosphorylation and PKC-θ-mediated up-regulation of Stat3 expression. Two recent reports illustrated that differentiated Th17 cells produce GM-CSF effector cytokines critical for development of EAE (5, 6). However, our results do not show defective GM-CSF production in PKC-θ−/− T cells. The resistance of PKC-θ−/− mice to EAE may be due to other defects observed in PKC-θ−/− T cells such as cell activation and survival. It worth mentioning that GM-CSF is unlikely to affect Th17 differentiation directly because T cells do not express GM-CSF receptors (5, 6), but it may indirectly affect Th17 differentiation via stimulation of other cells such as dendritic cells and macrophages. Given the potential involvement of Th17 cells in multiple autoimmune disorders, including multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease (4), our research identifies PKC-θ as a potential therapeutic target for treatment of Th17-dependent autoimmunity.
Acknowledgements
We thank Drs. Amnon Altman and Xin Lin for providing the PKC-θ expression plasmids, Dr. Yanfen Hu for the AP-1 expression plasmids, Dr. Richard Ye for the IKKβ expression plasmids, Dr. Toshio Hirano for providing the STAT3 promoter driven luciferase reporters and Dr. Margaret Morgan for expert editorial assistance.
This work was supported by grants NIH R01-AI053147, NIH R56-AI072554, the Nesvig lymphoma Fellowship and Research Fund and City of Hope.
Abbreviations used in this paper
- AcH4
acetylated histone H4
- ChIP
chromatin immnoprecipitation
- CRE
cAmp response element
- EAE
experimental autoimmune encephalomyelitis
- IKKβ
IκB kinase-β
- MIG
murine stem cell virus internal ribosome entry site green fluorescent protein (GFP)
- PKC-θ
Protein Kinase C-θ
- q-PCR
quantitative PCR
- RORγt
retinoic acid-related orphan receptor gamma t
- SEB
stat3-binding element
- WT
wild type
References
- 1.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 2.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 7.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 10.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 11.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, Shnier R, Portek IJ. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 12.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 13.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 15.Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM, Jr., Schreiber RD. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 21.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, Housseau F, Yu H, Pardoll DM, Drake CG. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, Shea JJO. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manicassamy S, Gupta S, Huang Z, Sun Z. Protein Kinase C-{theta}-Mediated Signals Enhance CD4+ T Cell Survival by Up-Regulating Bcl-xL. J Immunol. 2006;176:6709–6716. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 25.Manicassamy S, Gupta S, Huang Z, Molkentin JD, Shang W, Sun Z. Requirement of calcineurin a for the survival of naive T cells. J Immunol. 2008;180:106–112. doi: 10.4049/jimmunol.180.1.106. [DOI] [PubMed] [Google Scholar]
- 26.Manicassamy S, Gupta S, Sun Z. Selective function of PKC-theta in T cells. Cell Mol Immunol. 2006;3:263–270. [PubMed] [Google Scholar]
- 27.Manicassamy S, Sadim M, Ye RD, Sun Z. Differential Roles of PKC-theta in the Regulation of Intracellular Calcium Concentration in Primary T Cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 28.Manicassamy S, Sun Z. The critical role of protein kinase C-theta in Fas/Fas ligand-mediated apoptosis. J Immunol. 2007;178:312–319. doi: 10.4049/jimmunol.178.1.312. [DOI] [PubMed] [Google Scholar]
- 29.Manicassamy S, Yin D, Zhang Z, Molinero LL, Alegre ML, Sun Z. A critical role for protein kinase C-theta-mediated T cell survival in cardiac allograft rejection. J Immunol. 2008;181:513–520. doi: 10.4049/jimmunol.181.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman A, Kaminski S, Busuttil V, Droin N, Hu J, Tadevosyan Y, Hipskind RA, Villalba M. Positive feedback regulation of PLCgamma1/Ca(2+) signaling by PKCtheta in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol. 2004;34:2001–2011. doi: 10.1002/eji.200324625. [DOI] [PubMed] [Google Scholar]
- 31.Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Protein kinase Ctheta controls Th1 cells in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:7635–7641. doi: 10.4049/jimmunol.175.11.7635. [DOI] [PubMed] [Google Scholar]
- 33.Huang Z, Xie H, Wang R, Sun Z. Retinoid-related orphan receptor gamma t is a potential therapeutic target for controlling inflammatory autoimmunity. Expert Opin Ther Targets. 2007;11:737–743. doi: 10.1517/14728222.11.6.737. [DOI] [PubMed] [Google Scholar]
- 34.Tan SL, Zhao J, Bi C, Chen XC, Hepburn DL, Wang J, Sedgwick JD, Chintalacharuvu SR, Na S. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase C theta-deficient mice. J Immunol. 2006;176:2872–2879. doi: 10.4049/jimmunol.176.5.2872. [DOI] [PubMed] [Google Scholar]
- 35.Huang Z, Xie H, Ioannidis V, Held W, Clevers H, Sadim MS, Sun Z. Transcriptional regulation of CD4 gene expression by T cell factor-1/beta-catenin pathway. J Immunol. 2006;176:4880–4887. doi: 10.4049/jimmunol.176.8.4880. [DOI] [PubMed] [Google Scholar]
- 36.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon MJ, Wang R, Ma J, Sun Z. PKC-theta is a drug target for prevention of T cell-mediated autoimmunity and allograft rejection. Endocr Metab Immune Disord Drug Targets. 2010;10:367–372. doi: 10.2174/1871530311006040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vettese-Dadey M, Grant PA, Hebbes TR, Crane- Robinson C, Allis CD, Workman JL. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. Embo J. 1996;15:2508–2518. [PMC free article] [PubMed] [Google Scholar]
- 39.Ichiba M, Nakajima K, Yamanaka Y, Kiuchi N, Hirano T. Autoregulation of the Stat3 gene through cooperation with a cAMP-responsive element-binding protein. J Biol Chem. 1998;273:6132–6138. doi: 10.1074/jbc.273.11.6132. [DOI] [PubMed] [Google Scholar]
- 40.Narimatsu M, Maeda H, Itoh S, Atsumi T, Ohtani T, Nishida K, Itoh M, Kamimura D, Park SJ, Mizuno K, Miyazaki J, Hibi M, Ishihara K, Nakajima K, Hirano T. Tissue-specific autoregulation of the stat3 gene and its role in interleukin-6-induced survival signals in T cells. Mol Cell Biol. 2001;21:6615–6625. doi: 10.1128/MCB.21.19.6615-6625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baier G. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev. 2003;192:64–79. doi: 10.1034/j.1600-065x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Graham C, Li A, Fisher RJ, Shaw S. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem J. 2002;361:255–265. doi: 10.1042/bj3610255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee KY, D'Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- 44.Barouch-Bentov R, Lemmens EE, Hu J, Janssen EM, Droin NM, Song J, Schoenberger SP, Altman A. Protein kinase C-theta is an early survival factor required for differentiation of effector CD8+ T cells. J Immunol. 2005;175:5126–5134. doi: 10.4049/jimmunol.175.8.5126. [DOI] [PubMed] [Google Scholar]
- 45.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 47.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 49.Bi K, Altman A. Membrane lipid microdomains and the role of PKCtheta in T cell activation. Semin Immunol. 2001;13:139–146. doi: 10.1006/smim.2000.0305. [DOI] [PubMed] [Google Scholar]
- 50.Villalba M, Bi K, Hu J, Altman Y, Bushway P, Reits E, Neefjes J, Baier G, Abraham RT, Altman A. Translocation of PKC[theta] in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J Cell Biol. 2002;157:253–263. doi: 10.1083/jcb.200201097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 52.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]