Abstract
During development, facial branchiomotor (FBM) neurons, which innervate muscles in the vertebrate head, migrate caudally and radially within the brainstem to form a motor nucleus at the pial surface. Several components of the Wnt/planar cell polarity (PCP) pathway, including the transmembrane protein Vangl2, regulate caudal migration of FBM neurons in zebrafish, but their roles in neuronal migration in mouse have not been investigated in detail. Therefore, we analyzed FBM neuron migration in mouse looptail (Lp) mutants, in which Vangl2 is inactivated. In Vangl2 Lp/+ and Vangl2 Lp/Lp embryos, FBM neurons failed to migrate caudally from rhombomere (r) 4 into r6. Although caudal migration was largely blocked, many FBM neurons underwent normal radial migration to the pial surface of the neural tube. In addition, hindbrain patterning and FBM progenitor specification were intact, and FBM neurons did not transfate into other non-migratory neuron types, indicating a specific effect on caudal migration.
Since loss-of-function in some zebrafish Wnt/PCP genes does not affect caudal migration of FBM neurons, we tested whether this was also the case in mouse. Embryos null for Ptk7, a regulator of PCP signaling, had severe defects in caudal migration of FBM neurons. However, FBM neurons migrated normally in Dishevelled (Dvl) 1/2 double mutants, and in zebrafish embryos with disrupted Dvl signaling, suggesting that Dvl function is essentially dispensable for FBM neuron caudal migration. Consistent with this, loss of Dvl2 function in Vangl2 Lp/+ embryos did not exacerbate the Vangl2 Lp/+ neuronal migration phenotype. These data indicate that caudal migration of FBM neurons is regulated by multiple components of the Wnt/PCP pathway, but, importantly, may not require Dishevelled function. Interestingly, genetic-interaction experiments suggest that rostral FBM neuron migration, which is normally suppressed, depends upon Dvl function.
Keywords: Facial branchiomotor neuron migration, Planar Cell Polarity Signaling, Van gogh-like 2, Disheveled, Protein Tyrosine Kinase 7, Looptail
INTRODUCTION
Neuronal migration is an essential aspect of nervous system development, and contributes to the formation of functional neural networks and distinct neural layers in the mammalian brain. Radial migration, which accompanies the maturation of all neurons in the central nervous system, involves cell body translocation from the ventricular proliferative zone towards the outer (pial) surface of the brain, often along radial glial fibers. Several types of neurons also undergo tangential migration, which is glia-independent and orthogonal to the direction of radial migration (Hatten, 2002; Marin and Rubenstein, 2003; Nadarajah and Parnavelas, 2002).
In the embryonic hindbrain, facial branchiomotor (FBM) neurons undergo both radial and tangential migration, and thus make an excellent model system for studying neuronal migration mechanisms. In mice, FBM neurons are born in rhombomere 4 (r4) and by E10.5 begin to migrate caudally (tangentially) into r6, fully forming the facial motor nucleus near the pial surface of r6 by E14.5 (Carpenter et al., 1993; Fritzsch et al., 1993; Garel et al., 2000). Diverse classes of molecules, including members of the non-canonical Wnt/planar cell polarity (PCP) pathway, regulate FBM neuron migration in both zebrafish and mice (Song, 2007). In Xenopus and zebrafish, Wnt/PCP signaling components are required for proper convergence and extension cell movements during gastrulation (Darken et al., 2002; Park and Moon, 2002; Tada et al., 2002; Wallingford and Harland, 2002). In mice, Wnt/PCP proteins are additionally implicated in hair follicle orientation, stereocilia orientation in the inner ear, and neural tube closure (Curtin et al., 2003; Devenport and Fuchs, 2008; Doudney and Stanier, 2005; Kallay et al., 2006; Montcouquiol et al., 2003; Ravni et al., 2009; Torban et al., 2004a; Torban et al., 2004b; Ybot-Gonzalez et al., 2007).
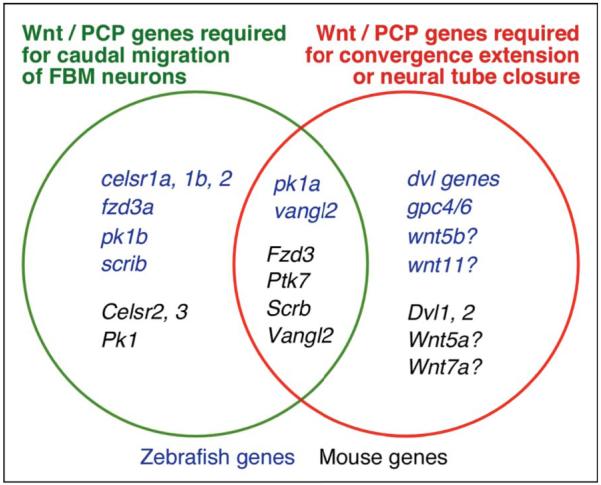
Given their roles in mediating directed cell migrations and cell polarity events in developing tissues, it is not surprising that Wnt/PCP genes also play critical roles in FBM neuron migration. However, it is not clear whether FBM neuron migration is truly a PCP process. In zebrafish, FBM neurons migrate normally in several Wnt/PCP mutants, defective for wnt5b, wnt11, and glypican4/6, which exhibit strong convergence and extension movement (PCP) defects (Bingham et al., 2002; Jessen et al., 2002) (Fig. 8). Conversely, FBM neurons fail to migrate caudally in other Wnt/PCP mutants, defective for fzd3a, celsr2, pk1b, and scrib, which exhibit no convergence and extension defects (Wada et al., 2005; Wada et al., 2006; Mapp et al., 2011) (Fig. 8). These observations suggest that FBM neuron migration and a PCP process like convergence and extension movements are regulated independently in zebrafish. Due to the importance of these cellular movements for nervous system development, we wondered whether these processes were also regulated independently in mammals. Therefore, we examined FBM neuron migration in mouse mutants looptail and chuzhoi, which inactivate Van Gogh-like 2 (Vangl2) and Protein Tyrosine Kinase 7 (Ptk7), respectively, and exhibit strong defects in several PCP processes (Kibar et al., 2001; Lu et al., 2004; Montcouquiol et al., 2006; Murdoch et al., 2001; Paudyal et al., 2010).
Figure 8.
Differential roles for Wnt/PCP genes in regulating FBM neuron migration and neural tube morphogenesis. Mouse (black) and zebrafish (blue) Wnt/PCP genes are categorized based on their roles in regulating caudal migration of FBM neurons or hallmark PCP processes like convergence and extension movements during gastrulation (zebrafish) and neural tube closure (mouse). These data are based on the phenotypes of mouse mutants and zebrafish mutants or morphants, with the exception of zebrafish dvl genes, which were tested using dominant-negative reagents. The “?”s after the Wnt genes signify that double mutants have not been tested, so a putative role in FBM migration cannot be ruled out. Zebrafish data from Wada et al., 2006 (celsr1a, 1b, 2, fzd3a), Mapp et al., 2010, 2011 (pk1b), Wada et al., 2005 (scrib), Carreira-Barbosa et al., 2003 (pk1a), Jessen et al., 2002 (vangl2, wnt5b, wnt11), Bingham et al., 2002 (gpc4/6), and this report (dvl genes). Mouse data from Vivancos et al., 2009 (Vangl2, Fzd3, Scrb, Wnt5a, Wnt7a), Qu et al., 2010 (Celsr2, 3, Fzd3), and this report (Vangl2, Ptk7, Dvl1, 2). Mouse Pk1 knockout phenotype (B. Fritzsch, personal communication).
Consistent with observations that FBM neuron migration and PCP processes may be regulated independently, it appears that Wnt/PCP signaling may itself play only a minor role in FBM neuron migration. First, although Wnt5a- and 7a-coated beads can attract FBM neurons in hindbrain explants, these neurons still migrate caudally in Wnt5a and Wnt7a mutant embryos (Vivancos et al., 2009), suggesting a redundant or minor role for Wnts in this process, as shown previously with zebrafish wnt5b and wnt11 mutants (Jessen et al., 2002). Second, abrogation of PCP-specific Dishevelled (Dvl) function in zebrafish using a dominant negative approach had no effect on FBM neuron migration (Jessen et al., 2002), suggesting that Dvl function may be dispensable for caudal migration. We have tested this hypothesis by extending the Dvl dominant-negative analysis in zebrafish, and by examining FBM neuron migration in Dvl knockout mice.
Vivancos et al. (2009) found that FBM neurons failed to migrate caudally in Vangl2 mutant mice harboring the looptail (Lp) S464N mutation (Murdoch et al., 2001). We report here that in a different Lp mutant (D255E; Kibar et al., 2001), and also in Vangl2 knockout mice (Song et al., 2010), caudal migration of FBM neurons was abolished. FBM neurons also failed to migrate in Ptk7 mutant embryos. Importantly, FBM neurons migrated normally in Dvl1/2 double mutants, and in zebrafish embryos with disrupted dvl signaling. Our data demonstrate that FBM neuron migration and PCP processes are regulated independently in mouse, as in zebrafish, and that Dvl function is largely dispensable for caudal FBM neuron migration (Fig. 8). Interestingly, genetic-interaction experiments suggest that rostral FBM neuron migration, which is normally suppressed, depends upon Dvl function.
MATERIALS AND METHODS
Animals
Mouse colonies were maintained and embryos collected according to the requirements of the Animals (Scientific Procedures) Act 1986 of the UK Government, and various institutional guidelines, including those of the University of Missouri Animal Care and Use Committee (ACUC). Zebrafish colonies were maintained and embryos collected following standard protocols approved by the University of Missouri ACUC.
Mouse lines and genotyping
The Vangl2Lp-m1Jus, Vangl2del, Celsr1Crsh, Dvl1tm1Awb, Dvl2tm1Awb, Ptk7chuzhoi, and SE1::gfp mouse lines have been described previously (Curtin et al., 2003; Hamblet et al., 2002; Kibar et al., 2001; Lijam et al., 1997; Shirasaki et al., 2006; Song et al., 2010). The Dvl1 and Dvl2 lines were purchased from Jackson Labs. Genotyping of the Vangl2Lp-m1Jus allele (referred to as Vangl2Lp, from here on) was performed using primers 5′-GGTTCAGTTTGCCGTTTCTC-3′ and 5′-CCCTCCTTCCCCTAAACCTT-3′, followed by sequencing of the PCR product to identify the T1228A mutation. Genotyping of Celsr1Crsh was performed similarly using primers 5′-AATTCAGGTGAGTGTGTTGGA-3′ and 5′-GTCACCACTCAGTAGGTCCAG-3′ to identify the A3119G mutation.
For timed matings, noon on the day of a copulation plug was defined as embryonic day (E)0.5. Embryos were staged using standard morphological criteria (Nagy, 2003) before fixation.
Zebrafish
Maintenance of zebrafish stocks, and collection and development of embryos in embryo medium were carried out as described previously (Bingham et al., 2002; Westerfield, 1995). To facilitate analysis of branchiomotor neuron development, Tg(isl1:GFP) fish (Higashijima et al., 2000) were used for all experiments.
RNA injection
The expression constructs encoding different Dishevelled (Dvl) and Daam1 proteins in pCS2 were obtained from indicated sources by Drs. Oni Mapp and Victoria Prince (University of Chicago) and kindly provided to us: Xenopus Dvl full-length, and Dvl deltaC (Tada and Smith, 2000); Dvl delta PDZ (Xdd1), Dvl delta N, and human N-Daam1 (Habas et al., 2001). RNAs were prepared and injected as described previously (Sittaramane et al., 2009). Embryos at the 1-4 cell stage were injected with RNA (200 pg/embryo) and examined at 24-48 hours post fertilization for FBM neuron migration phenotypes. To monitor the amounts of protein produced from the various RNAs, ~50 embryos per treatment were collected at 20 hpf, when FBM neurons are migrating, solubilized in lysis buffer, and processed for Western blot analysis with anti-myc (all Dvl constructs) and anti-FLAG (N-Daam1) antibodies (anti-myc: Cell Signaling Technologies; anti-FLAG: Sigma-Aldrich) (Fig. S6).
In situ hybridization
Hindbrains were processed for in situ hybridization as described previously (Qu et al., 2010). For imaging, hindbrains were either mounted in open book preparations or embedded in OCT (Ted Pella) for cryostat sectioning. Riboprobes used in this study were Dvl3 (Tissir and Goffinet, 2002), Gata3 (Karis et al., 2001), Hb9 (Qu et al., 2010), Mash1 and Math3 (Tiveron et al., 2003), Pk1 (Song et al., 2006), Tbx20 (Kraus et al., 2001), Vangl2 (Tissir and Goffinet, 2006), and Wnt5a (Song et al., 2006).
Immunohistochemistry
For NF160 neurofilament staining, mouse embryos were processed as previously described (Qu et al., 2010). Embryos were stored in 75% glycerol and imaged on an Olympus SZX12 stereomicroscope. Whole-mount immunohistochemistry of zebrafish embryos (zn5, GFP, myc antibodies) was performed and imaged as described previously (Sittaramane et al., 2009).
Demarcation of Rhombomere Boundaries
Rhombomere boundaries in the mouse wild-type hindbrain were defined using several criteria: In E12.5 embryos processed for Tbx20 in situs, the caudal limit of the trigeminal (nV) motor neurons was defined as the r2/3 boundary, the rostral edge of the FBM neuron expression domain was defined as the r3/4 boundary, and the sharp indentation in the lateral edge of the migrating column was defined as the r4/5 boundary (Fig. 2D). For E14.5 embryos, the approximate locations of r2, r5 and r6 were defined by the positions of the nV (r2) and FBM (r5, r6) neurons, respectively (Fig. 2G). For E12.5 embryos expressing GFP in cranial motor neurons, the r3/4 and r4/5 boundaries were defined as for Tbx20 in situs. These boundaries also coincided with the fan-shaped genu of FBM axons extending laterally toward their exit points (Fig. 2A), and with the location of the abducens motor nucleus (nVI) in r6 (Fig. 2B).
Figure 2.
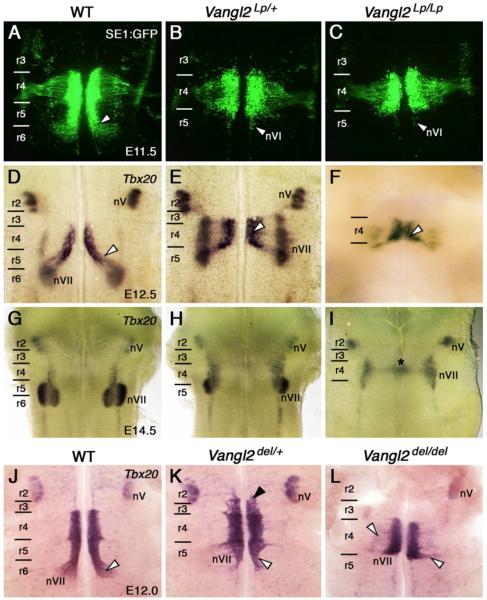
FBM neurons fail to migrate caudally in looptail mutants and Vangl2 knockout embryos. The trigeminal motor nucleus (nV) is located in r2 in all embryos. A-C, Ventricular views of SE1::gfp hindbrains. In a WT embryo (A), FBM neurons (white arrowhead) migrate caudally from r4 into r6 and radially away from the midline. In Vangl2Lp/+ (B) and Vangl2Lp/Lp embryos (C), FBM neurons fail to migrate out of r4. GFP-expressing cells in r5 are abducens motor neurons (nVI; white arrowheads). D-F, Ventricular views of hindbrains processed for Tbx20 ISH. In a WT embryo (D), FBM neurons migrate caudally from r4 into r6 (white arrowhead), then migrate radially to form the facial motor nucleus (nVII) in r6. In Vangl2Lp/+ embryos (E), most Tbx20-expressing cells remain in r4 in medial and lateral positions, reflecting a near-failure of caudal migration. In Vangl2Lp/Lp embryos (F), caudal migration is completely abolished, with all FBM neurons located medially within r4. The apparent fusion of FBM populations across the midline may result from the defective floorplate and open neural tube in mutants. G-I, Pial views. In a WT embryo (G), FBM neurons have completed migration into r5/r6 to form the facial motor nucleus (nVII). In Vangl2Lp/+ embryos (H), the facial motor nucleus (nVII) is elongated within r4/r5, and is confined entirely to r4 in Vangl2Lp/Lp embryos (I), with some fused clusters (asterisk) at the midline. J-K, Ventricular views of hindbrains processed for Tbx20 ISH. In a WT embryo (J), FBM neurons (white arrowhead) migrate in characteristic fashion. In a Vangl2del/+ embryo (K), FBM neurons span r3 (black arrowhead) to r5 (white arrowhead), and do not migrate into r6, with most of the neurons confined to r4. In a Vangl2del/del embryo (L), FBM neurons do not migrate out of r4, with some cells migrating radially within r4 (white arrowheads).
Rhombomere boundaries in mouse mutant hindbrains were assumed to be coincident with those in wild-type embryos because the identities and sizes of rhombomere compartments (r3-r5) were not affected in Vangl2Lp/+, Vangl2Lp/Lp, Celsr1Crsh/+ and Celsr1Crsh/Crsh embryos (Qu et al., 2010; Thoby-Brisson et al., 2012). One exception was Dvl1/2 double mutant embryos where r3 appeared to be longer at the expense of r4 (Fig. 6D), with no appreciable change relative to wild-type in the overall length of the hindbrain region spanning r2-r6.
Figure 6.
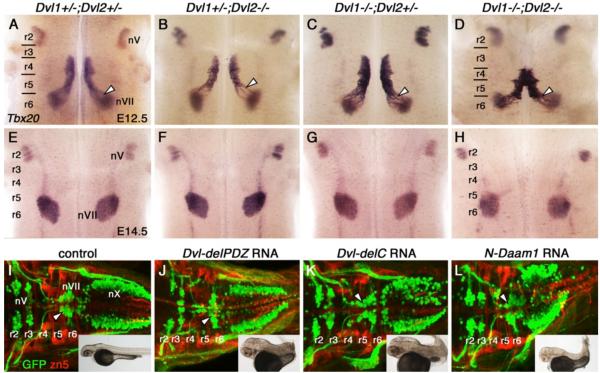
Caudal migration of FBM neurons is not affected by loss of Dishevelled function. Ventricular (A-D) and pial (E-H) views of embryos processed for Tbx20 ISH. In E12.5 control Dvl1+/-;Dvl2+/- embryos (A), FBM neurons migrate caudally from r4 into r6 (arrowhead) to form the facial motor nucleus (nVII). This caudal migration occurs normally in Dvl1+/-;Dvl2-/- (B), Dvl1-/-;Dvl2+/- (C), and Dvl1-/-;Dvl2-/- mutants (D), inspite of neural tube closure defects in many of these embryos. By E14.5, FBM neurons in control embryos (E) have completed their caudal and radial migrations to form facial motor nuclei (nVII) in r6. Likewise, these nuclei are formed in r6 in Dvl-deficient embryos including Dvl1+/-;Dvl2-/- (F, 2/2 embryos), Dvl1-/-;Dvl2+/- (G, 6/6 embryos), and Dvl1-/-;Dvl2-/- mutants (H, 4/4 embryos). Neural tube defects were seen in Dvl1-/-;Dvl2-/- mutants. I-L, Dorsal views of 48 hpf Tg(isl1:gfp) zebrafish embryos processed for anti-GFP and zn5 immunohistochemistry. In control embryos (I), GFP-expressing FBM neurons migrate caudally from r4 into r6 (arrowhead) and r7 to form the facial motor nucleus (nVII). Zn5 staining labels rhombomere boundaries. The trigeminal (nV) and vagal (nX) motor neurons are located in r2 and r3, and the caudal hindbrain, respectively. Inset shows intact embryo with normal extension of the body axis. In embryos injected with Dvl-delPDZ (J), Dvl-delC (K), and N-Daam1 (L) RNA (200 pg/embryo), FBM neurons are able to migrate caudally (arrowheads) out of r4 despite convergence and extension defects resulting in a shortened body axis (insets). Positions of the nV and nX neurons are not affected by these treatments.
Rhombomere boundaries in zebrafish were established based on the characteristic patterns of zn5+ve commissural axon fascicles (Bingham et al., 2002).
RESULTS
FBM neurons fail to migrate caudally in Vangl2 mutants
In mouse, facial branchiomotor (FBM) neurons start differentiating in rhombomere (r) 4 at E10.5, as shown by expression of Tbx20, a marker for branchiomotor and visceromotor neurons (Coppola et al., 2005), (Fig. 1A; Song et al., 2006), and start migrating caudally shortly thereafter. By E12, there is a continuous stream of FBM neurons migrating caudally out of r4 into r5 and r6, where they undergo radial migration toward the pial surface (Fig. 1B; Garel et al., 2000). Previous studies in zebrafish and mice have demonstrated roles for several Wnt/PCP genes in FBM neuron migration (Bingham et al., 2002; Carreira-Barbosa et al., 2003; Qu et al., 2010; Vivancos et al., 2009; Wada et al., 2006). The Wnt/PCP gene Vangl2 is expressed in FBM neurons and in adjacent tissues during the migration period (Fig. 1C-F; 6 embryos per time point; compare Fig. 1F and Fig. S2A’ to visualize FBM neuron location in hindbrain cross section; see also Fig. 8 in Song et al., 2006). Therefore, we tested whether FBM neuron migration was affected in looptail (Lp) mutant embryos harboring a D255E mutation in the C-terminal cytoplasmic domain of Vangl2 (Torban et al., 2004b) that abolishes localization to the plasma membrane (Torban et al., 2007).
Figure 1.
Vangl2 expression in the hindbrain. A-D, Ventricular views of flat-mounted hindbrains processed for in situ hybridization (ISH) with Tbx20 (A-B) and Vangl2 (C-D) probes. At E10.5 (A), Tbx20 is expressed in trigeminal branchiomotor neurons in r2-r3 and facial branchiomotor (FBM) neurons in r4. By E12.0 (B), Tbx20 expression is maintained in laterally-migrated trigeminal branchiomotor neurons in r2-r3 and caudally migrating FBM neurons spanning r4-r6. At the onset of FBM neuron migration, E10.5 (C) and E11.5 (D), Vangl2 mRNA is expressed at all axial levels of the hindbrain. Stronger expression is seen near the midline corresponding to the location of the FBM neurons. E-F, Coronal sections (r4 level) of embryos in C and D. At E10.5 (E), Vangl2 is expressed in the ventricular zone (white arrowhead) and in the FBM neuron domain (dotted circle), with low expression in the floorplate (black arrowhead). At E11.5 (F), Vangl2 expression is maintained in the ventricular zone (white arrowhead) and in the FBM neuron domain (dotted circle), and reduced in floorplate cells (black arrowhead).
We first examined FBM neuron migration in the SE1::gfp background, in which all cranial motor neurons express GFP (Song et al., 2006). In WT embryos at E11.5 and E12.5, FBM neurons formed longitudinal streams of migrating cells spanning r4 to r6, then migrated dorso-laterally and radially within r6 (Fig. 2A, Fig. S1A; n=10 embryos). In contrast, FBM neurons largely failed to migrate caudally out of r4 in both Vangl2Lp/+ and Vangl2Lp/Lp embryos (Fig. 2B, C; Lp/+ (n=17), Lp/Lp (8); Table S1), although some neurons migrated dorso-laterally, especially in the vicinity of the r4/r5 boundary (Fig. S1B, C). Interestingly, some GFP+ve cells were also found laterally in r3 in several Vangl2Lp/+ and Vangl2Lp/Lp embryos, but never in WT embryos, suggestive of aberrant rostral migration (Fig. S1; Lp/+ (39/47), Lp/Lp (9/26); Table S1). Tbx20 in situs revealed that by E12.5, WT FBM neurons migrated radially towards the pial surface within r6 to form the facial motor nucleus (nVII) (Fig. 2D; Fig. S2A-A”; n=7), which was fully formed by E14.5 (Fig. 2G; n=3). Interestingly, although FBM neurons in Vangl2Lp/+ remained mostly confined to r4, they still underwent their dorso-lateral and radial migrations to form elongated nuclei at the pial surface, spanning r3 to r5 (Fig. 2E, H; Fig. S2B-B”; E12.5 (n=8), E14.5 (4)). In Vangl2Lp/Lp mutants, FBM neurons remained within r4 and most failed to migrate radially to the pial surface of the neural tube (Fig. 2F, I; Fig. S2C-C”; E12.5 (n=3), E14.5 (6); see also Vivancos et al., 2009). Since craniorachischisis (open neural tube) occurs in Vangl2Lp/Lp, but not Vangl2Lp/+ embryos (Murdoch et al., 2001; Torban et al., 2004b; Ybot-Gonzalez et al., 2007), the failure of FBM neurons to migrate caudally in looptail mutants is not a secondary consequence of neural tube defects (Table S1).
The failure of FBM neuron migration in Vangl2Lp/+ embryos may reflect a dominant negative nature of the Vangl2Lp allele, which encodes a protein that fails to reach the plasma membrane (Iliescu et al., 2011; Torban et al., 2007). Therefore, we also examined FBM neuron migration in Vangl2 knockout mice (Vangl2del/del), where no Vangl2 protein is detected (Song et al., 2010). No FBM neurons migrated caudally in Vangl2del/del embryos (Fig. 2L; n=5). While a significant number of FBM neurons migrated caudally in Vangl2del/+ embryos and formed a facial motor nucleus in r6, a large number of neurons also failed to migrate out of r4 (Fig. 2K; data not shown; n=11). In a few Vangl2del/+ embryos (3/11), Tbx20+ve cells were found in r3 and r2 (Fig. 2K), suggestive of aberrant rostral migration (Table S1). Together, the Vangl2Lp/+ and Vangl2del/+ phenotypes suggest that the caudal migration defects in these embryos result from haploinsufficiency.
FBM neurons are specified and differentiate normally in Vangl2Lp mutants
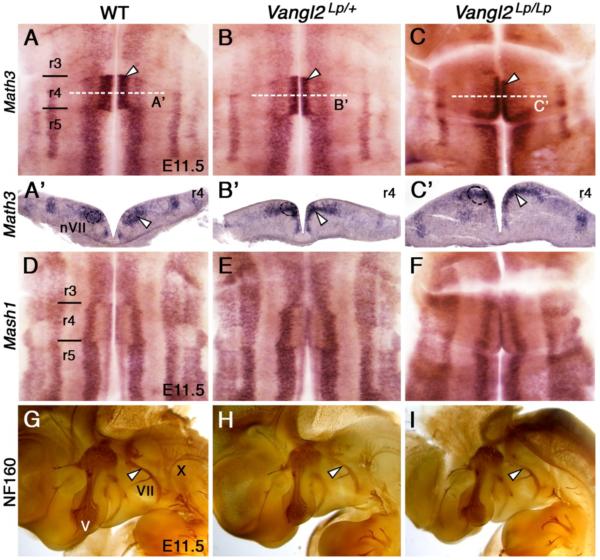
We tested whether the FBM migration defects in Vangl2Lp mutants were a consequence of improper progenitor specification. Math3 and Mash1 encode transcription factors essential for FBM progenitor specification and their subsequent migration, and are expressed in longitudinal domains in the ventricular zone (Ohsawa et al., 2005; Tiveron et al., 2003). These expression domains were not affected in Vangl2Lp/+ and Vangl2Lp/Lp embryos (Fig. 3B-F; Math3: Lp/+ (n=3), Lp/Lp (2); Mash1: Lp/+ (2), Lp/Lp (2)), indicating that mutant FBM neurons are specified correctly. Furthermore, FBM neurons in both WT and Vangl2Lp mutants expressed Phox2b, another branchiomotor and visceromotor differentiation marker (Pattyn et al., 2000), indicating that the non-migrating cells in the Vangl2Lp mutants differentiate normally (Fig. S3A-C; WT (n=8), Lp/+ (7), Lp/Lp (3)). Interestingly, Ret7, a GDNF receptor that is normally expressed by FBM neurons in r6 (Garel et al., 2000), was expressed by non-migrated neurons in r4 of Vangl2Lp/+ and Vangl2Lp/Lp embryos (Fig. S3H, I; WT (n=3), Lp/+ (4), Lp/Lp (2)), suggesting that the entire program of FBM neuron differentiation proceeds normally in the absence of caudal migration.
Figure 3.
Normal hindbrain development in looptail mutants. A-F, ventricular views; A’-C’, coronal sections. Math3 expression in r4 (white arrowhead) is predominantly in the motor neuron progenitor domain (pMN, dotted circle) in WT (A, A’) embryos, and is unaffected in Vangl2Lp/+ (B, B’) and Vangl2Lp/Lp (C, C’) embryos. Mash1 expression in medial aspect of r4 is also limited to the pMN domain in the WT embryo (D), and is unaffected in Vangl2Lp/+ (E), and Vangl2Lp/Lp (F) embryos. G-I, Lateral views, rostral to the left, of NF160-stained embryos. In a WT embryo (G), the trigeminal (V) and facial (VII, white arrowhead) nerves project into the first and second branchial arches, respectively, and the vagus (X) nerve exits from the caudal hindbrain. These projections are essentially normal in Vangl2Lp/+ (H) or Vangl2Lp/Lp (I) embryos.
Immunostaining of peripheral nerves with a neurofilament antibody (NF160) revealed similar projection patterns for the trigeminal (nV) and facial (nVII) nerves in E11.5 embryos of all genotypes (Fig. 3G-I; WT (n=2), Lp/+ (3), Lp/Lp (2)), indicating that despite a failure of FBM neurons to migrate, their axons project and branch correctly, and innervate peripheral targets in mutant embryos. To investigate this further, we applied NeuroVue dyes (Fritzsch et al., 2005) in the second branchial arch to retrogradely label FBM neuron cell bodies through their axonal projections. Consistent with NF160 staining, FBM neurons were found in r4 in Vangl2Lp/+ and Vangl2Lp/Lp mutants, although they were reduced in number in the latter (Fig. S3D-F; Lp/+ (n=3), Lp/Lp (2)). This reduction may result from open neural tube defects in Vangl2Lp/Lp embryos rather than due to failure of caudal migration, since the numbers of back-filled FBM neurons were qualitatively similar in wild-type and Vangl2Lp/+ embryos even though caudal migration was nearly absent in heterozygotes (Fig. S3D, E).
Finally, we investigated whether the Vangl2Lp mutation affected the development of other neuronal types in the r4-r5 region. The rhombomere markers Hoxb1 (r4) and Krox20 (r3/r5) were expressed normally in Vangl2Lp/+ and Vangl2Lp/Lp embryos (see Fig. 1 in Thoby-Brisson et al., 2012), indicating that rhombomere patterning is not affected by the failure of the neural tube to close in Vangl2Lp/Lp mutants, and that FBM neuron migration defects are not due to defective hindbrain patterning. Rhombomere 4 generates both FBM and inner ear efferent (IEE) neurons. Whereas FBM neurons migrate caudally out of r4, IEE neurons, which express Gata3 (Karis et al., 2001), are confined to r4 (Fritzsch et al., 1993). In r5, the non-migratory abducens motor neurons express the somatomotor neuron marker Hb9 (Thaler et al., 1999). In WT embryos, Gata3 was found in a high-expressing medial domain adjacent to the floorplate and a weaker-expressing lateral domain, but not expressed in FBM neurons (Fig. S4A, A’; n=6). This expression pattern was not affected in Vangl2Lp/+ embryos (Fig. S4B, B’; Lp/+ (n=8)), and was slightly reduced in the r4 medial domain in Vangl2Lp/Lp embryos (Fig. S4C, C’; Lp/Lp (n=1)), correlating with the absence of Tbx20-expressing IEE neurons (Fig. S2C, C’). Importantly, however, Gata3 was not expressed ectopically in FBM neurons (Fig. S4B-C’). Similarly, Hb9 was not ectopically expressed in FBM neurons in r4 of Vangl2Lp/+ and Vangl2Lp/Lp embryos (Fig. S4D-F; Lp/+ (n=10), Lp/Lp (5)). Together, these results indicate that the failure of FBM neurons to migrate caudally in Vangl2Lp mutants is not due to a misspecification as non-migratory neurons.
Wnt/PCP genes are expressed normally in Vangl2Lpmutants
In mouse Tbx20 mutants, FBM neurons fail to migrate caudally and poorly express several Wnt/PCP genes, including Vangl2, Prickle1 (Pk1) and Wnt11 (Song et al., 2006). In zebrafish, pk1b is expressed in FBM neurons, and is necessary for caudal migration (Rohrschneider et al., 2007). In addition, Wnt5a is expressed in the vicinity of FBM neurons and may function as an attractive cue for caudal migration (Song et al., 2006; Vivancos et al., 2009). Therefore, we examined the expression of Wnt5a and Pk1 in Vangl2Lp mutants. In WT hindbrains, Wnt5a was expressed at the midline up to r3, then broadly in the neuroepithelium from r5 and caudally, with a sharp boundary at the r4/r5 border (Fig. 4A; n=4; Vivancos et al., 2009). In Vangl2Lp/Lp mutants (Fig. 4C; n=4), the boundary was less clear and overall expression levels appeared lower. However, Wnt5a expression in Vangl2Lp/+ embryos (Fig. 4B; n=4), which have FBM migration defects, was indistinguishable from WT, indicating that the caudal migration defect in Vangl2Lp/Lp mutants is not due to altered Wnt5a expression. Pk1 was expressed in FBM neurons in both WT and Vangl2Lp mutants (Fig. 4D-F; WT (n=2), Lp/+ (2), Lp/Lp (2)). Together, these data suggest that defective FBM neuron migration in Vangl2Lp mutants does not result from altered Wnt/PCP gene expression.
Figure 4.
Normal expression of Wnt/PCP genes in looptail mutants. In a WT embryo (A), Wnt5a is expressed in r5 and caudally, with a sharp r4/r5 boundary (black arrowhead). The rostral domain of Wnt5a expression (black arrow) extends into r3. Wnt5a expression is normal in Vangl2Lp/+ embryos (B). In a Vangl2Lp/Lp embryo (C), Wnt5a expression is reduced, although a boundary and the medial expression domain (arrow) are intact despite neural tube defects. In a WT embryo (D), Prickle1 is expressed in FBM neurons along their migratory route spanning r4 through r6. In Vangl2Lp/+ (E) and Vangl2Lp/Lp (F) embryos, Prickle1-expressing FBM neurons are confined to r4.
Ptk7, which interacts genetically with Vangl2, is necessary for FBM neuron migration
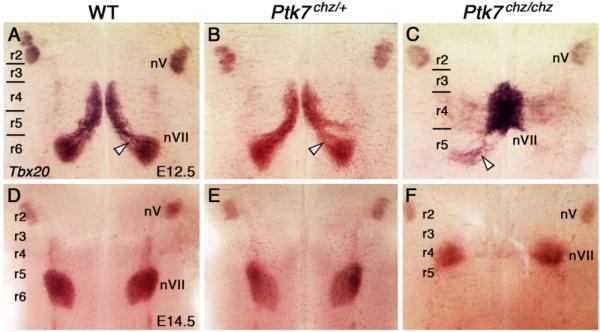
Ptk7 is a PCP gene encoding a transmembrane, tyrosine kinase-like protein (Mossie et al., 1995) that genetically interacts with Vangl2 during PCP events like neural tube closure and stereociliary bundle orientation in the inner ear (Lu et al., 2004; Paudyal et al., 2010). Therefore, we examined FBM neuron migration in the mouse Ptk7 mutant chuzhoi (chz), in which the level of soluble Ptk7 is greatly reduced (Paudyal et al., 2010). In E12.5 Ptk7chz/chz mutants (n=6), Tbx20 in situs showed that most FBM neurons failed to migrate caudally out of r4 (Fig. 5). In some embryos (4/6), a few FBM neurons migrated caudally to a similar extent as WT (compare Fig. 5C to 5A). By E14.5, FBM neurons had completed dorso-lateral migration to form the facial motor nucleus (nVII) near the pial surface in r6 of WT and Ptk7chz/+ embryos (Fig. 5D, E; WT (n=8), chz/+ (7)), and in r4 of Ptk7chz/chz embryos (Fig. 5F; n=8), demonstrating a clear role for Ptk7 in caudal, but not dorso-lateral, migration of FBM neurons in mice.
Figure 5.
FBM neurons fail to migrate caudally in chuzhoi mutants. Ventricular (A-C) and pial (D-F) views of embryos processed for Tbx20 ISH. In E12.5 WT (A) and Ptk7chz/+ (B) embryos, FBM neurons migrate caudally from r4 into r6 (arrowheads) to form the facial motor nucleus (nVII). In Ptk7chz/chz mutants (C), FBM neurons completely or largely fail to migrate out of r4, with a few neurons migrating caudally into r5 (white arrowhead). By E14.5, FBM neurons in WT (D) and Ptk7chz/+ (E) embryos have completed their caudal and radial migrations to form facial motor nuclei (nVII) in r6. In Ptk7chz/chz mutants (F), the facial motor nuclei are rostrally displaced and located in r4 (8/8 embryos). The location of the trigeminal motor neurons (nV) in r2 is not affected in mutants.
Dishevelled function is dispensable for caudal migration of FBM neurons
In mice, Wnt5a and Wnt7a can act as chemoattractants for FBM neurons in an explant assay; however, their in vivo roles are less clear since FBM neurons migrate caudally out of r4 in Wnt5a and Wnt7a mutants, with a slight defect in dorso-lateral migration in r5/r6 in Wnt5a mutants (Vivancos et al., 2009). In zebrafish, several Wnt/PCP genes (vangl2, pk1a, pk1b, fzd3a, celsr1-3, scrib) are necessary for FBM neuron migration (Bingham et al., 2002; Carreira-Barbosa et al., 2003; Jessen et al., 2002; Rohrschneider et al., 2007; Wada et al., 2005; Wada et al., 2006), but wnt5b and wnt11 appear to be dispensable (Jessen et al., 2002). Importantly, over-expression of Xdd1 (Sokol, 1996), a dominant negative form of Dishevelled (Dvl), the central downstream signaling mediator of the Wnt/PCP pathway, has no effect on caudal migration of FBM neurons (Jessen et al., 2002). To test directly a role for Dvl function in mice, we examined FBM neuron migration in Dvl knockout mice (Etheridge et al., 2008; Hamblet et al., 2002; Wang et al., 2005).
E12.5 and E14.5 embryos from Dvl1+/-; Dvl2+/- incrosses were collected and processed for Tbx20 in situs. Combinatorial deletion of multiple copies of Dvl1 and Dvl2 did not disrupt caudal migration of FBM neurons (E12.5: Fig. 6A-C; E14.5: Fig. 6E-G), even in Dvl1+/-; Dvl2-/- embryos (n=6), some of which exhibit hindbrain neural tube defects (3/6). Importantly, FBM neurons also migrated caudally into r6 in Dvl1/2 double mutants (Fig. 6D, H; n=4 for each age), which exhibit profound PCP defects including a fully open neural tube and misorientation of stereociliary bundles (Etheridge et al., 2008; Hamblet et al., 2002; Wang et al., 2005; Table S1). Similar to Vangl2Lp/Lp, FBM neurons in r4 of double mutants were initially fused at the midline (Fig. 6D). By E14.5, however, no Tbx20-expressing cells were found at the midline, and a well-defined facial motor nucleus was formed in r6 (Fig. 6H), indicating that FBM neurons undergo essentially normal caudal and radial (dorso-lateral) migrations in Dvl1; Dvl2 double knockout embryos. Since three Dvl genes (1-3) are expressed in partially overlapping patterns in the mouse embryo (Tissir and Goffinet, 2006), Dvl3 could potentially compensate for loss of Dvl1/2 function in FBM neurons in double mutants. However, Dvl3 expression is not affected in double mutants, and is restricted to the ventricular zone adjacent to the migrating FBM neurons at E12.5 (Fig. S5B; n=2) and post-migrated neurons at E14.5 (Fig. S5D; n=1). These results suggest strongly that caudal migration does not require Dvl function in FBM neurons.
Our previous studies with a dominant negative Dishevelled construct (Xdd1) in zebrafish embryos also suggested that FBM neuron migration is Dvl-independent (Jessen et al., 2002). To further validate these findings, we interfered with Dvl signaling using additional constructs, and examined their effects on caudal migration of FBM neurons using Tg(isl1:gfp) zebrafish embryos, which express GFP in branchiomotor neurons (Higashijima et al., 2000). Western blot analysis confirmed the presence of mutant proteins in 20 hpf embryos during the period of neuronal migration (Fig. S6B). For embryos injected with Dvl-delPDZ RNA, we confirmed by whole-mount immunostaining the presence of myc-tagged protein both in the motor neurons and in adjacent cells (Fig. S6C). Similar to mice, zebrafish FBM neurons are born in r4 and migrate caudally into r6 and r7 (Fig. 6I; Chandrasekhar, 2004; Chandrasekhar et al., 1997; Higashijima et al., 2000). While over-expression of the dominant negative Dvl construct (Xdd1/Dvl-delPDZ; Tada and Smith, 2000) generated convergence and extension (CE) defects during gastrulation as expected, resulting in a shortened body axis, caudal migration of FBM neurons was not affected (Fig. 6J; Jessen et al., 2002). Importantly, over-expression of additional dominant negative constructs, Dvl-delC (lacking the DEP domain and C-terminal; Tada and Smith, 2000) and N-Daam1 (lacking Formin-homology domains to bind Dvl and activate downstream signaling; Habas et al., 2001), generated CE defects, but failed to disrupt caudal FBM neuron migration (Fig. 6K, L; summarized in Fig. S6A). Since the dominant negative constructs would interfere with Dvl-mediated signaling notwithstanding the expression of multiple Dvl genes, these data suggest strongly that caudal migration of FBM neurons does not require Dishevelled function.
Since FBM neuron migration is Vangl2-dependent (Fig. 2), and Vangl2 and Dvl physically interact (Torban et al., 2004b), we further tested a potential role for Dvl function in migration by examining whether Dvl2 and Vangl2 interact genetically for this process. It was demonstrated previously that Dvl2 and Vangl2Lp interact genetically during neurulation, with Vangl2Lp/+; Dvl2-/- embryos showing craniorachischisis while Vangl2Lp/+; Dvl2+/- embryos have closed neural tubes (Wang et al., 2006). Therefore, we tested whether a reduction in Dvl signaling by lowering Dvl2 copy number exacerbated the FBM migration defect of Vangl2Lp/+ embryos. Removal of one or both copies of Dvl2 in a Vangl2+/+ background had no effect on migration of FBM neurons out of r4 (Fig. 7B, C; Dvl2+/- (n=14), Dvl2-/- (14); pooled data), although Dvl2-/- mice exhibited mild PCP defects (Hamblet et al., 2002). Importantly, many FBM neurons migrated in thick streams into r5 in both Vangl2Lp/+; Dvl2+/- (5/5 embryos; Fig. 7E) and Vangl2Lp/+; Dvl2-/- embryos (5/5 embryos; Fig. 7F), similar to the phenotype of Vangl2Lp/+ embryos (Fig. 7D; n=4), suggesting that Vangl2 and Dvl2 do not genetically interact to regulate caudal FBM neuron migration. Since Vangl2Lp/+; Dvl2-/- embryos had fully open neural tubes (Wang et al., 2006; data not shown), the clusters of non-migrated FBM neurons in r4 were fused across the midline (Fig. 7F), as in Vangl2Lp/Lp (Fig. 7G) and Dvl1-/-; Dvl2-/- embryos (Fig. 6D), giving the appearance of a stronger migration defect than in Vangl2Lp/+; Dvl2+/- embryos (Fig. 7E). Interestingly, putative migration of FBM neurons into r3, noted in some Vangl2Lp/+ embryos (Fig. 7D; 3/4), was never seen in Vangl2Lp/+; Dvl2-/- embryos, suggesting that the ectopic migration may be Dvl-dependent.
Figure 7.
Genetic interactions between Vangl2Lp, Dvl2, and Celsr1Crsh for FBM neuron migration. The caudal migratory streams of FBM neurons are labeled with white arrowheads, while rostrally-migrating neurons in Celsr1-deficient embryos are labeled with black arrowheads. The location of the trigeminal (nV) motor nucleus (demarcating r2) is noted with an asterisk in every preparation except G. A-C, Removing Dvl2 genes from a wild-type background (A) did not affect FBM neuron migration (B, C). D-F, Removing Dvl2 genes from a Vangl2Lp/+ background (D), did not exacerbate the migration defect of Vangl2Lp/+ embryos, with thick streams of FBM neurons migrating caudally into r5 (E, F), compared to Vangl2Lp/Lp embryos (G). Fusion of FBM clusters across the midline may be a consequence of defects in neural tube and floorplate development in these embryos. H-J, While removing one copy of Dvl2 had no effect on the abnormal rostral migration of FBM neurons (black arrowhead, I), as seen in Celsr1Crsh/+ embryos (H), removing the second copy of Dvl2 completely blocked rostral migration (J), but not caudal migration. Rostral migration was also blocked in Vangl2Lp/+; Celsr1Crsh/+ embryos (K).
To test this possibility, we crossed Dvl2 mutants with Crash mice, which carry a mutation in an extracellular cadherin repeat of the atypical cadherin Celsr1 (Torban et al., 2007) that results in a subset of FBM neurons migrating rostrally into r2 and r3 (Qu et al., 2010). In Celsr1Crsh/+ embryos, a significant number of FBM neurons migrated rostrally into r2/r3, although most migrated caudally into r6 (Fig. 7H; n=13; Table S1; Qu et al., 2010). Importantly, while many FBM neurons migrated rostrally in Celsr1Crsh/+; Dvl2+/- embryos (Fig. 7I; 12/14), this defect was completely suppressed in Celsr1Crsh/+; Dvl2-/- embryos (Fig. 7J; n=5). These data suggest that while caudal FBM neuron migration is likely independent of Dvl activity, the aberrant rostral migration seen in Celsr1Crsh/+ embryos requires Dvl function. Finally, the rostral migration defect was also suppressed in Vangl2Lp/+; Celsr1Crsh/+ transheterozygotes (Fig. 7K; n=3; Table S1), indicating further that aberrant rostral migration is a response to Wnt/PCP signaling since it is sensitive to reduction in both Dvl and Vangl2 function.
DISCUSSION
Our detailed characterization of FBM neuron migration defects in looptail mutants has highlighted the specificity of the phenotype within the hindbrain. We have shown that the failure of motor neuron migration is not a result of defective specification or differentiation of the neurons. Moreover, expression of some Wnt/PCP genes is not affected in mutants. Importantly, we find that caudal migration of FBM neurons is insensitive to severe reduction of Dishevelled function, suggesting that this mode of migration may be independent of Wnt/PCP signaling (Fig. 8).
Effects of Vangl2 mutations on FBM neuron migration and PCP events
In both Vangl2del/+ and Vangl2Lp/+ embryos, most FBM neurons fail to migrate caudally, a fully penetrant phenotype for both alleles. Since no Vangl2 protein is detected in Vangl2del/del mice (Song et al., 2010), the Vangl2del/+ phenotype is likely due to haploinsufficiency, with a high level of Vangl2 activity required for caudal FBM neuron migration. The Vangl2Lp/+ migration phenotype also results from reduction of Vangl2 activity, because the Vangl2Lp allele encodes either a non-functional or a dominant negative protein. Some studies suggest that the Vangl2Lp allele is null because the mutant protein fails to reach the plasma membrane (Iliescu et al., 2011; Merte et al., 2010; Torban et al., 2007) and is unlikely to compete with WT Vangl2 for intracellular binding partners, especially since mutant Vangl2 does not bind Dvl in vitro (Torban et al., 2004b). Other studies suggest that the Vangl2Lp allele is dominant negative since the mutant protein decreases wild-type Vangl2 phosphorylation, which is essential for its function (Gao et al., 2011; Song et al., 2010). Nonetheless, regardless of the nature of the Vangl2Lp allele, FBM neurons largely fail to migrate caudally in Vangl2Lp/+ embryos suggesting that FBM neuron migration is quite sensitive to the level of Vangl2 function. Similar migration defects were noted in Vangl2Lp/+ embryos in a previous study (Vivancos et al., 2009) using a different Lp allele (S464N; Murdoch et al., 2001), consistent with a dose-dependent role for Vangl2 in this process. Interestingly, no defects in caudal migration were seen in zebrafish embryos heterozygous for either of two mutations in vangl2 (trilobite alleles: tri tk50f and tri tc240a; Bingham et al., 2002) including one that results in no detectable transcript (tri tk50f; Jessen et al., 2002). However, since biochemical or genetic compensatory mechanisms cannot be ruled out to explain the zebrafish data, we favor the idea that high levels of Vangl2 activity are required for caudal migration of FBM neurons.
Although some PCP events like stereocilia orientation are affected in Vangl2Lp/+ and Vangl2del/+ embryos (Montcouquiol et al., 2006; Song et al., 2010), other PCP processes are not affected since both Vangl2del/+ and Vangl2Lp/+ embryos have normal, closed neural tubes. Importantly, however, both exhibit strong and comparable caudal migration defects (Table S1). If one assumes that Vangl2 regulates FBM neuron migration and PCP processes through the same molecular pathways, these data suggest that FBM neuron migration is more sensitive to levels of Vangl2 than neural tube closure. Alternatively, Vangl2 may perform unique and separable functions, acting through independent pathways, for neuronal migration and neural tube closure, since these cellular processes have been uncoupled in Vangl2 heterozygotes.
Site of Vangl2 function for FBM neuron migration
In zebrafish embryos, vangl2 is expressed ubiquitously in the neural tube and non-neural tissues at the onset and early stages of FBM neuron migration (Jessen and Solnica-Krezel, 2004; Park and Moon, 2002). Genetic mosaic analysis with zebrafish trilobite mutants indicates that vangl2 functions primarily in a non-cell autonomous manner to regulate FBM neuron migration (Jessen et al., 2002; Walsh et al., 2011). In contrast to zebrafish, mouse Vangl2 expression in the hindbrain is restricted to the ventricular zone and to differentiating FBM neurons (Song et al., 2006; Fig. 1). Therefore, it is possible that Vangl2 functions within FBM neurons in mice. Consistent with this idea, conditional inactivation of Tbx20 in facial motor neurons leads to the failure of caudal migration, concomitant with the loss of Vangl2 expression in these neurons (Song et al., 2006).
Alternatively, Vangl2 may function non-cell autonomously in mice, as in zebrafish, to regulate FBM neuron migration. Cell transplantation experiments in zebrafish indicate a role for vangl2 in floorplate cells for neuronal migration, and FBM neurons fail to migrate out of r4 in a mouse mutant lacking floorplate cells (Sittaramane, V., Glasco, D. et al., in preparation). Since Vangl2 is transiently expressed in mouse floorplate cells between E9.5-10.5, prior to FBM neuron migration, we cannot rule out a floorplate-associated role for Vangl2 in this process. The role of Vangl2 in specific hindbrain cell types can be addressed by generating tissue-specific knockouts using the Vangl2flox allele (Song et al., 2010).
In zebrafish, FBM neurons use the laminin-expressing basement membrane as a substrate for their caudal migration from r4 to r6/r7 (Grant and Moens, 2010). In contrast, mouse FBM neurons migrate caudally in close proximity to the ventricular zone from r4 into r6, where they migrate radially to the pial surface. Interestingly, Laminin 5 is expressed along the ventricular zone in the mouse hindbrain (Coles et al., 2006), whereas in zebrafish, laminins are restricted to the basement membrane (Grant and Moens, 2010). The hindbrain expression domains of mouse Laminin 5, Vangl2 and Celsr1 overlap, and in zebrafish, laminin 1 genetically interacts with vangl2 to regulate FBM neuron migration (Sittaramane et al., 2009). Therefore, it is possible that Vangl2 and Celsr1 interact with Laminins and other molecules at the ventricular surface of the mouse hindbrain to guide the caudal migration of FBM neurons.
Caudal migration of FBM neurons may be independent of Wnt/PCP and Dishevelled signaling
FBM neuron migration defects have been described in multiple Wnt/PCP mutants in both mice and zebrafish (Bingham et al., 2002; Carreira-Barbosa et al., 2003; Mapp et al., 2010; Qu et al., 2010; Vivancos et al., 2009; Wada et al., 2005; Wada et al., 2006). However, it is unclear whether FBM neuron migration is a PCP-dependent process. Our finding that caudal FBM neuron migration is blocked in Ptk7 mutants is consistent with a role for Wnt/PCP signaling since Ptk7 genetically interacts with Vangl2, and is a regulator of PCP signaling (Lu et al., 2004; Paudyal et al., 2010). Furthermore, Wnt5a is expressed in a graded fashion from r5 and caudally, suggesting that it may act as an attractive cue to migrating FBM neurons (Vivancos et al., 2009). In hindbrain explant cultures, some FBM neurons migrate towards Wnt-coated beads. However, Wnt5a mutant embryos show only very minor defects in caudal FBM neuron migration and Wnt7a mutants show no migration defects. These observations suggest that Wnts (and PCP signaling) may play a minor or redundant role in regulating FBM neuron migration (Vivancos et al., 2009). Interestingly, mosaic analyses of vangl2, scrib, pk1b, and Nancy-Horan syndrome-like 1b (nhsl1b) in particular host environments have uncovered PCP-dependent and PCP-independent mechanisms underlying FBM neuron migration (Walsh et al., 2011).
To directly test the role of PCP signaling in FBM neuron migration, we examined Dishevelled-deficient embryos. FBM neurons migrate normally in Dvl1/2 double mutants despite a fully open neural tube, a hallmark PCP defect (Table S1). This suggests either that FBM neuron migration and PCP events such as neural tube closure are regulated independently, or that caudal neuronal migration requires relatively low levels of Dvl/PCP signaling. Although mice express three Dvl genes in partially overlapping patterns (Tissir and Goffinet, 2006), Dvl3 expression is not affected in Dvl1/2 double mutants, and is excluded from FBM neurons (Fig. S6), suggesting strongly that caudal migration does not require Dvl function within FBM neurons. However, we cannot rule out that Dvl3 compensates for loss of Dvl1-2 function in Dvl1/2 double mutants by acting non-autonomously during neuronal migration. Since Dvl2/3 double mutants die by E9.5, prior to FBM neuron migration (Etheridge et al., 2008), testing a putative role for low-level, non-autonomous Dvl signaling in neuronal migration would necessitate generating triple mutants using conditional alleles. Importantly, however, disruption of Dvl signaling in zebrafish embryos with three different dominant-negative reagents generated only PCP defects (shortened body axis) and had no effect on FBM neuron migration. Since these constructs have the potential to interfere with the functions of multiple Dvl genes not only in motor neurons but also in surrounding tissues, our results suggest strongly that FBM neuron migration is independent of Dvl function (Fig. 8).
Whereas our Dvl data suggest that FBM neuron migration requires lower (or zero) levels of Dvl/PCP signaling (relative to that needed for neural tube closure), our Vangl2Lp/+ data indicate that higher levels of PCP signaling are required (Table S1). Since these putative signaling requirements are mutually exclusive, we instead favor the model that FBM neuron migration and PCP processes are regulated independently of each other, with neuronal migration being independent of Dvl function. In this scenario, Vangl2 may possess a unique and separate function, not required for PCP, which acts in an alternate Dvl-independent pathway to regulate FBM neuron migration. Consistent with this idea, vangl2 genetically interacts with non-PCP genes such as tag1, laminin 1, and hdac1 to regulate FBM neuron migration in zebrafish (Nambiar et al., 2007; Sittaramane et al., 2009). Intriguingly, Vangl2 genetically and physically interacts with Pk1b (unpublished data cited in Mapp et al., 2010), which is expressed in migrating FBM neurons (Rohrschneider et al., 2007), and regulates migration primarily through a novel PCP-independent pathway at the nucleus (Mapp et al., 2011).
Migration of FBM neurons at r3/r4 boundary is Dvl-dependent
Whereas the ability of FBM neurons to migrate caudally appears to be Dvl-independent, our data suggest that Dvl signaling must be reduced to prevent them from migrating rostrally into r3. We showed previously that in Celsr1Crsh/+ mutants, a subset of FBM neurons migrates rostrally into r2/r3, though most FBM neurons migrate caudally into r6 (Qu et al., 2010). Since Wnt5a is also expressed along the midline in r2/r3 in addition to the caudal hindbrain (Fig. 4; Vivancos et al., 2009), we propose that mouse Celsr1 normally functions to prevent FBM neurons at the r3/r4 boundary from migrating rostrally (in a Dvl-dependent manner) towards the chemoattractive Wnt5a source in r2/r3. Consistent with this, we found that loss of Dvl2 function rescued the rostral migration defect in Celsr1Crsh/+ embryos (Fig. 7). Similarly, loss of Vangl2 function in Celsr1Crsh/+ embryos (Vangl2Lp/+; Celsr1Crsh/+ transheterozygotes) also rescued the rostral migration defect (Fig. 7). Importantly, anterograde NeuroVue labeling experiments indicate that the FBM neurons migrating rostrally in Celsr1Crsh/+ mutants originate exclusively from the r4 territory immediately adjacent to the r3/r4 boundary rather than from random or more caudal locations in r4 (D. Glasco and A. Chandrasekhar, unpublished observations). These results suggest that migration of FBM neurons at the r3/r4 boundary is especially sensitive to Dvl/Vangl2-dependent signaling, and that Celsr1 function is necessary to block inappropriate rostral migration towards the Wnt5a source in r3. In contrast, migration of FBM neurons arising in the rest of r4 is either only weakly sensitive to or independent of Dvl signaling. This model may also explain why FBM neurons are attracted to Wnt-coated beads placed laterally near the r3/r4 boundary (Vivancos et al., 2009), since FBM neurons at this boundary would be most sensitive to Wnts, and free to migrate laterally toward the beads through a Celsr1-negative domain. Consistent with this model, Wnt5a-coated beads fail to attract FBM neurons in Dvl2 mutants (L. Reustle, W. Bryant, and A. Chandrasekhar, unpublished observations). Thus, it appears that subsets of mouse FBM neurons may exhibit differential sensitivity to Dvl signaling depending upon their position within r4.
In conclusion, our work has defined a role for Vangl2 in the caudal migration of FBM neurons that appears to be independent of PCP signaling mediated by Dishevelled. Our data also suggest that Wnt/PCP signaling can induce some FBM neurons to migrate rostrally but that this response is normally suppressed. It will be of interest to understand the mechanisms underlying the suppression of the rostral migration response, as well as the nature of the PCP-independent pathways regulating caudal migration.
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Chandrasekhar lab for discussion and fish care. We thank Olivier Pourquie and Sam Pfaff for the looptail and SE1::gfp mice, respectively. We thank Jane Johnson, Oni Mapp, Victoria Prince, Michelle Studer, Fadel Tissir, and Paul Trainor for probes and expression constructs. This work was supported by an NIH predoctoral fellowship 1F31NS063513 (DMG), the Bioimaging Research Center at GIST (MRS), the Medical Research Council (JNM), and NIH grant NS040449 (AC).
ROLE OF FUNDING SOURCE
The funders played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bingham S, Higashijima S, Okamoto H, Chandrasekhar A. The Zebrafish trilobite gene is essential for tangential migration of branchiomotor neurons. Dev Biol. 2002;242:149–60. doi: 10.1006/dbio.2001.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter EM, Goddard JM, Chisaka O, Manley NR, Capecchi MR. Loss of Hox-A1 (Hox-1.6) function results in the reorganization of the murine hindbrain. Development. 1993;118:1063–75. doi: 10.1242/dev.118.4.1063. [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–46. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A. Turning heads: development of vertebrate branchiomotor neurons. Dev Dyn. 2004;229:143–61. doi: 10.1002/dvdy.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar A, Moens CB, Warren JT, Jr., Kimmel CB, Kuwada JY. Development of branchiomotor neurons in zebrafish. Development. 1997;124:2633–44. doi: 10.1242/dev.124.13.2633. [DOI] [PubMed] [Google Scholar]
- Coles EG, Gammill LS, Miner JH, Bronner-Fraser M. Abnormalities in neural crest cell migration in laminin alpha5 mutant mice. Dev Biol. 2006;289:218–28. doi: 10.1016/j.ydbio.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Coppola E, Pattyn A, Guthrie SC, Goridis C, Studer M. Reciprocal gene replacements reveal unique functions for Phox2 genes during neural differentiation. EMBO J. 2005;24:4392–403. doi: 10.1038/sj.emboj.7600897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 2002;21:976–85. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–68. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudney K, Stanier P. Epithelial cell polarity genes are required for neural tube closure. Am J Med Genet C Semin Med Genet. 2005;135:42–7. doi: 10.1002/ajmg.c.30052. [DOI] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Christensen MA, Nichols DH. Fiber pathways and positional changes in efferent perikarya of 2.5- to 7-day chick embryos as revealed with DiI and dextran amines. J Neurobiol. 1993;24:1481–99. doi: 10.1002/neu.480241104. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue Green and their use for double and triple labeling of neuronal profile. Brain Res Bull. 2005;66:249–58. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, et al. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Dev Cell. 2011;20:163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Garcia-Dominguez M, Charnay P. Control of the migratory pathway of facial branchiomotor neurones. Development. 2000;127:5297–307. doi: 10.1242/dev.127.24.5297. [DOI] [PubMed] [Google Scholar]
- Goodrich LV. The plane facts of PCP in the CNS. Neuron. 2008;60:9–16. doi: 10.1016/j.neuron.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PK, Moens CB. The neuroepithelial basement membrane serves as a boundary and a substrate for neuron migration in the zebrafish hindbrain. Neural Dev. 2010;5:9. doi: 10.1186/1749-8104-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–3. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–18. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliescu A, Gravel M, Horth C, Kibar Z, Gros P. Loss of membrane targeting of Vangl proteins causes neural tube defects. Biochemistry. 2011;50:795–804. doi: 10.1021/bi101286d. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Solnica-Krezel L. Identification and developmental expression pattern of van gogh-like 1, a second zebrafish strabismus homologue. Gene Expr Patterns. 2004;4:339–44. doi: 10.1016/j.modgep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem. 2006;99:647–64. doi: 10.1002/jcb.20992. [DOI] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–30. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Underhill DA, Canonne-Hergaux F, Gauthier S, Justice MJ, Gros P. Identification of a new chemically induced allele (Lp(m1Jus)) at the loop-tail locus: morphology, histology, and genetic mapping. Genomics. 2001;72:331–7. doi: 10.1006/geno.2000.6493. [DOI] [PubMed] [Google Scholar]
- Kraus F, Haenig B, Kispert A. Cloning and expression analysis of the mouse T-box gene tbx20. Mech Dev. 2001;100:87–91. doi: 10.1016/s0925-4773(00)00499-8. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Mapp OM, Walsh GS, Moens CB, Tada M, Prince VE. Zebrafish Prickle1b mediates facial branchiomotor neuron migration via a farnesylation-dependent nuclear activity. Development. 2011;138:2121–32. doi: 10.1242/dev.060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp OM, Wanner SJ, Rohrschneider MR, Prince VE. Prickle1b mediates interpretation of migratory cues during zebrafish facial branchiomotor neuron migration. Dev Dyn. 2010;239:1596–608. doi: 10.1002/dvdy.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–83. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, Ginty DD. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2010;12:41–6. doi: 10.1038/ncb2002. sup pp 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossie K, Jallal B, Alves F, Sures I, Plowman GD, Ullrich A. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene. 1995;11:2179–84. [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3:423–32. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nagy A. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2003. [Google Scholar]
- Nambiar RM, Ignatius MS, Henion PD. Zebrafish colgate/hdac1 functions in the non-canonical Wnt pathway during axial extension and in Wnt-independent branchiomotor neuron migration. Mech Dev. 2007;124:682–98. doi: 10.1016/j.mod.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, Sakuma R, Luga V, Roncari L, Attisano L, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Ohsawa R, Ohtsuka T, Kageyama R. Mash1 and Math3 are required for development of branchiomotor neurons and maintenance of neural progenitors. J Neurosci. 2005;25:5857–65. doi: 10.1523/JNEUROSCI.4621-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–5. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Hirsch M, Goridis C, Brunet JF. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000;127:1349–58. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- Paudyal A, Damrau C, Patterson VL, Ermakov A, Formstone C, Lalanne Z, Wells S, Lu X, Norris DP, Dean CH, et al. The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC Dev Biol. 2010;10:87. doi: 10.1186/1471-213X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Glasco DM, Zhou L, Sawant A, Ravni A, Fritzsch B, Damrau C, Murdoch JN, Evans S, Pfaff SL, et al. Atypical Cadherins Celsr1-3 Differentially Regulate Migration of Facial Branchiomotor Neurons in Mice. J Neurosci. 2010;30:9392–9401. doi: 10.1523/JNEUROSCI.0124-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravni A, Qu Y, Goffinet AM, Tissir F. Planar cell polarity cadherin celsr1 regulates skin hair patterning in the mouse. J Invest Dermatol. 2009;129:2507–9. doi: 10.1038/jid.2009.84. [DOI] [PubMed] [Google Scholar]
- Rohrschneider MR, Elsen GE, Prince VE. Zebrafish Hoxb1a regulates multiple downstream genes including prickle1b. Dev Biol. 2007;309:358–72. doi: 10.1016/j.ydbio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 Promotes Wnt/Planar Cell Polarity-like Signaling by Antagonizing Dvl1-Mediated Feedback Inhibition in Growth Cone Guidance. Dev Cell. 2011;20:177–91. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Lewcock JW, Lettieri K, Pfaff SL. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron. 2006;50:841–53. doi: 10.1016/j.neuron.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Sittaramane V, Sawant A, Wolman MA, Maves L, Halloran MC, Chandrasekhar A. The cell adhesion molecule Tag1, transmembrane protein Stbm/Vangl2, and Lamininalpha1 exhibit genetic interactions during migration of facial branchiomotor neurons in zebrafish. Dev Biol. 2009;325:363–73. doi: 10.1016/j.ydbio.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–67. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–82. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MR. Moving cell bodies: understanding the migratory mechanism of facial motor neurons. Arch Pharm Res. 2007;30:1273–82. doi: 10.1007/BF02980268. [DOI] [PubMed] [Google Scholar]
- Song MR, Shirasaki R, Cai CL, Ruiz EC, Evans SM, Lee SK, Pfaff SL. T-Box transcription factor Tbx20 regulates a genetic program for cranial motor neuron cell body migration. Development. 2006;133:4945–55. doi: 10.1242/dev.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–60. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–87. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Bouvier J, Glasco DM, Stewart ME, Dean C, Murdoch JN, Champagnat J, Fortin G, Chandrasekhar A. Brainstem respiratory oscillators develop independently of neuronal migration defects in the Wnt/PCP mouse mutant looptail. PLoS ONE. 2012;7:e31140. doi: 10.1371/journal.pone.0031140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Expression of planar cell polarity genes during development of the mouse CNS. Eur J Neurosci. 2006;23:597–607. doi: 10.1111/j.1460-9568.2006.04596.x. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Pattyn A, Hirsch MR, Brunet JF. Role of Phox2b and Mash1 in the generation of the vestibular efferent nucleus. Dev Biol. 2003;260:46–57. doi: 10.1016/s0012-1606(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Torban E, Kor C, Gros P. Van Gogh-like2 (Strabismus) and its role in planar cell polarity and convergent extension in vertebrates. Trends Genet. 2004a;20:570–7. doi: 10.1016/j.tig.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004b;279:52703–13. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- Torban E, Wang HJ, Patenaude AM, Riccomagno M, Daniels E, Epstein D, Gros P. Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: effect of the Lp mutation. Gene Expr Patterns. 2007;7:346–54. doi: 10.1016/j.modgep.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Vivancos V, Chen P, Spassky N, Qian D, Dabdoub A, Kelley M, Studer M, Guthrie S. Wnt activity guides facial branchiomotor neuron migration, and involves the PCP pathway and JNK and ROCK kinases. Neural Dev. 2009;4:7. doi: 10.1186/1749-8104-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Iwasaki M, Sato T, Masai I, Nishiwaki Y, Tanaka H, Sato A, Nojima Y, Okamoto H. Dual roles of zygotic and maternal Scribble1 in neural migration and convergent extension movements in zebrafish embryos. Development. 2005;132:2273–85. doi: 10.1242/dev.01810. [DOI] [PubMed] [Google Scholar]
- Wada H, Tanaka H, Nakayama S, Iwasaki M, Okamoto H. Frizzled3a and Celsr2 function in the neuroepithelium to regulate migration of facial motor neurons in the developing zebrafish hindbrain. Development. 2006;133:4749–59. doi: 10.1242/dev.02665. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Walsh GS, Grant PK, Morgan JA, Moens CB. Planar polarity pathway and Nance-Horan syndrome-like 1b have essential cell-autonomous functions in neuronal migration. Development. 2011;138:3033–3042. doi: 10.1242/dev.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio) M. Westerfield; Eugene, OR: 1995. [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–99. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.