Abstract
Protein Kinase C-θ (PKC-θ) has been shown to be a critical T cell receptor (TCR) signaling molecule that promotes the activation and differentiation of naïve T cells into inflammatory effector T cells. We demonstrate here that PKC-θ-mediated signals inhibit iTreg differentiation via an AKT-Forkhead Box O1/3a (FoxO1/3A) pathway. Transforming growth factor β-induced iTreg differentiation was enhanced in PKC-θ−/− T cells or WT cells treated with a specific PKC-θ inhibitor, but was inhibited by the PKC-θ activator PMA, or by CD28 crosslinking which enhances PKC-θ activation. PKC-θ−/− T cells had reduced activity of the AKT kinase, and the expression of a constitutively active form of AKT in PKC-θ−/− T cells restored ability to inhibit iTreg differentiation. Furthermore, knockdown or over expression of the AKT downstream targets FoxO1 and FoxO3a was found to inhibit or promote iTreg differentiation in PKC-θ−/− T cells accordingly, indicating that the AKT-FoxO1/3A pathway is responsible for the inhibition of iTreg differentiation of iTreg downstream of PKC-θ. We conclude that PKC-θ is able to control T cell-mediated immune responses by shifting the balance between the differentiation of effector T cells and inhibitory Tregs.
Introduction
Naive CD4+ T cells can differentiate into either inflammatory effector T cells or be induced to form regulatory T cells (iTregs) (1, 2), two distinct subsets of T cell helpers with opposite functions. A fine balance between these two opposing T cell types is required for a functional immune system. Understanding the pathways that control the balance between the differentiation of naïve T cells into inflammatory effector T cells and iTregs facilitates the development of novel therapies for treatment of T cell-mediated autoimmunity. Activation of naïve T cells in the presence of TGF-β1 induces expression of Forkhead Box P3 (Foxp3), a master transcription factor instructing iTregs differentiation, and thus a marker for iTreg (3). In contrast to iTregs, natural Tregs (nTregs) are not induced but develop in the thymus. That naive T cells can be differentiated or converted to inhibitory iTregs suggests there is a therapeutic value for such a conversion in the treatment of autoimmunity. However, at present little is known about the mechanisms for regulating this conversion process. One regulatory candidate is AKT, a serine/threonine kinase that is activated following TCR engagement (4). Activation of AKT is significantly reduced in Tregs (5) and studies have shown AKT activation prevents iTreg differentiation by inhibiting the up-regulation of Foxp3 (6, 7). This result was further confirmed by a study showing that Phosphoinositide-3-Kinase (PI3K), an upstream kinase responsible for AKT activation, also inhibited Foxp3 up-regulation (8), supporting that AKT negatively regulates iTreg differentiation. Among the downstream targets of AKT, the mammalian target of rapamycin (mTOR) and Forkhead Box O1 and 3a (FoxO1/3a) have been shown to regulate Treg differentiation (9). mTOR signals through two functionally distinct complexes, mTORC1 and mTORC2. AKT acts as an upstream molecule of mTORC1 to regulate the activation of dwonstream p70 ribosomal S6 kinase (S6K). Little is known about both upstream and downstream signaling events involved in mTORC2, although it is clear that mTORC1 and mTORC2 work together but independently to regulate iTreg differentiation (10). Activated AKT also prevents Treg differentiation via the inactivation of FoxO1 and FoxO3a, both of which are thought to promote Treg differentiation through the direct activation of Foxp3 transcription (11, 12). When activated, AKT phosphorylates FoxO1 and FoxO3a, which leads to their exclusion from the nucleus and prevents them from activating transcription of Foxp3. Thus, AKT is an important molecule upstream of FoxO1/3a that regulates Treg differentiation. Little is known however, about the molecules upstream of AKT that are involved in the regulation process.
PKC-θ is a critical TCR signaling molecule required for the activation and differentiation of naïve T cells into inflammatory T effector cells (13–16). Our own studies have contributed to the understanding of PKC-θ function in vivo through the creation of a PKC-θ−/− mouse knockout strain (13, 17–22). The availability of PKC-θ−/− mice has facilitated the study of PKC-θ-regulated T cell function in vivo. PKC-θ is essential for the full development of effective Th2 responses to Helminth infection (15) and exposure to model allergens (15, 23). PKC-θ is also required for Th17 responses based on studies using an experimental autoimmune encephalomyelitis (EAE) model (24–26). In a previous study we also demonstrated PKC-θ plays an important role in T cell-mediated cardiac allograft rejection (22). Altogether, previous studies demonstrate a critical role for PKC-θ in the differentiation of naïve T cells into effector T cells that mediate actual immune responses. However, little is known about the function of PKC-θ in the regulation of iTreg differentiation.
In this study, we have shown that PKC-θ-mediated CD28 signaling prevented iTreg differentiation via an AKT-dependent pathway. Deletion of PKC-θ or a PKC-θ inhibitor potentiated differentiation of T cells into iTregs, suggesting that PKC-θ negatively regulates iTreg differentiation. We showed that AKT activation was impaired in PKC-θ−/− T cells under iTreg priming conditions. As a consequence of impaired AKT activity, phosphorylation of the downstream AKT targets, S6K and FoxO1/3a, was reduced in PKC-θ−/− T cells. Forced expression of FoxO1 or FoxO3a, but not active S6K, enhanced iTreg formation in PKC-θ−/− T cells, suggesting that PKC-θ inhibits iTreg differentiation via an AKT/FoxO1/3a pathway. Therefore, PKC-θ promotes the differentiation of naïve T cells into inflammatory effectors but prevents inhibitory Treg formation.
Materials and Methods
Mice
PKC-θ−/− mice were described previously (13) and backcrossed with C57BL/6 mice for more than 10 generations. Foxp3-GFP mice were obtained from Dr. Vijay Kuchroo (Harvard University) and Dr. Defu Zeng (Beckman Research Institute of City of Hope). B6.SJL (CD45.1) and OTII mice were purchased from The Jackson Laboratory. OTII/PKC-θ−/− mice were generated by crossing OTII with PKC-θ−/− mice. All mice were housed under specific pathogen-free conditions and experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the Beckman Research Institute of City of Hope (IACUC#07023).
Plasmids
An empty retroviral expression plasmid MSCV-IRES-Thy1.1 (MIT) and one that encodes constitutively active AKT (MIT-AKT*) were obtained from Dr. Christophe Benoist (Harvard University) (27). LMP vector based retroviral FoxO1 shRNA expressing vectors (LMP-FoxO1 shRNA1 and LMP-FoxO1 shRNA2), MSCV-IRES-GFP (MIG) vector-based retroviral FoxO1, FoxO3a and constitutively active FoxO3a-expressing vectors [MIG-FoxO1, MIG-FoxO3a, and MIG-FoxO3a (3A)] were obtained from Dr. Yun-cai Liu (La Jolla Institute for Allergy and Immunology) (28). MSCV-IRES-human CD4 (MIC) vector based retroviral P70-S6 Kinase 1 (S6K) and constitutively active S6K expressing vectors [MIC-S6K and MIC-S6K (CA)] were obtained from Dr. Craig Walsh (University of California, Irvine).
T-cell isolation and in vitro differentiation of iTreg cells
Naïve (CD4+CD25−CD44lowCD62Lhi) T cells were isolated from lymph nodes and spleens of mice by cell sorting using a FACSAria II cell sorter (BD Bioscience). For in vitro differentiation of naïve T cells into iTreg cells, 2 × 105 cells were cultured with anti-CD3 (145–2C11, eBioscience), anti-CD28 (37.51, eBioscience), 5 ng/ml TGF-β1 (Peprotech) and 10 ng/ml IL-2 (Peprotech) in goat-anti-hamster IgG (0.2 mg/ml, MP Biomedicals) pre-coated 24-well plates. T cells were cultured for 3 days in RPMI 1640 (Cellgro) containing 2 mM L-glutamine, 50 µM β-mercaptoethanol, 100 U/ml penicillin, 100 µg/ml streptomycin and 10% FBS at 37°C with 5% CO2. PKC-θ inhibitor was obtained from Rigel, Inc.
In vivo differentiation of iTreg cells
The method was adapted from previous studies (28, 29). In brief, 3 × 106 naïve T cells sorted from OTII or OTII/PKC-θ−/− mice were transferred into syngeneic B6.SJL (CD45.1) mice. After 24 h, recipient mice were fed grade VI OVA (20 mg/ml; Sigma-Aldrich) in drinking water for 5 days. Drinking water containing OVA was changed every two days.
Flow cytometry
Antibodies were purchased from eBioscience, unless otherwise indicated. After blocking the Fc receptor with anti-CD16/32 (clone 93), cells were incubated with anti-mouse CD4 (clone L3T4) and anti-mouse TCR-β (clone H57-597, BD Bioscience) or anti-CD45.2 (clone 104, BioLegend), and fixed/permeabilized in Foxp3 Staining Buffer (eBioscience). Cells were intracellularly stained with anti-mouse Foxp3 (clone FJK-16S), and analyzed using a BD FACSCanto II flow cytometer (BD Biosciences) and FlowJo software (Treestar).
Retroviral Packaging and Transduction
Retroviral expression plasmids were transfected into Phoenix-Eco cells using lipofectamine 2000 (Invitrogen). After 48 hours, viral supernatants were collected, passed through 0.4 µM filters and stored at −80°C until use. For transduction, naïve T cells were first activated with anti-CD3 (1 µg/ml) and anti-CD28 (1 µg/ml) antibodies in presence of 10 ng/ml IL-2 for 24 hours, then spin infected with viral supernatants (2500 rpm, 30°C for 2 hours) in the presence of 8 µg/ml of polybrene (Sigma-Aldrich). After spin infection, TGF-β1 was added to the culture media to induce iTreg differentiation.
Immunoblot analysis
Naïve T cells (5 × 106) sorted from WT or PKC-θ−/− mice were incubated with 1 µg/ml anti-CD3 and anti-CD28 in the presence of 5 ng/ml TGF-β1 for 1 h on ice. Secondary goat-anti-hamster IgG (8 µg/ml) was added and the cells were then incubated at 37°C for variable times. After incubation, cells were lysed in RIPA buffer (Sigma-Aldrich) with phosphatase inhibitor (Sigma-Aldrich) and protease inhibitor (Roche). Soluble proteins were separated on a NuPAGE 4–12% Bis-Tris gel and transferred to 0.45 µM PVDF membrane. Immunoblot analysis was done with following antibodies (all from Cell Signaling): anti-phospho-AKT (T308, 4056), anti-AKT (4685), anti-phospho-Zap70 (Tyr493, 2704), anti-Zap70 (2705), anti-phospho-S6K (T389, 9234), anti-S6K (2708), anti-phospho-FoxO1 (S256, 9461), anti-FoxO1 (2880), anti-phospho-FoxO3a (S253, 9466), anti-FoxO3a (2497), and anti-β-actin (4970). Band intensity was quantitatively analyzed by ImageJ software (NIH).
Apoptosis assay
WT or PKC-θ−/− naïve T Cells were differentiated to iTreg for 3 days, then washed once with ice-cold annexin V binding buffer (10 mM HEPES (pH 7.5), 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2) and stained with PE-conjugated annexin V and 7-aminoactinomycin D (7-AAD, BD Pharmingen), according to the manufacturer’s protocol. Detection of apoptotic cells was performed on a BDCantoII cytometer (BD Biosciences).
Statistical analysis
Statistical analysis was performed using the unpaired, two-tailed Student's t-test. P values < 0.05 were considered significant.
Results
PKC-θ inhibitor promotes, whereas PKC-θ activator prevents, iTreg differentiation
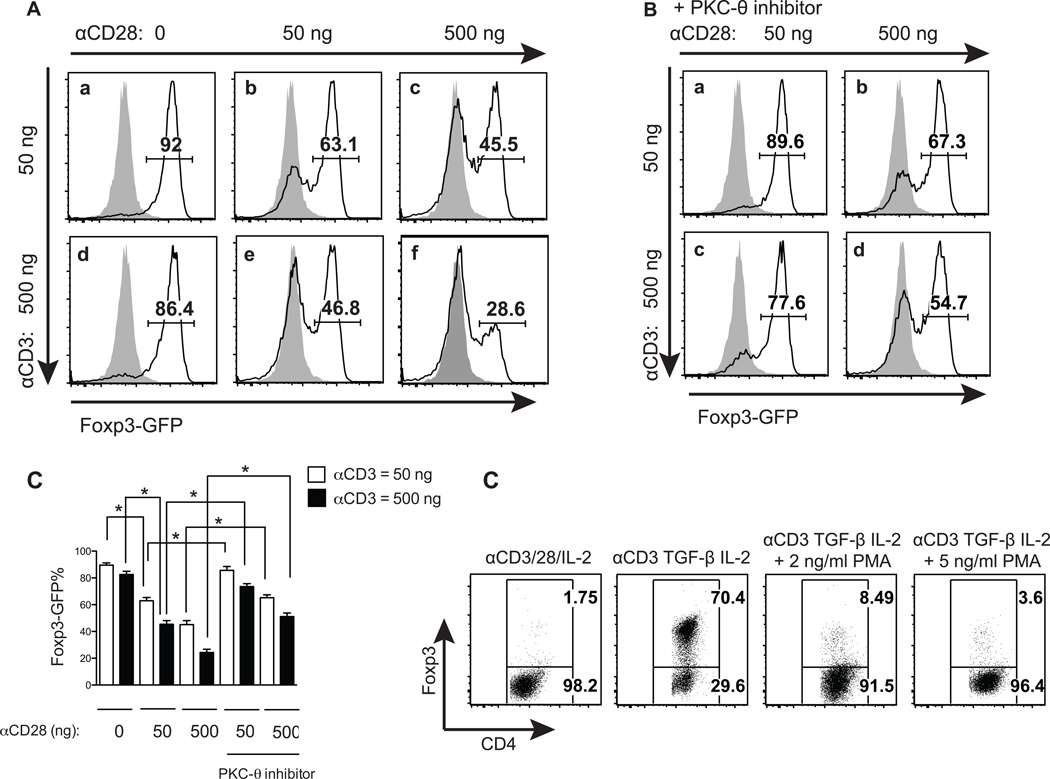
Previous studies have demonstrated a role of PKC-θ in the differentiation of naïve T cells into inflammatory T helper cells (15, 26). Here we investigated the function of PKC-θ in the differentiation of inhibitory iTreg cells. A strain of Foxp3-GFP knock-in mice (1) was used in this experiment, so that GFP could be used to monitor live Foxp3 expression as a marker for iTregs. Naïve T cells were first stimulated by 50 ng/ml of anti-CD3 antibody and TGF-β1/IL-2 in combination with increasing amounts of anti-CD28 antibody (0, 50 and 500 ng/ml) which is known to enhance PKC-θ activation significantly (30, 31) (Fig. 1A, top panels). A large proportion of iTregs (92%) were induced by TGF-β1in the absence of anti-CD28 antibody (Fig. 1A-a), indicating the dispensable role of CD28 in iTreg differentiation. However, stimulation of CD28 by 50 ng/ml of anti-CD28 antibody led to a reduction of the iTreg in the differentiated cell populations from 92% (Fig. 1A-a) to 63.1% (Fig. 1A-b). Increasing the anti-CD28 antibody concentration to 500 ng/ml further reduced iTregs to 45.5% (Fig. 1A-c). Similar inhibitory effects of CD28 on iTreg formation were also observed when T cells were stimulated with 500 ng/ml of anti-CD3 antibody. In the absence of anti-CD28 antibody, there were 86.4% iTreg (Fig. 1A-d), which was decreased to 46.8% and 28.6% after treatment with 50 ng/ml and 500 ng/ml anti-CD28 antibody, respectively (Fig. 1A-e and f). Therefore, iTreg formation was inhibited by CD28 co-stimulation in a dose dependent manner. We then tested the effects of a specific PKC-θ inhibitor, REG134, on CD28-mediated inhibition of iTreg differentiation (Fig. 1B). REG134 is 150 and 1200 fold more potent for PKC-θ than for PKCδ and PKCε (data not show), which are the most homologous members of PKC-θ, and belong to the same PKC subfamily (novel PKC). In addition, this inhibitor prevented PKC-θ-mediated function such as CD69 up-regulation and proliferation in WT but not PKC-θ−/− T cells (data not shown). Under the same conditions, using 50 ng/ml anti-CD3 antibody (Fig. 1B, top panels), the PKC-θ inhibitor blocked the inhibitory action of 50 ng/ml anti-CD28 antibody and increased the percentage of iTregs from 63.1% (Fig. 1A-b) to 89.6% (Fig. 1B-a), which is almost equal to the percentage of iTregs induced in the absence of CD28 co-stimulation (92%, Fig. 1A-a). The PKC-θ inhibitor therefore abolishes CD28-mediated inhibition under this condition. The use of the PKC-θ inhibitor also reduced the inhibitory effects of 500 ng/ml of anti-CD28 antibody and increased iTreg from 45.5% (Fig. 1A-c) to 67.3 % (Fig. 1B-d) in treated populations. Similarly, when T cells were stimulated with 500 ng/ml of anti-CD3 antibody, the PKC-θ inhibitor blocked or prevented the inhibitory action of anti-CD28 and increased iTreg from 46.8% (Fig. 1A-e) to 77.6% in cells treated with 50 ng/ml anti-CD28 antibody (Fig. 1B-c), and from 28.6% (Fig. 1A-f) to 54.7% in cells treated with 500 ng/ml anti-CD28 antibody (Fig. 1B-d). Above results were reproducible as shown in figure 1C which are averaged from three independent experiments. The data therefore suggest that PKC-θ mediates the CD28 signals to inhibit iTreg formation. This hypothesis was further confirmed by an experiment which showed that phorbal ester (PMA), which activates multiple isoforms of PKC including PKC-θ, greatly reduced the percentage of TGF-β1-induced iTregs from 70.4% (Fig. 1D, second panel from left) to 8.49% (2 ng/ml PMA, Fig. 1D, third panel from left) and 3.6% (5 ng/ml PMA, Fig. 1D, fourth panel from left). Due to lack of specificity for PKC-θ, PMA treatment alone is not sufficient to indicate PKC-θ function in iTreg differentiation. However, the fact that PMA and PKC-θ inhibitor have opposite effects on iTreg formation strongly supports that PKC-θ activated by CD28 inhibits iTreg differentiation.
Figure 1.
PKC-θ negatively regulates iTreg differentiation. (A) The effects of TCR signaling strength on iTreg differentiation. Naïve T cells from Foxp3-GFP mice were stimulated with 5 ng/ml TGF-β1, 10 ng/ml IL-2 and the indicated amounts of anti-CD3 and anti-CD28 antibodies for 3 days. Foxp3-GFP expression was detected by flow cytometry. Black lines show the expression of Fox3p-GFP in TGF-β1-induced cells, while the grey shaded areas are GFP in naïve T cells from Foxp3-GFP stimulated with the same anti CD3 and anti-CD28 concentrations but without TGF-β1 and IL2. (B) PKC-θ inhibitor promotes iTreg differentiation. Naïve T cells from Foxp3-GFP mice were differentiated into iTreg cells using the same conditions described in (A), but in the presence of 200 nM of the specific PKC-θ inhibitor REG134. (C) Summary of (A) and (B) averaged from three independent experiments. (D) The PKC-θ activator PMA suppresses iTreg differentiation. Naïve T cells were differentiated into iTreg using the same conditions in (A) plus in the presence of the indicated amount of PMA for 3 days. Tregs were detected by intracellular staining of Foxp3. Plots shown are representative of at least three independent experiments.
PKC-θ negatively regulates iTreg differentiation
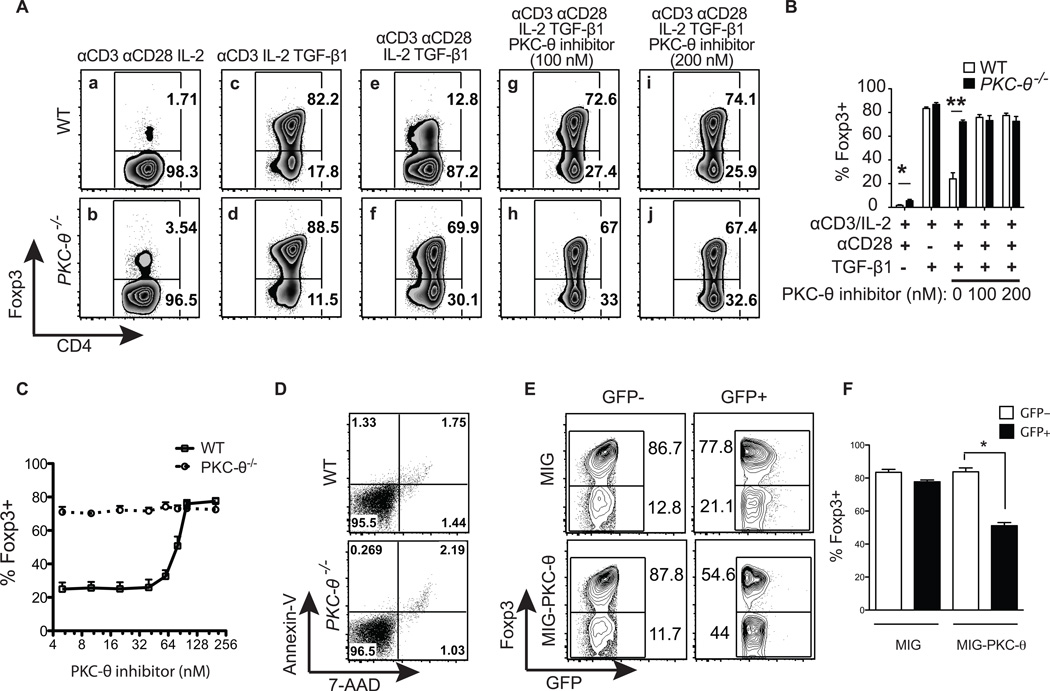
To exclude off-target effects of PKC-θ pharmacological regulators, T cells obtained from WT (Fig. 2A, top panels) and PKC-θ−/− (Fig. 2A, bottom panels) mice were compared to determine the function of PKC-θ in iTreg differentiation. Although low percentages of iTregs were detected after naïve T cells were stimulated without TGF-β1, more iTregs were detected in the differentiated PKC-θ−/− T cell population (3.54%) than WT T cells (1.71%) (Fig. 2A-a and b). Stimulation with anti-CD3 antibody alone in the presence of TGF-β1 induced similar percentages of iTregs in WT (82.2%) and PKC-θ−/− T cells (88.5%) (Fig. 2A-c and d), suggesting that PKC-θ is not required for formation of iTregs. Consistent with previous results (Fig. 1A), in the presence of anti-CD28 antibody, the iTreg was significantly reduced to 12.8% in WT T cells (Fig. 2A-e), whereas there were still 69.9% iTregs PKC-θ−/− T cells (Fig. 2A-f), revealing the essential function of PKC-θ in CD28-mediated inhibition of iTreg differentiation. Also consistent with previous results (Fig. 1B), the addition of the PKC-θ inhibitor abolished CD28-mediated inhibition and increased the iTregs from 12.8% to 72.6% (100 nM of inhibitor) and 74.1% (200 nM inhibitor) in WT T cells after treatment (Fig. 2A-e, g and i). However, the presence or absence of PKC-θ inhibitor, had no effect on iTreg differentiation in PKC-θ−/− T cells; the percentage of iTregs was 69.9% in the absence of PKC-θ inhibitor compared to 67% and 67.4% in those treated with 100 nM and 200 nM PKC-θ inhibitor respectively (Fig. 2A-f, h and j), demonstrating the specificity of the PKC-θ inhibitor and the essential role of PKC-θ in the inhibition of iTreg differentiation. These results were highly reproducible; Figure 2B are the results averaged from four independent experiments that are described in Figure 2A. We also examined the effects of different concentrations of PKC-θ inhibitor on iTreg differentiation (Fig. 2C), and showed that PKC-θ inhibitor could promote iTreg formation in a dose-dependent manner. To exclude the possibility that increased PKC-θ−/− iTreg is due to changes in apoptosis, we examined apoptotic cells by annexin V and 7-AAD staining (Fig. 2D). There were very few dead cells, and no obvious difference in apoptosis between WT and PKC-θ−/− T cells, which was expected due to presence of IL-2, a critical survival factor for T cells. Apoptosis therefore, is unlikely to be responsible for the huge difference in TGF-β1-induced iTregs observed between WT and PKC-θ−/− T cells. Lastly, PKC-θ was introduced back into PKC-θ−/− T cells using a retrovirus expressing PKC-θ (MIG-PKC-θ) and compared with an empty virus (MIG) that only expressed GFP. When gated on GFP- cells that were not transduced and therefore did not express viral genes, no significant difference in iTregs was observed between the empty viral control (86.7%) and the PKC-θ expressing virus (87.8%) infected PKC-θ−/− T cells (Fig. 2E, two left panels and Fig. 2F). In contrast, when gated on successfully transduced GFP+ cells that expressed viral genes, the PKC-θ-expressing virus-infected cells had significantly reduced iTreg (54.6%) compared to those (77.8%) infected by empty virus (Fig. 2E, two right panels and Fig. 2F). Because the restoration of PKC-θ expression restored the ability to inhibit iTreg differentiation in PKC-θ−/− T cells, the enhanced iTreg differentiation observed in PKC-θ−/− T cells is not due to developmental defects, but due to lack of PKC-θ-mediated inhibition. Taken together, these results confirm the inhibitory role of PKC-θ in iTreg differentiation.
Figure 2.
PKC-θ−/− T cells have enhanced potential to differentiate into iTregs. (A) Isolated naïve CD4+ T cells from WT or PKC-θ−/− mice were differentiated into iTregs for 3 days under the conditions indicated. Intracellular Foxp3 detected with a specific antibody was then analyzed by flow cytometry. (B) The percentage of Foxp3+ cells were averaged (mean ± SEM) from four independent experiments described in (A), *, p<0.05; **, p<0.01; error bars indicate ±SD. (C) PKC-θ inhibitor promotes iTreg formation in a dose dependent manner. CD4+ T cells from WT (solid line) or PKC-θ−/− (dotted line) mice were differentiated into iTregs for 3 days in the presence different concentrations of PKC-θ inhibitor (X-axies), and percentage of iTreg induced is indicated (Y-axies). (D) No obvious difference in apoptosis was observed between WT and PKC-θ−/− T cells. Naïve CD4+ T cells from WT control or PKC-θ−/− mice were differentiated into iTreg cells with 5 ng/ml TGF-β1 and 10 ng/ml IL-2 for 3 days. Apoptotic cells were then detected by Annexin-V and 7-AAD staining. (E) Restoration of PKC-θ in PKC-θ−/− T cells restored the inhibition of iTreg differentiation. Naïve PKC-θ−/− T cells infected with MIG-PKC-θ or an empty virus control were differentiated into iTreg with anti-CD3/28, TGF-β1 and IL-2. Percentage of Foxp3- or Foxp3+ cells in GFP- and GFP+ cell populations are indicated. The plots shown are representative of at least three independent experiments. (F) Summary of (E) averaged from three independent experiments.
PKC-θ inhibits iTreg formation in vivo
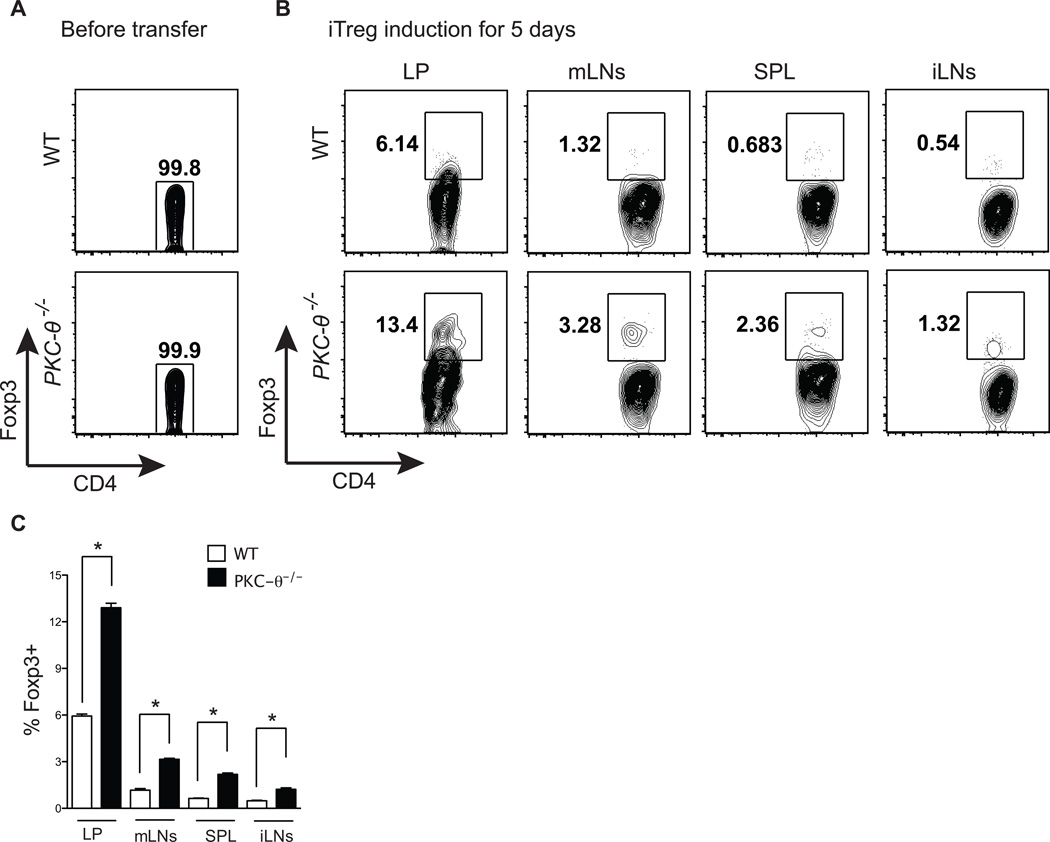
In the above studies, purified naïve T cells were cultured in defined medium optimal for iTreg differentiation in vitro. To study the role of PKC-θ in iTreg differentiation in vivo, iTregs were induced in mice using a model previously described (1, 32). T cells from OT-II TCR transgenic mice with congenic marker CD45.2 were used as donor cells, because these T cells respond to a single ligand, Ova. Sorted naïve OT-II WT and OT-II PKC-θ−/− T cells, that lacked Foxp3+ positive cells (Fig. 3A), were adoptively transferred into congenic CD45.1 recipients that then received Ova in the drinking water for five days. Consistent with previous reports (1, 32), Tregs were mostly induced in gut-associated lymphoid tissues such as the lamina propria (LP, 6.14%) and the mesenteric lymph nodes (mLNs, 1.32%) (Fig. 3B, upper two left panels), whereas relatively fewer iTregs were found in other lymphoid tissues such as spleen (SPL, 0.68%) and inguinal lymph nodes (iLNs, 0.54%) (Fig. 3B, upper two right panels). Significantly more iTregs were differentiated from adoptively transferred CD45.1 PKC-θ−/− T cells in all the lymphoid tissues examined; 13.4% in LP, 3.23% in mLN, 2.36% in SPL and 1.32% in iLNs (Fig. 3B, lower panels), suggesting that lack of PKC-θ promotes induction of Tregs in vivo. These results are reproducible as shown in figure 3C averaged from three independent experiments. Therefore, our data indicate that PKC-θ negatively regulates iTreg formation both in vitro and in vivo.
Figure 3.
PKC-θ−/− T cells form more iTreg cells in vivo. (A) Lack of Foxp3 expression in sorted naïve T cells prior to adoptive transfer. Foxp3 expression in freshly sorted CD4+CD25− CD44loCD62Lhi cells from OTII or OTII/PKC-θ−/− mice was detected by flow cytometry. (B) Sorted donor cells (CD45.2+) as indicated in (A), were transferred into CD45.1 syngeneic hosts that were then fed OVA in the drinking water for 5 days. Intracellular Foxp3 in CD45.2+CD4+TCR-β+ cells in the LP, mLNs, iLNs and SPL were detected by flow cytometric analysis. Plots shown are representative of three independent experiments. (C) Summary of (B) averaged from three independent experiments.
Impaired activation of AKT is responsible for enhanced iTreg differentiation in PKC-θ−/− T cells
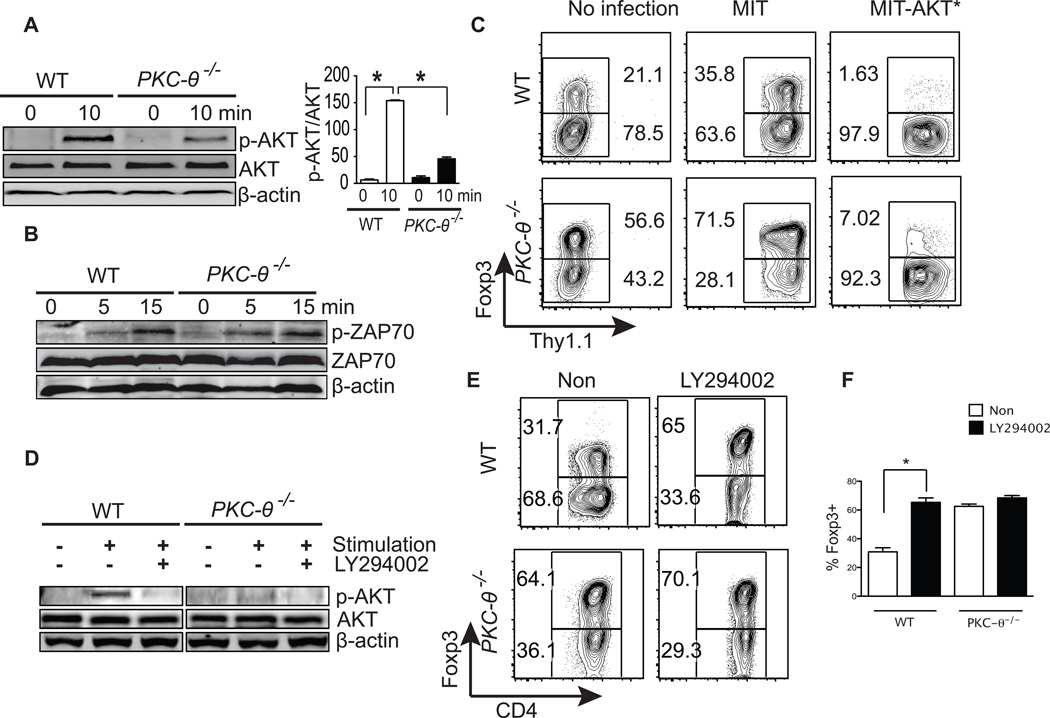
AKT has been shown to negatively regulate iTreg differentiation (6, 7). We therefore examined the phosphorylation of threonine 308 (T308) of AKT, which activates the kinase (33), in WT and PKC-θ−/− T cells cultured under iTreg priming conditions (Fig. 4A). In response to stimulation, AKT was strongly phosphorylated in WT cells, however, phosphorylation was greatly impaired in PKC-θ−/− T cells (Fig. 4A, left panel). Impaired activation of AKT in PKC-θ−/− T cells was confirmed by the quantification of phosphorylated AKT versus total AKT, averaged from several independent assays (Fig. 4A, right panel). In contrast, activation of ZAP70, another critical TCR signaling molecule, used here as a control, was normal in PKC-θ−/− T cells (Fig. 4B) which is consistent with our previous results (20). To determine whether impaired AKT activation is responsible for enhanced iTreg differentiation in PKC-θ−/− T cells, an active form of AKT was introduced into T cells via retrovirus-mediated transduction. In untreated cells (Fig. 4C, two left panels) and empty virus (MIT)-infected cells (Fig. 4C, two middle panels), PKC-θ−/− T cells had significantly higher percentages of iTregs (56.6%–71.5%) (Fig. 4C, two lower left panels) than those of the WT T cells (21%–35.8%) (Fig. 4C, two upper left panels). However, retrovirally expressed active AKT (MIT-AKT*) greatly decreased iTregs to 7.02% in PKC-θ−/− T cells (Fig. 4C, lower right panel), although it was still higher than the percentage of similarly treated WT T cells (1.63%) (Fig. 4C, upper right panel). These results suggest that AKT regulate iTreg differentiation even in the absence of PKC-θ. To determine whether endogenous AKT regulates iTreg differentiation, LY294002, an inhibitor for PI3 kinase (PI3K), an upstream kinase required for AKT activation, was tested. LY294002 treatment blocked phosphorylation of T308 in the AKT protein in stimulated T cells (Fig. 4D), suggesting effective inhibition of PI3K-AKT pathway. In WT T cells, treatment with LY294002 increased iTreg cells from 31.7% to 65% (Fig. 4E, upper two panels and Fig. 4F), which was close to the percentage of iTregs observed in PKC-θ−/− T cells (64.1%), suggesting that endogenous PI3K pathway regulates iTreg differentiation. As expected, LY294002 had minimum effects on iTreg differentiation in PKC-θ−/− T cells which already had lower AKT activity; the percentage of iTregs was 64.1% in the absence of LY294002 compared to 70.1% in its presence (Fig. 4D, lower two panels and Fig. 2F). The fact that active AKT inhibits iTreg formation in WT and PKC-θ−/− T cells suggests that ATK regulates iTreg differentiation either downstream of PKC-θ, or independent of PKC-θ. However, AKT activity is lower in PKC-θ−/− T cells which have enhanced potential to form iTreg, and forced activation of AKT inhibited such potential in PKC-θ−/− T cells. Altogether, our data favor the possibility that AKT is downstream of PKC-θ in the regulation of iTreg differentiation.
Figure 4.
Impaired activation of AKT in PKC-θ−/− T cells is responsible for enhanced iTreg differentiation. (A) Impaired AKT activation in PKC-θ−/−. Naïve WT and PKC-θ−/− T cells were either untreated or stimulated with 1 µg/ml anti-CD3, 1 µg/ml anti-CD28 and 5 ng/ml TGF-β1. Phosphorylation of AKT (T308), expression of total AKT, and the β-actin control were detected by Western blot analysis. The ratio between phosphorylated-AKT and total AKT bands were averaged (mean ± SEM) from three experiments; *, p<0.01; error bars indicate ±SD. (B) Normal activation of ZAP70 in PKC-θ−/− T cells. WT and PKC-θ−/− T cells were either untreated or stimulated with similar conditions as in (A) and phosphorylation of ZAP70 (Tyr493), expression of total ZAP70, and the β-actin control were detected by Western blot analysis. (C) Forced expression of constitutively active AKT (AKT*) inhibits iTreg differentiation. WT and PKC-θ−/− T cells were either untreated or infected with control virus (MIT) or virus expressing activated AKT (MIT-AKT*). T cells were then differentiated into iTreg cells with anti-CD3/28, TGF-β1 and IL-2. Thy1.1 was a marker for successful retroviral transduction, and thus expression of the protein of interest. Percentages of Foxp3 negative or positive cells are indicated. (D) LY294002 prevents AKT phosphorylation. WT and PKC-θ−/− T cells were either untreated or stimulated with the conditions described in (A) in the presence or absence of 10 µM LY294002. Phosphorylation of AKT (T308), expression of total AKT and the β-actin control were detected by Western blot analysis. (E) LY294002 potentiates iTreg differentiation. Naïve WT and PKC-θ−/− T cells were differentiated into iTreg cells with or without 10 µM LY294002. Tregs were detected by flow cytometric analysis of intracellular Foxp3 detected with a specific antibody. Results shown are representative of at least three independent experiments. (F) Summary of (E) averaged from three independent experiments.
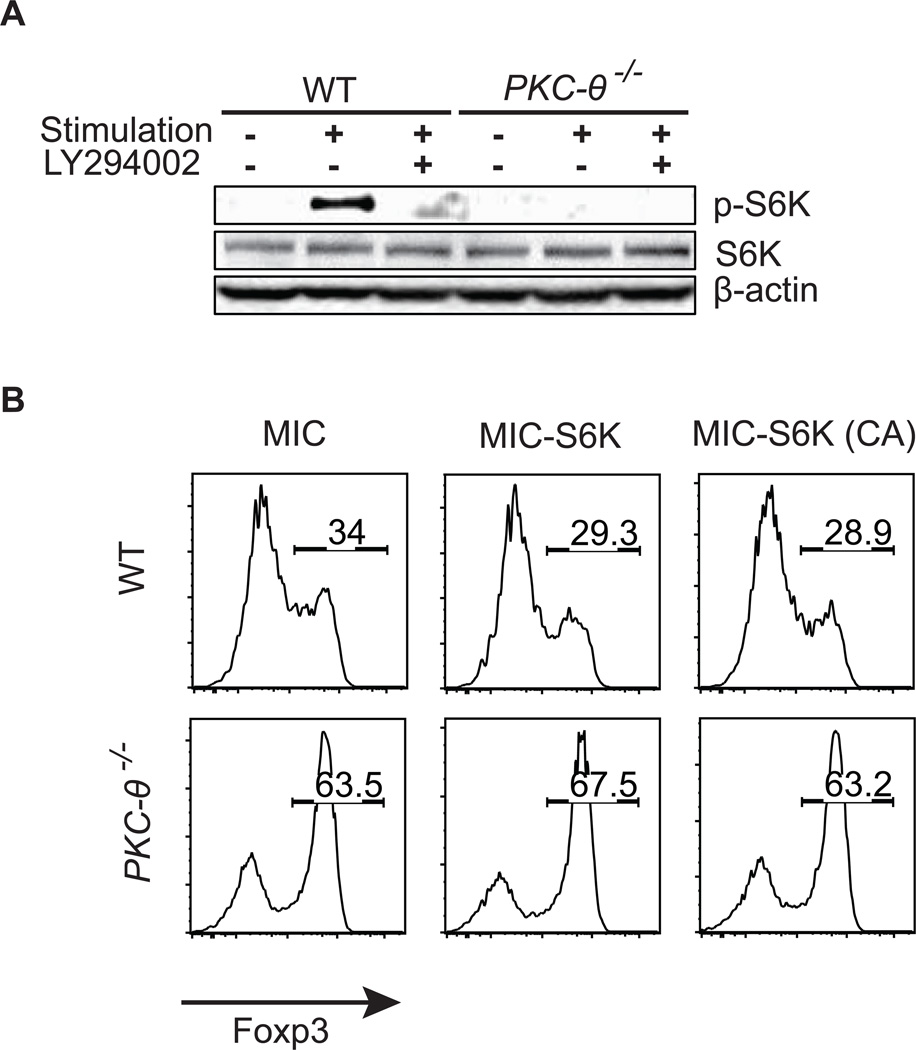
PKC-θ-regulated iTreg differentiation is independent of S6K, an AKT downstream target
Because S6K is one of the AKT downstream target molecules that may play a role in iTreg differentiation (10, 34), we investigated S6K function in PKC-θ-regulated iTreg differentiation. Upon activation, S6K is phosphorylated at serine 389 (35), which was detected by western blot analysis using a specific antibody that only recognizes this phosphorylated form of S6K (Fig. 5A). Consistent with AKT being the upstream signaling molecule, the LY294002 prevented phosphorylation of S6K in WT T cells. PKC-θ−/− T cells also had lower levels of the phosphorylated form of S6K which was expected due to its lower AKT activity. To determine whether lower S6K activation is responsible for the enhanced iTreg differentiation in PKC-θ−/− T cells, we evaluated the effects of the WT and the constitutively active form of S6K [S6K(CA)] (36) on iTreg formation using retroviral expression system (Fig. 5B). The percentage of iTregs was 28.9–34% in WT cells (Fig. 5B, top three panels) and 63.2%–67.5% in PKC-θ−/− T cells (Fig. 5B, lower three panels) regardless of whether S6K or S6K (CA) was absent or present. Therefore, forced expression of the WT or activated form of S6K did not affect iTreg differentiation in WT cells or PKC-θ−/− T cells. This is in striking contrast to the forced expression of AKT, which dramatically affected iTreg differentiation (Fig 4C). The results suggest that S6K is unlikely to be a signaling molecule downstream of PKC-θ-mediated inhibitory pathway of iTreg differentiation.
Figure 5.
S6K is not involved in PKC-θ-mediated iTreg differentiation. (A) Activation of S6K is impaired in PKC-θ−/− T cells. T cells were either untreated or stimulated with 1 µg/ml anti-CD3, 1 µg/ml anti-CD28, 5 ng/ml TGF-β1 in the presence or absence of LY294002 (10 µM). Phosphorylation of S6K (T389), expression of total S6K and the β-actin control were detected by Western blot analysis. (B) Forced expression of WT S6K or active S6K [S6K (CA)] does not affect iTreg differentiation. T cells infected with MIC-S6K, MIC-S6K (CA) or control virus were differentiated into iTreg cells for 3 days with anti-CD3/28, TGF-β1 and IL-2. Tregs were detected by flow cytometric analysis of intracellular Foxp3. Results shown are representative of at least three independent experiments.
PKC-θ regulates iTreg differentiation via AKT downstream molecules FoxO1/3a
The other AKT downstream target molecules that may play a role in iTreg differentiation are FoxO1 and FoxO3a (FoxO1/3a) (11, 12). Therefore, we investigated their functions in PKC-θ-enhanced iTreg differentiation. AKT is thought to phosphorylate FoxO1 at serine 256 and FoxO3a at serine 253. This results in their export from the nucleus and prevents activation of their target genes, including Foxp3, in the nucleus (37). We first examined phosphorylation of FoxO1/3a by western blot assays (Fig. 6A-B). In WT cells, stimulation by iTreg priming conditions leads to phosphorylation of FoxO1/3a. Consistent with the lower upstream AKT activity, in PKC-θ−/− T cells the phosphorylated forms of FoxO1 (Fig. 6A, upper panel) and FoxO3a (Fig. 6A, third panel) were decreased. This was confirmed by quantification of phosphorylated FoxO1 versus total FoxO1 (Fig. 6B, left panel) and phosphorylated FoxO3a versus total FoxO3a (Fig. 6B, right panel) averaged from three independent assays. The results suggest that PKC-θ−/− T cells contain higher levels of the non-phosphorylated forms of FoxO1/3a, which are retained in nucleus and thus capable of activating their target gene Foxp3. This finding is consistent with the increased iTreg ratio in PKC-θ−/− T cells after stimulation. Next, we determined whether changes in the levels of FoxO1/3a caused corresponding changes in iTreg differentiation, using knockdown and overexpression approaches. Two different shRNAs that can effectively knockdown FoxO1 (Fig. 6C) were expressed using a retrovirus containing a selectable puromycin resistance gene and GFP as the reporter molecule (11). The empty virus (LMP) infected PKC-θ−/− T cells had a higher percentage of iTreg cells (41.8%) than the WT cells (24.6%) (Fig. 6D, two left panels), which is consistent with, but not as dramatic as, previous results. This may be due to the different method used in order to accommodate the puromycin selection step. Knockdown by the two shRNAs (LMP-FoxO1 shRNA1 and LMP-FoxO1 shRNA2) led to a decrease in the percentage of iTregs from 24.6% to 16.8% and 17.6% in the respective WT cells (Fig.6D, two upper right panels), suggesting that endogenous FoxO1 stimulates iTreg differentiation. Knockdown of FoxO1 was also able to decrease the percentage of iTregs from 41.8% to 30.3% (shRNA1) and 28.2% (shRNA2) in the PKC-θ−/− T cells (Fig. 6D, two lower right panels), suggesting that FoxO1 is likely downstream of PKC-θ, in the regulation of iTreg differentiation. These results were reproducible as shown in figure 6E averaged from three independent experiments. We then determined the effects of overexpression of FoxO1 and FoxO3a on iTreg formation (Fig. 6F). In WT T cells, the percentage of iTregs (8.95%) was significantly increased in cells transduced with virus expressing FoxO1 (32.1%), FoxO3a (27.1%) and FoxO3a (3A) (32.3%) (Fig. 6F, upper panels). The constitutively active form of FoxO3a which has a mutation in the AKT phosphorylation site that prevents its phosphorylation and nuclear export (11). The percentage of iTregs in PKC-θ−/− T cells (41%) was also increased by infection and expression of FoxO1 (50.2%), FoxO3a (55.7%) and FoxO3a (3A) (65%) (Fig. 6F, lower panels). These results were reproducible as shown in figure 6G averaged from three independent experiments. In contrast to S6K, FoxO1/3a is able to affect iTreg differentiation in both WT and PKC-θ−/− T cells. These results indicate that the decreased phosphorylation of FoxO1/3a, which leads to its nuclear accumulation and availability to stimulate its target gene, Foxp3, is responsible for the enhanced iTreg differentiation in PKC-θ−/− T cells.
Figure 6.
FoxO1 and FoxO3a mediate PKC-θ-enhanced iTreg differentiation. (A) Reduced phosphorylation of FoxO1 and FoxO3a in PKC-θ−/− T cells. Naïve WT and PKC-θ−/− T cells were either untreated or stimulated with 1 µg/ml anti-CD3, 1 µg/ml anti-CD28 and 5 ng/ml TGF-β1. Phosphorylation of FoxO1 (S-256) and FoxO3a (S-253), expression of total FoxO1, FoxO3a and the β-actin control were detected by Western blot analysis. (B) Ratio of bands representing phosphorylated-FoxO1 and total FoxO1, phosphorylated-FoxO3a and total FoxO3a. Bands were averaged (mean ± SEM) from three experiments; *, p<0.01; error bars indicate ±SD. (C) Knockdown of FoxO1 by shRNA. T cells were transduced with LMP expression shRNA1 and shRNA 2 targeting FoxO1, and FoxO1 expression was detected by westernblot analysis. (D) Knock down of FoxO1 impairs iTreg differentiation. T cells infected with two different LMP-FoxO1 shRNAs or a control virus were differentiated into iTreg cells. Expression of Foxp3 was detected by intracellular staining. (E) Summary of (D) averaged from three independent experiments. (F) Forced expression of FoxO1, FoxO3a or constitutively active FoxO3a (FoxO3a [3A]) enhances iTreg differentiation. Naïve WT and PKC-θ−/− T cells infected with MIG-FoxO1, MIG-FoxO3a, MIG-FoxO3a (3A), or a control virus were differentiated into iTreg cells. Tregs were detected by flow cytometric analysis of intracellular Foxp3. Results shown are representative of at least three independent experiments. (G) Summary of (F) averaged from three independent experiments.
Discussion
Previous studies of PKC-θ have almost always focused on its role in the stimulation of T cell activation and differentiation (16, 19). In vitro assays initially showed that PKC-θ mediates the critical TCR signals required for T cell activation and differentiation into effector T cells (13, 14, 20, 38). PKC-θ’s function in T cell activation has also been confirmed in vivo. Studies have shown there are defective Th2 and Th17 responses and allograft rejection in PKC-θ−/− mice (15, 22–26). PKC-θ, therefore, is known to promote the differentiation of naïve T cells into inflammatory effector T cells that carry out immune responses in vivo. In this study, we investigated the role of PKC-θ in Treg differentiation, and our results indicate that PKC-θ-mediated signals inhibited the differentiation of naïve T cells into iTregs. This suggests that activation of PKC-θ is able to boost the immune response by both promoting the differentiation of inflammatory T cells and blocking the differentiation of inhibitory iTregs. In contrast, inhibition of PKC-θ is likely to restrict the immune response by both inhibiting the formation of inflammatory T cells and also promoting iTreg differentiation. PKC-θ inhibitors can also enhance Treg effector function, since PKC-θ inhibits Treg-mediated suppression (39). PKC-θ has long been considered a drug target (16, 19, 40), and highly specific inhibitors similar to the one used in this study are expected to have efficacy in treatment of T cell-mediated autoimmunity and allograft rejection via both the inflammatory and inhibitory arms of the immune system.
Another question addressed in this study was determination of the downstream molecular mechanisms that played a role in PKC-θ-mediated inhibition of iTreg differentiation. Our results support the hypothesis that PKC-θ inhibits iTreg differentiation via an AKT-dependent pathway. The initial evidence for involvement of AKT came from the observation that PKC-θ−/− T cells have impaired activation of AKT. This result appears inconsistent with previous results that PKC-θ and AKT works independently to regulate T cell activation (41–43). In contrast to previous studies examining signaling events in T cell activation, here we focused on iTreg differentiation. Therefore, T cells were stimulated by iTreg priming conditions to mimic signaling events during iTreg differentiation. Different signaling processes are involved in T cell activation and iTreg differentiation due to presence of specific cytokines required to drive iTreg formation. Crosstalk between PKC and AKT pathway is widely reported in non-hematopoietic cells (44–49) as well as in B (50) and T cells (51, 52). In one study, PKCα in T cells was showed to be able to activate AKT by direct phosphorylation (52). However, PKC pathway can also stimulate AKT function via other mechanisms. For example, Bauer et al showed that PKC-θ and AKT cooperatively stimulated NF-κB (51), which appears to be consistent with the notion that PKC-θ and AKT work independently. However, PKC-θ and AKT were in the same complexes, and dominant negative AKT was found to inhibit PKC-θ-mediated activation of NF-κB, suggesting crosstalk between PKC-θ and AKT. Their research showed that PKC-θ regulated AKT function via promoting its recruitment to the lipid rafts critical for TCR signals. Our results favor that PKC-θ regulates AKT under iTreg priming conditions. The involvement of AKT in PKC-θ-regulated iTreg differentiation was also supported by that LY294002, the PI3K inhibitor, potentiated iTreg formation in WT but not PKC-θ−/− T cells. PDK1, downstream of PI3K, is believed to activate PKC-θ by phosphorylation of T538 site (53, 54). In this case, LY294002 is expected to inhibit both AKT and PKC-θ, and therefore, cannot differentiate the effects of AKT and PKC-θ. However, the function of PDK1 in the activation of PKC-θ is questioned by Baier et al (55). To make it more complicated, a recent report suggests that GLK phopshorylates T538 to activate PKC-θ (56). If this is the case, LY294002 does not likely affect PKC-θ activation via inhibition of PI3K-PDK1 pathway. Although these results are not sufficient to determine whether AKT is downstream of PKC-θ or acting independently of PKC-θ to regulate iTreg differentiation, the fact that active AKT inhibits iTreg formation in PKC-θ−/− T cells that have lower AKT activity strongly favors the possibility that PKC-θ regulates iTreg differentiation via AKT. Interestingly, the activation of both PKC-θ and AKT are enhanced by CD28 signaling (9, 30, 43, 57), which raises the possibility that stimulation of CD28 inhibits iTreg formation via a PKC-θ-AKT mediated pathway. Indeed, our results demonstrated that crosslinking CD28 inhibited iTreg formation, which is consistent with a previous report (58). Furthermore, crosslinking CD28 failed to inhibit iTreg differentiation in PKC-θ−/− T cells or WT cells treated with PKC-θ inhibitor or LY294002 suggesting that a PKC-θ-AKT pathway mediates the CD28 signals essential for inhibiting iTreg formation. Yu’s group (59) showed that CD28 is required for iTreg differentiation, which appears to be inconsistent with our results. In contrast to our experiments, iTreg differentiation was performed in the absence of exogenous IL-2 in their report. CD28 stimulation is required for production of IL-2, an essential factor for iTreg formation (60). Indeed, stimulation of CD28 in the presence of exogenous IL-2 inhibited iTreg differentiation in another report from Yu’s group (58), which is consistent with our results.
AKT controls S6K, a target of mTOR, and FoxO1/3a, which may regulate iTreg differentiation (9, 11, 12). We asked which of the AKT-regulated pathways are required for PKC-θ-mediated inhibition of iTreg differentiation. mTOR signals through two complexes, mTORC1 and mTORC2, and S6K is an downstream target of mTORC1 (9). Lower S6K activity was detected in PKC-θ−/− T cells that had impaired AKT activation, suggesting defective mTORC1 complexes. However, forced expression of either WT or a constitutively active S6K did not affect iTreg differentiation in PKC-θ−/− T cells, suggesting that S6K is not involved in the iTreg differentiation pathway. mTORC1 and mTORC2 works together to regulate iTreg differentiation, indicated by defects in both the mTORC1 and mTORC2, but not either one of the two complexes, affected Treg differentiation (10). Therefore, our result that forced activation of kinase S6K alone did not change iTreg differentiation in either WT or PKC-θ−/− T cells is consistent with the notion that defects in mTORC1 alone is not sufficient to affect iTreg differentiation. In contrast, iTreg differentiation was changed by both over expression and knockdown of FoxO1/3a, which are also regulated by AKT. Mice deficient in both FoxO1 and FoxO3a develop autoimmunity due to a significant decrease in Treg cells (61), suggesting a stimulatory role for FoxO1/3a in Treg differentiation. FoxO1/3a promotes Treg differentiation, most likely via the direct binding and stimulation of Foxp3 transcription (11, 12). Activation of AKT leads to phosphorylation of FoxO1/3a, which results in their export from the nucleus, preventing them from activating Foxp3 transcription (9, 11). Consistent with impaired AKT activity in PKC-θ−/− T cells, FoxO1/3a phosphorylation was reduced in these cells, allowing accumulation of more FoxO1/3a in the nucleus to stimulate Foxp3 expression. Knockdown of FoxO1 prevented its accumulation and inhibited formation of Foxp3+ iTregs, whereas overexpression of FoxO1 or FoxO3a increased iTreg differentiation in both PKC-θ−/− and WT T cells. Our results suggest that PKC-θ inhibits iTreg differentiation via AKT-mediated phosphorylation of FoxO1/3a, leading to their export from the nucleus and failure to stimulate Foxp3 transcription. PKC-θ is known to stimulate NF-κB via CARM1/BCL10/MALT1 complexes (62). Blockade of NF-κB activation by deletion of CARMA1 or c-Rel prevents iTreg differentiation (63). The reason is that production of IL-2, essential for iTreg differentiation (64, 65), is dependent on NF-κB. Since PKC-θ is required for IL-2 production via activation of NF-κB pathway (66). PKC-θ also likely promotes iTreg differentiation via stimulation of IL-2 production. However, we used exogenous IL-2 in our study. Therefore, the defects we observed are not due to lack of IL-2 production in PKC-θ−/− T cells.
We as well as others have shown that PKC-θ−/− mice have reduced nTreg development in the thymus (67, 68), suggesting that PKC-θ promotes nTreg development in vivo. nTreg development in the thymus depends on positive selection by stronger TCR signals than that required for the development of conventional CD4 and CD8 T cells (69, 70). Therefore, the reduced nTreg development is likely to be due to the overall reduction of TCR signaling strength in the absence of PKC-θ. Development of conventional T cells is normal in PKC-θ−/− mice (13), since these cells require weaker TCR signals which do not appear to be affected by the absence of PKC-θ. Conversely, the activation of peripheral T cells, which also requires a strong TCR stimulus, is defective in PKC-θ−/− mice (13). Differentiation of naïve T cells to iTreg also requires TCR stimulation. Our results showed that PKC-θ is dispensable for the TCR signaling required for iTreg differentiation, suggesting that iTreg differentiation may require weaker TCR signals. Whereas, stronger TCR signals such as those enhanced by co-stimulation with CD28, actually inhibit iTreg differentiation. Thus, PKC-θ is a critical molecule that controls the threshold of TCR signals which determines the fate of T cell development and differentiation. Manipulation of PKC-θ activity using inhibitors or activators can adjust immune responses by affecting both inflammatory and inhibitory T cell differentiation.
Acknowledgements
We are grateful to Dr. Christophe Benoist for the AKT expression plasmids, Dr. Yun-cai Liu for FoxO1/31 shRNA and expression plasmids, Drs. John Blenis and Craig Walsh for the various S6K expression plasmids, Dr. Chen Dong for helping with retrovirus-mediated transduction of T cells and Drs. Vijay Kuchroo and Defu Zeng for the Foxp3-GFP knock-in mice.
This work was supported by NIH R01-AI053147, NIH R56-AI072554, the Nesvig lymphoma Fellowship and Research Fund, and City of Hope.
References
- 1.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Lopes JE, Chong MM, Ivanov, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Cantrell DA. T-cell antigen receptor signal transduction. Immunology. 2002;105:369–374. doi: 10.1046/j.1365-2567.2002.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 6.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med. 2010;207:1347–1350. doi: 10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 14.Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman A, Isakov N, Baier G. Protein kinase Ctheta: a new essential superstar on the T-cell stage. Immunol Today. 2000;21:567–573. doi: 10.1016/s0167-5699(00)01749-7. [DOI] [PubMed] [Google Scholar]
- 17.Manicassamy S, Gupta S, Huang Z, Molkentin JD, Shang W, Sun Z. Requirement of calcineurin a for the survival of naive T cells. J Immunol. 2008;180:106–112. doi: 10.4049/jimmunol.180.1.106. [DOI] [PubMed] [Google Scholar]
- 18.Manicassamy S, Gupta S, Huang Z, Sun Z. Protein Kinase C-{theta}-Mediated Signals Enhance CD4+ T Cell Survival by Up-Regulating Bcl-xL. J Immunol. 2006;176:6709–6716. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- 19.Manicassamy S, Gupta S, Sun Z. Selective function of PKC-theta in T cells. Cell Mol Immunol. 2006;3:263–270. [PubMed] [Google Scholar]
- 20.Manicassamy S, Sadim M, Ye RD, Sun Z. Differential Roles of PKC-theta in the Regulation of Intracellular Calcium Concentration in Primary T Cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Manicassamy S, Sun Z. The critical role of protein kinase C-theta in Fas/Fas ligand-mediated apoptosis. J Immunol. 2007;178:312–319. doi: 10.4049/jimmunol.178.1.312. [DOI] [PubMed] [Google Scholar]
- 22.Manicassamy S, Yin D, Zhang Z, Molinero LL, Alegre ML, Sun Z. A critical role for protein kinase C-theta-mediated T cell survival in cardiac allograft rejection. J Immunol. 2008;181:513–520. doi: 10.4049/jimmunol.181.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 24.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Protein kinase Ctheta controls Th1 cells in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:7635–7641. doi: 10.4049/jimmunol.175.11.7635. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Xie H, Wang R, Sun Z. Retinoid-related orphan receptor gamma t is a potential therapeutic target for controlling inflammatory autoimmunity. Expert Opin Ther Targets. 2007;11:737–743. doi: 10.1517/14728222.11.6.737. [DOI] [PubMed] [Google Scholar]
- 26.Tan SL, Zhao J, Bi C, Chen XC, Hepburn DL, Wang J, Sedgwick JD, Chintalacharuvu SR, Na S. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase C theta-deficient mice. J Immunol. 2006;176:2872–2879. doi: 10.4049/jimmunol.176.5.2872. [DOI] [PubMed] [Google Scholar]
- 27.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. The Journal of experimental medicine. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. The Journal of experimental medicine. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nature immunology. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Lo PF, Zal T, Gascoigne NR, Smith BA, Levin SD, Grey HM. CD28 plays a critical role in the segregation of PKC theta within the immunologic synapse. Proc Natl Acad Sci U S A. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin X, O'Mahony A, Mu Y, Geleziunas R, Greene WC. Protein kinase C-theta participates in NF-kappaB activation induced by CD3–CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 34.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- 36.Schalm SS, Blenis J. Identification of a conserved motif required for mTOR signaling. Curr Biol. 2002;12:632–639. doi: 10.1016/s0960-9822(02)00762-5. [DOI] [PubMed] [Google Scholar]
- 37.Arden KC. FoxO: linking new signaling pathways. Mol Cell. 2004;14:416–418. doi: 10.1016/s1097-2765(04)00213-8. [DOI] [PubMed] [Google Scholar]
- 38.Altman A, Kaminski S, Busuttil V, Droin N, Hu J, Tadevosyan Y, Hipskind RA, Villalba M. Positive feedback regulation of PLCgamma1/Ca(2+) signaling by PKCtheta in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol. 2004;34:2001–2011. doi: 10.1002/eji.200324625. [DOI] [PubMed] [Google Scholar]
- 39.Zanin-Zhorov A, Ding Y, Kumari S, Attur M, Hippen KL, Brown M, Blazar BR, Abramson SB, Lafaille JJ, Dustin ML. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science. 2010;328:372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi K, Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol Res. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, Zhang J. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 44.Greco S, Storelli C, Marsigliante S. Protein kinase C (PKC)-delta/-epsilon mediate the PKC/Akt-dependent phosphorylation of extracellular signal-regulated kinases 1 and 2 in MCF-7 cells stimulated by bradykinin. J Endocrinol. 2006;188:79–89. doi: 10.1677/joe.1.06433. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Zhang J, Flechner L, Hyun T, Yam A, Franke TF, Pierce JH. Protein kinase C-alpha overexpression stimulates Akt activity and suppresses apoptosis induced by interleukin 3 withdrawal. Oncogene. 1999;18:6564–6572. doi: 10.1038/sj.onc.1203065. [DOI] [PubMed] [Google Scholar]
- 46.Lu D, Huang J, Basu A. Protein kinase Cepsilon activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-alpha-induced cell death. J Biol Chem. 2006;281:22799–22807. doi: 10.1074/jbc.M603390200. [DOI] [PubMed] [Google Scholar]
- 47.Preiss S, Namgaladze D, Brune B. Critical role for classical PKC in activating Akt by phospholipase A2-modified LDL in monocytic cells. Cardiovasc Res. 2007;73:833–840. doi: 10.1016/j.cardiores.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE, Gao B, Krepinsky JC. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol. 2009;20:554–566. doi: 10.1681/ASN.2008040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 50.Barragan M, de Frias M, Iglesias-Serret D, Campas C, Castano E, Santidrian AF, Coll-Mulet L, Cosialls AM, Domingo A, Pons G, Gil J. Regulation of Akt/PKB by phosphatidylinositol 3-kinase-dependent and -independent pathways in B-cell chronic lymphocytic leukemia cells: role of protein kinase C{beta} J Leukoc Biol. 2006;80:1473–1479. doi: 10.1189/jlb.0106041. [DOI] [PubMed] [Google Scholar]
- 51.Bauer B, Krumbock N, Fresser F, Hochholdinger F, Spitaler M, Simm A, Uberall F, Schraven B, Baier G. Complex formation and cooperation of protein kinase C theta and Akt1/protein kinase B alpha in the NF-kappa B transactivation cascade in Jurkat T cells. J Biol Chem. 2001;276:31627–31634. doi: 10.1074/jbc.M103098200. [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Qiao G, Ying H, Zhang J, Yin F. TCR-induced Akt serine 473 phosphorylation is regulated by protein kinase C-alpha. Biochem Biophys Res Commun. 2010;400:16–20. doi: 10.1016/j.bbrc.2010.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KY, D'Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- 54.Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruber T, Freeley M, Thuille N, Heit I, Shaw S, Long A, Baier G. Comment on "PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation". Science. 2006;312:55. doi: 10.1126/science.1115362. author reply 55. [DOI] [PubMed] [Google Scholar]
- 56.Chuang HC, Lan JL, Chen DY, Yang CY, Chen YM, Li JP, Huang CY, Liu PE, Wang X, Tan TH. The kinase GLK controls autoimmunity and NF-kappaB signaling by activating the kinase PKC-theta in T cells. Nat Immunol. 2011;12:1113–1118. doi: 10.1038/ni.2121. [DOI] [PubMed] [Google Scholar]
- 57.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 58.Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, Yu XZ. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood. 2011;117:3096–3103. doi: 10.1182/blood-2010-08-301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J Immunol. 2008;181:2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 62.Lin X, Wang D. The roles of CARMA1, Bcl10, and MALT1 in antigen receptor signaling. Semin Immunol. 2004;16:429–435. doi: 10.1016/j.smim.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-kappaB-dependent manner. J Immunol. 2011;186:4609–4617. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Visekruna A, Huber M, Hellhund A, Bothur E, Reinhard K, Bollig N, Schmidt N, Joeris T, Lohoff M, Steinhoff U. c-Rel is crucial for the induction of Foxp3(+) regulatory CD4(+) T cells but not T(H)17 cells. Eur J Immunol. 2010;40:671–676. doi: 10.1002/eji.200940260. [DOI] [PubMed] [Google Scholar]
- 65.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 66.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 67.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]