ABSTRACT
Purpose: To summarize the existing literature examining constraint-induced movement therapy (CIMT), relative to dose-matched control interventions, for upper-limb (UL) dysfunction in adult survivors of stroke. Methods: CINAHL, Cochrane Library, Embase, NARIC/CIRRIE—Rehabdata, PEDro, PubMed, Scopus, and Web of Science were searched from their inception to February 2011. Trial quality was described using the PEDro scale. The findings were summarized with meta-analysis. Results: For the 22 trials identified, the mean (SD) PEDro score was 6.4 (1.2). Meta-analysis showed CIMT to be superior to dose-matched interventions based on indicators of UL motor capacity (15 trials, n=432; standardized mean difference [SMD]=0.47, 95% CI, 0.27–0.66) and UL ability (14 trials, n=352; SMD=0.80, 95% CI, 0.57–1.02); Functional Independence Measure scores (6 trials, n=182; mean difference [MD]=5.05, 95% CI, 2.23–7.87); and Motor Activity Log scores (Amount of Use: 12 trials, n=318; MD=1.05, 95% CI, 0.85–1.24; Quality of Movement: 11 trials, n=330; MD=0.89, 95% CI, 0.69–1.08). Conclusions: Compared to control interventions of equal duration and dose, CIMT produced greater improvements in a variety of indicators of UL function in adult survivors of a stroke with residual movement of their upper limb.
Key Words: stroke, rehabilitation, constraint-induced movement therapy, upper extremity
RÉSUMÉ
Objectif : Faire la synthèse de la littérature existante traitant de la thérapie par contrainte induite (TCI) par rapport aux interventions de contrôle avec dosage équivalent dans les cas de dysfonctions des membres supérieurs chez les adultes ayant survécu à un accident vasculaire cérébral. Méthode : Une recherche a été effectuée dans CINAHL, dans la bibliothèque de Cochrane, dans Embase, NARIC/CIRRIE—Rehabdata, PEDro, PubMed, Scopus, et Web of Science depuis leur mise en ligne jusqu'en février 2011. La qualité des recherches a été évaluée à l'aide de l'échelle PEDro. Les conclusions ont été résumées à l'aide d'une méta-analyse. Résultats : Au total, 22 essais ont été répertoriés; la moyenne de leur cote à l'échelle PEDro était de 6,4 (SD1 de 0,2). La méta-analyse a révélé que la TCI est supérieure aux interventions de contrôle avec dosage équivalent pour les indicateurs de capacité motrice des membres supérieurs (15 essais, n=432; MDS=0,47; 95%IC, 0,27–0,66); capacité des membres supérieurs (14 essais, n=352; MDS=0,80; 95%IC, 0,57–1,02); mesure de l'autonomie fonctionnelle [Functional Independence Measure] (6 essais, n=182; DM=5,05; 95%IC, 2,23–7,87); et score du journal de l'activité motrice [Motor Activity Log] (quantité d'utilisation : 12 essais, n=318; DM=1,05; 95%IC, 0,85–1,24; qualité du mouvement : 11 essais, n=330; DM=0,89; 95%IC 0,69–1,08). Conclusions : Si on la compare aux interventions de contrôle de durée et avec dosage équivalents, la TCI suscite de plus grandes améliorations dans une variété d'indicateurs de la fonction des membres supérieurs chez les adultes ayant survécu à un AVC avec mouvement résiduel de leurs membres supérieurs.
Mots clés : accident vasculaire cérébral, AVC, revue, réadaptation, membres supérieurs
The term constraint-induced movement therapy (CIMT) describes a package of interventions designed to decrease the impact of a stroke on the upper-limb (UL) function of some stroke survivors.1 CIMT has three main components: (1) supervised repetitive practice of UL tasks calibrated to challenge the participant's motor capacity; (2) restraint of the less-affected limb for prolonged periods to force use of the more-affected limb; and (3) use of adjunctive behavioural strategies, such as contracts and adherence logs, to promote transfer of skill from the clinical setting to the participant's everyday life. The original rationale for CIMT was based on the assumption that for a significant number of people living with the effects of stroke, deficits in UL ability could be attributed to learned non-use.2 This hypothesis suggests that limited use of the upper limb after a stroke is, to some degree, a behaviour learned over time through positive reinforcement of compensatory use of the less-involved limb and negative reinforcement of less efficient and unsuccessful use of the more-involved limb.
The efficacy of CIMT has been examined using a variety of research designs to investigate its impact on different indicators of UL function. Several systematic reviews have summarized this body of literature,3–8 notably a Cochrane Review7 that synthesized results of 19 suitable randomized controlled trials (RCTs) available as of June 2008. Meta-analyses conducted for this review showed statistically significant positive effects of CIMT (vs. control interventions) immediately after treatment for the following measures: (1) performance-based indicators of global disability (data from 6 studies, total sample size of 184 participants; standardized mean difference [SMD]=0.36, z=2.36, p=0.018); (2) performance-based indicators of UL motor function (14 studies, n=436; SMD=0.72, z=3.51, p<0.001); (3) self-report indicators of arm use (16 studies, n=541; weighted mean difference [WMD]=1.16, z=20.71, p<0.001); (4) self-reported arm motor function (16 studies, n=541; WMD=0.87, z=14.63, p<0.001); and (5) performance-based measures of arm motor impairment (11 studies, n=192; SMD=0.65, z=2.54, p=0.011). As in earlier systematic reviews, the conclusions drawn from these findings were limited by methodological issues in the trials available and the consequent difficulty in rationalizing the pooling of findings. For example, the validity of pooling the findings of RCTs that studied different CIMT regimens has been questioned by most reviews. Fortunately, as more RCTs of CIMT have been reported, it has become possible to address some of these issues. For example, Shi and colleagues'8 literature search for specific CIMT regimens (0.5–3 h/d of supervised repetitive practice and less than 6 h/d of restraint) found 13 suitable trials that could plausibly be pooled for synthesis with meta-analytic techniques.
Another issue that has limited the strength of conclusions is variability among the control interventions, especially with respect to time spent in therapy. Since amount of practice has been proposed as a crucial factor in determining outcomes in physical rehabilitation for stroke survivors,9 and CIMT interventions generally call for increased time in therapy relative to conventional practice, it would be worthwhile to determine whether the apparent difference in effect for CIMT interventions can be attributed to differences in amount of practice, as reflected by differences in time spent in therapy. To our knowledge, the literature comparing CIMT with a dose-matched control intervention (i.e., a control intervention of equivalent time) has not been reviewed.
The purpose of this systematic review, therefore, was to summarize the available trials examining the impact of CIMT on the UL function of adults living with the effects of stroke. In particular, we sought trials designed with a reference group receiving the same amount of therapy time as the CIMT group. Whenever possible, we used meta-analytic techniques to summarize the findings.
METHODS
Literature search and screening
We searched the following databases from their inception to February 2011: CINAHL, Cochrane Library, Embase, National Rehabilitation Information Centre (NARIC/CIRRIE—Rehabdata), PEDro, PubMed, Scopus, and Web of Science. The specific search strategy for CINAHL is given in the Appendix; all search strategies are available upon request. We also hand-searched the tables of contents for Archives of Physical Medicine and Rehabilitation, American Journal of Physical Medicine and Rehabilitation, American Journal of Occupational Therapy, Australian Journal of Occupational Therapy, Canadian Journal of Occupational Therapy, Clinical Rehabilitation, Disability and Rehabilitation, (Australian) Journal of Physiotherapy, Journal of Rehabilitation, Journal of Rehabilitation Medicine, NeuroRehabilitation, Neurorehabilitation and Neural Repair, Physical Therapy, Physiotherapy Canada, Physiotherapy Theory and Practice, and Stroke for January 2010 through February 2011. We looked for studies that (1) used a randomized controlled or crossover design; (2) studied adult survivors of a stroke; (3) examined a CIMT protocol with both a formal supervised training element and a restraint component; and (4) included a dose-matched control group (i.e., one receiving a formal supervised intervention equal in therapy time to the CIMT group).
Titles of all studies identified by the search strategy were reviewed by two authors (LT, HC) for obviously unsuitable publications. The abstracts of the remaining studies were reviewed by the same two authors to exclude inappropriate studies. Finally, the remaining studies were reviewed in full text by the same two authors; at this stage we excluded studies in which the reference group did not receive a dose-matched intervention. The remaining trials formed the pool upon which analyses were performed. As a final effort to identify suitable trials, we examined the reference lists of available narrative and systematic reviews and the included trials. Throughout the screening process, a publication was pushed to the next level of screening if either reviewer questioned its suitability for this review. Any discrepancy between reviewers at the final stage was resolved through discussion, with a third author (TS) serving as arbiter as necessary.
Quality of trials
The quality of each trial was assessed using the PEDro scale,10 which considers 11 key design and reporting elements of RCTs specific to physiotherapy and produces a summary score out of 10 (higher scores indicate higher quality of design). Evidence that the PEDro scale can be administered in a reliable11 way to give a valid indicator of the quality of an intervention trial12,13 is available; the scale has been used widely in systematic reviews of physiotherapy interventions.14–19 Two authors (LT, HC) independently scored all trials, resolving any discrepancy in scoring by negotiation; a third author (TS) served as arbiter, as necessary.
Data extraction
The relevant study characteristics and outcome data for each trial were extracted by one author (TS) using a data-extraction form developed for this review. A second author (HC) later confirmed the accuracy of this process. Each trial's randomization method and the number of groups formed by randomization were determined. We reviewed the relevant PEDro items to determine whether allocation concealment and blinding of assessors were adequate. Study participants were described by age; time since stroke onset; and presence/absence of minimum criteria for motor ability, cognitive ability, and/or degree of use of the more-affected upper limb. The constituent elements of the CIMT (amount and frequency of training, use of shaping principles, therapist:participant ratio during training , type and amount of restraint) and control interventions (amount, frequency, and content) were noted.
Each trial's evaluation methodology was described in terms of the specific instruments used and the timing of assessments. We anticipated four categories of instruments, based on the International Classification of Functioning, Disability and Health20 (ICF; see Box 1): UL Motor Capacity, UL Ability, Comprehensive Function, and Self-Report. We considered UL Motor Capacity and UL Ability to be the primary outcomes; secondary outcomes were measures from the Comprehensive Function and Self-Report categories. After extracting outcome data (group means and associated standard deviations for each instrument) for the CIMT and control groups at all evaluation points following completion of the intervention, we examined the pre-intervention scores for each instrument to determine whether any baseline differences were present that would invalidate use of post-intervention scores for meta-analysis. If necessary, we compared baseline scores between groups using Student's t-test or the Mann–Whitney rank-sum test with a critical p-value of 0.05. We attempted to obtain any missing data from the contact author(s) listed in the publication.
Box 1.
Categories of Instruments
| Category | Description | ICF domain | Example instruments |
|---|---|---|---|
| UL motor capacity | Measures of the degree of motor control of the upper limb | Body structure and function |
|
| UL ability | Measures reflecting the ability of the upper limb to contribute to daily life | Activity | |
| Comprehensive function | Instruments that provide a comprehensive indicator of the function of the individual | Activity | |
| Self-report measures | Instruments that use a self-report format | Potentially any ICF domain |
UL=upper limb; ICF=International Classification of Functioning, Disability and Health.
Statistical analysis
We used meta-analysis to summarize the findings when data reflecting the same outcome category were available from three or more trials. Where trials used the same instrument, the mean difference statistic (MD) was used to summarize the magnitude of any effect; where different trials used different instruments to measure the same outcome, the standardized mean difference (SMD) was used. The 95% confidence interval (95% CI) was calculated for each MD and SMD point estimate; a finding was considered statistically significant if the 95% CI did not contain zero. We assumed a fixed-effects model as the basis for all calculations unless significant heterogeneity among the findings from pooled trials was found.
Heterogeneity among pooled studies was evaluated using χ2 and I2 statistics: substantial heterogeneity was deemed to be present if the χ2 finding was significant (p<0.10) and the I2 estimate exceeded 50%. We performed sub-group analyses where a comparison showed substantial heterogeneity, to explore possible clinical and methodological reasons for the heterogeneity, and repeated analyses using a random-effects model for those comparisons that showed significant heterogeneity among included trials.
The strategy for our statistical analyses was guided by the Cochrane Handbook for Systematic Reviews of Interventions29 and performed using Review Manager 5.0 software (Nordic Cochrane Centre, Cochrane Collaboration, 2008). Our review process and its reporting were guided by the PRISMA Statement.30
RESULTS
Figure 1 outlines the literature search and screening process that identified 22 studies meeting our criteria.31–52 Note that two publications,53,54 although deemed potentially appropriate at the abstract review stage, were not included because we were unable to obtain the full text and therefore could not establish either suitability or outcomes. Table 1 shows the elements of the included trials' research designs. The majority of trials (19/22) reported blinding their assessors to participants' group assignment. Most formed two groups: an experimental group receiving the CIMT intervention and a control group receiving a dose-matched intervention; five trials31,42–44,46 added a third group that received no intervention, and two39,52 had a control group that received a specific bilateral arm-training regimen. Dromerick and colleagues33 formed a third group that received a longer-duration CIMT regimen, and Wang and colleagues48 formed a control group that received usual care; neither of these groups was used in our analyses. Five trials32,33,35,41,48 conducted follow-up assessments. The mean (SD) PEDro score for the included trials was 6.4 (1.2, range 3–8).
Figure 1.
Flow diagram showing accounting of studies through the search process.
Table 1.
Elements of Research Design for the Included Trials
| Study | Total patients/group |
Follow-up assessments? |
Blinded assessors? |
Allocation concealment? |
PEDro score (/10) |
|---|---|---|---|---|---|
| Atteya (2004)31 | CIMT=2 CONV=2 Nil=2 |
Post only | Unclear | Unclear | 3 |
| Boake et al. (2007)32 | CIMT=10 CONV=13 |
Post 3–4 mo |
Yes | Unclear | 6 |
| Dromerick et al. (2009)33 | CIMT=19 Hi-CIMT=16 CONV=17 |
Post 3 mo |
Yes | Unclear | 7 |
| Dromerick et al. (2000)34 | CIMT=11 CONV=9 |
Post only | Yes | Unclear | 7 |
| Hayner et al. (2010)35 | CIMT=6 CONV=6 |
Post 6 mo |
No | Unclear | 6 |
| Lin et al. (2007)36 | CIMT=17 CONV=15 |
Post only | Yes | Yes | 8 |
| Lin et al. (2008)37 | CIMT=12 CONV=10 |
Post only | Yes | Unclear | 7 |
| Lin et al. (2009a)38 | CIMT=16 CONV=16 |
Post only | Yes | Unclear | 7 |
| Lin et al. (2009b)39 | CIMT=20 BAT=20 CONV=20 |
Post only | Yes | Yes | 8 |
| Lin et al. (2010)40 | CIMT=5 CONV=8 |
Post only | Unclear | Unclear | 6 |
| Myint et al. (2008)41 | CIMT=23 CONV=20 |
Post 3 mo |
Yes | Yes | 7 |
| Page et al. (2001)42 | CIMT=2 CONV=2 Nil=2 |
Post only | Yes | Unclear | 4 |
| Page et al. (2002)43 | CIMT=4 CONV=5 Nil=5 |
Post only | Yes | Unclear | 6 |
| Page et al. (2004)44 | CIMT=7 CONV=4 Nil=6 |
Post only | Yes | Unclear | 5 |
| Page et al. (2005)45 | CIMT=5 CONV=5 |
Post only | Yes | Unclear | 5 |
| Page et al. (2008)46 | CIMT=13 CONV=12 Nil=10 |
Post only | Yes | Unclear | 7 |
| Suputtitada et al. (2004)47 | CIMT=33 CONV=36 |
Post only | Yes | Unclear | 7 |
| Wang et al. (2011)48 | CIMT=10 CONV=10 CONTROL=10 |
2 wk 4 wk |
Yes | Unclear | 7 |
| Wu et al. (2007a)49 | CIMT=13 CONV=13 |
Post only | Yes | Unclear | 7 |
| Wu et al. (2007b)50 | CIMT=15 CONV=15 |
Post only | Yes | Unclear | 7 |
| Wu et al. (2007c)51 | CIMT=24 CONV=23 |
Post only | Yes | Unclear | 7 |
| Wu et al. (2011)52 | CIMT=22 BAT=22 CONV=22 |
Post only | Yes | Unclear | 7 |
CIMT=constraint-induced movement therapy; CONV=conventional rehabilitation given for same duration as CIMT group; Nil=control therapy group that received no intervention; Hi-CIMT=CIMT intervention provided for longer duration; BAT=a specific bilateral arm-training regimen; CONTROL=conventional rehabilitation given for shorter duration than CIMT and CONV groups.
Study participants are described in Table 2. All trials used a screening method to ensure that all participants had some residual volitional movement of the affected upper limb, and 10 trials used the Amount of Use sub-scale of the Motor Activity Log (MAL-AoU) to exclude participants who made frequent use of their more-affected limb. The combination of these two criteria may have served to identify individuals demonstrating the learned non-use phenomenon (i.e., those who did not make spontaneous use of the upper limb despite having the motor ability to do so). Four trials31,42–44 that did not employ a criterion MAL-AoU score included an explicit statement that the researchers were satisfied that all included participants demonstrated learned non-use. Thus, 14/22 trials appear to have recruited adult survivors of stroke who satisfy the requirements for the learned non-use phenomenon. Twelve trials excluded participants because of the presence of spasticity in the arm or hand. All but two reported a formal screening process for establishing a minimum degree of cognition.
Table 2.
Participant Inclusion and Exclusion Criteria for Included Trials
| Study | Patient age | Time post event at recruitment |
Minimum motor requirement | Minimum cognitive requirement | MAL- AoU ≤2.5/5? |
|---|---|---|---|---|---|
| Atteya (2004)31 | 18–75 y | 1–6 mo |
|
Modified MMSE ≥70/100 | No |
| Boake et al. (2007)32 | “adult” | ≤2 wk |
|
MMSE ≥24/30 | No |
| Dromerick et al. (2009)33 | NS – mean age 63.9 y |
<28 d |
|
|
No |
| Dromerick et al. (2000)34 | NS – all patients >47 y |
<2 wk |
|
NIHSS–consciousness and communication ≤1 | No |
| Hayner et al. (2010)35 | 18–100 y | >6 mo | “trace movement of the hand” | MMSE ≥24/30 | No |
| Lin et al. (2007)36 | NS – all patients >43 y |
>1 y |
|
Modified MMSE ≥70/100 | Yes |
| Lin et al. (2008)37 | NS | NS – mean time post-stroke 18.9 mo | Brunnstrom–arm >III | “ability to understand and follow instructions” | Yes |
| Lin et al. (2009a)38 | NS – all patients >30 y |
NS – all patients recruited >6 mo post-CVA |
|
MMSE >24/30 | Yes |
| Lin et al. (2009b)39 | NS – all patients >23 y |
>6 mo |
|
MMSE >24/30 | Yes |
| Lin et al. (2010)40 | NS – mean age 49.6 y |
>3 mo |
|
MMSE ≥24/30 | No |
| Myint et al. (2008)41 | NS – mean age ∼63 y |
2–16 wk |
|
MMSE (Cantonese version) ≥17/30 |
No |
| Page et al. (2001)42 | NS – all patients >44 y |
1–6 mo |
|
Modified MMSE ≥70/100 | No |
| Page et al. (2002)43 | 19–95 y | 4 wk–6 mo |
|
Modified MMSE ≥70/100 | No |
| Page et al. (2004)44 | 18–95 y | >1 y |
|
Modified MMSE ≥70/100 | No |
| Page et al. (2005)45 | 18–95 y | <2 wk |
|
Modified MMSE ≥70/100 | Yes |
| Page et al. (2008)46 | 18–80 y | >1 y |
|
Modified MMSE ≥69/100 | Yes |
| Suputtitada et al. (2004)47 | 18–80 y | >1 y |
|
“no severe cognitive impairments” | No |
| Wang et al. (2011)48 | NS – mean age ∼63 y |
Described as “acute/subacute”; mean time since stroke ∼11 wk |
|
MMSE >24/30 | No |
| Wu et al. (2007a)49 | “Elderly” – all patients >65 y |
2 wk–31 mo |
|
Modified MMSE ≥63/100 | Yes |
| Wu et al. (2007b)50 | NS – all patients >45 y |
1–3 y |
|
Modified MMSE ≥70/100 | Yes |
| Wu et al. (2007c)51 | NS – all patients >40 y |
3 wk–37 mo | Brunnstrom–arm>III | Modified MMSE ≥70/100 | Yes |
| Wu et al. (2011)52 | NS – mean age 53.1 y |
>6 mo |
|
MMSE ≥23/30 | Yes |
MAL-AoU=Amount of Use sub-scale of Motor Activity Log; NS=not specified; MCP=metacarpophalangeal joint; MAS=Modified Ashworth Scale; MMSE=Mini-Mental Status Examination; NIHSS=National Institutes of Health Stroke Scale; SBMOCS=Short Blessed Memory Orientation and Concentration Scale; ARAT=Action Research Arm Test.
The components of the CIMT regimens are shown in Table 3. A clear majority of the trials (15/22) administered a supervised training regimen of either 20 or 30 hours. Three trials35,47,48 provided substantially more training time (60 h); six trials31,42–46 administered the training component over 10 weeks rather than the more common 2 or 3 weeks. One trial47 had a single therapist administer the training to groups of three or four participants; we assumed all other training was conducted with a 1:1 participant:therapist supervision ratio, although this was not always stated explicitly. All but four trials34,37,47,51 explicitly commented on the use of “shaping” principles, which suggests that the activities performed during the training component were recalibrated on an ongoing basis to present an achievable challenge. The restraint component most commonly involved wearing a padded mitt for 5 or 6 hours/day, 5 days/week. Three trials32,41,48 implemented the restraint for 90% of waking hours for the duration of the regimen. Of the 12 trials31,35,36,38,39,41–45,48,52 that described a strategy to measure adherence to the restraint component, eight31,36,38,41–43,45,49 commented on the actual degree of adherence. Four trials35,43–45 commented on performance of activities at home; none of the identified trials described a formal home programme. The content of the control regimens is shown in Table 3; none of the trials provided details on the strategy used to progress the difficulty of exercises/activities. Two trials37,38 included a restraint component in the control intervention.
Table 3.
Description of CIMT and Control Regimens Studied in Identified Trials
| Experimental (CIMT) regimen |
||||
|---|---|---|---|---|
| Study | Time in supervised practice (total hours) |
“Shaping” principles used? |
Restraint component |
Content of control regimen |
| Atteya (2004)31 | 1 h/d, 3 d/wk×10 wk (30 h) | Yes | 5 h/d, 5 d/wk×10 wk with a sling/mitt | Facilitation of more-affected limb and practice of compensation with less-affected limb |
| Boake et al. (2007)32 | 3 h/d 6 d/wk×14–15 d (42–45 h) | Yes | 90% of waking hours with a mitt | Therapeutic activities for more-affected hand and performance of daily activities with either hand |
| Dromerick et al. (2009)33 | 2 h/d, 5 d/wk×2 wk (20 h) | Yes | 6 h/d, 5 d/wk×2 wk with a mitt | Compensatory techniques for ADL, strengthening, and ROM |
| Dromerick et al. (2000)34 | 2 h/d, 5 d/wk×2 wk (20 h) | Unclear | 6 h/d, 5 d/wk×2 wk with a mitt | Compensatory techniques for ADL, strengthening, ROM, and positioning |
| Hayner et al. (2010)35 | 6 h/d, 5 d/wk×2 wk (60 h) | Yes | Minimum of 6 h/d, 5 d/wk×2 wk with a mitt | Bilateral performance of activities |
| Lin et al. (2007)36 | 2 h/d, 5 d/wk×3 wk (30 h) | Yes | 6 h/d, 5 d/wk×3 wk with a mitt | Strength, balance, and fine-motor training; practice of activities; stretching of more-affected arm |
| Lin et al. (2008)37 | 2 h/d, 5 d/wk×3 wk (30 h) | Unclear | 3 h/d, 5 d/wk×3 wk with a mitt | Weight bearing and practice of fine-motor activities; restraint of less-affected hand 3 h/d |
| Lin et al. (2009a)38 | 2 h/d, 5 d/wk×3 wk (30 h) | Yes | 6 h/d, 5 d/wk×3 wk with a mitt | Weight bearing and practice of fine-motor activities; restraint of less-affected hand 3 h/d |
| Lin et al. (2009b)39 | 2 h/d, 5 d/wk×3 wk (30 h) | Yes | 6 h/d, 5 d/wk×3 wk with a mitt | Training for hand function, coordination, balance, and movements of more-affected arm; uni- and bilateral practice of tasks |
| Lin et al. (2010)40 | 2 h/d, 5 d/wk×3 wk (30 h) | Yes | 6 h/d, 5 d/wk×3 wk with a mitt | Weight bearing and practice of fine-motor activities |
| Myint et al. (2008)41 | 4 h/d, 5 d/wk×2 wk (40 h) | Yes | 90% of waking hours with a padded shoulder sling | Bimanual UL tasks, practice of compensatory techniques, strengthening and ROM exercises |
| Page et al. (2001)42 | 1 h/d, 3 d/wk×10 wk (30 h) | Yes | 5 h/d, 5 d/wk×10 wk with a sling/mitt | Facilitation of more-affected limb; practice of compensation with less-affected limb |
| Page et al. (2002)43 | 1 h/d, 3 d/wk×10 wk (30 h) | Yes | 5 h/d, 5 d/wk×10 wk with a sling/splint | Facilitation of more-affected limb; practice of compensation with less-affected limb |
| Page et al. (2004)44 | 1 h/d, 3 d/wk×10 wk (30 h) | Yes | 5 h/d, 5 days/week×10 wk with a mitt | Facilitation of more-affected limb; practice of compensation with less-affected limb |
| Page et al. (2005)45 | 0.5 h/d, 3 d/wk×10 wk (15 h) | Yes | 5 h/d, 5 d/wk×10 wk with a mitt | Stretching of and weight bearing on more-affected limb; manual dexterity exercises and practice of compensatory techniques with less-affected limb |
| Page et al. (2008)46 | 0.5h/d, 3 d/wk×10 wk (15 h) | Yes | 5h/d, 5 d/wk×10 wk with a sling/mitt | Facilitation of more-affected limb; practice of compensation with less-affected limb |
| Suputtitada et al. (2004)47 | 6 h/d, 5 d/wk×2 wk (60 h) | Unclear | “Minimum of 60 h” with a mitt | Bimanual performance of activities |
| Wang et al. (2011)48 | 3 h/d, 5 d/wk×4 wk (60 h) | Yes | 90% of waking hours with a wrist/hand splint | Strength, balance, manual dexterity exercises; task practice; practice of activities using less-affected side |
| Wu et al. (2007a)49 | 2 h/d, 5 d/wk×3 wk (30 h) | Yes | 6 h/d, 5 d/wk×3 wk with a mitt | Functional task practice, stretching of and weight bearing on more-affected limb; manual dexterity exercises, practice of compensatory techniques with less-affected limb |
| Wu et al. (2007b)50 | 2 h/d, 5 d/wk×3 wk (30 h) | Yes | 6 h/d, 5 d/wk×3 wk with a mitt | Balance training, stretching of and weight bearing on the more-affected limb; manual dexterity exercises, practice of compensatory techniques with less-affected limb |
| Wu et al. (2007c)51 | 2 h/d, 5 d/wk×3 wk (30 h) | Unclear | 6 h/d, 5 d/wk×3 wk with a mitt | Functional task practice, stretching of and weight bearing on more-affected limb, manual dexterity exercises |
| Wu et al. (2011)52 | 2 h/d, 5 d/wk×3 wk (30 h) | Yes | 6 h/d, 5 d/wk×3 wk with a mitt | Functional task practice, coordination, balance, stretching of and weight bearing on more-affected limb; practice of compensatory techniques with less-affected limb |
CIMT=constraint-induced movement therapy; ADL=activities of daily living; ROM=range of motion; UL=upper limb.
Meta-analyses of primary outcomes
Upper-limb motor capacity
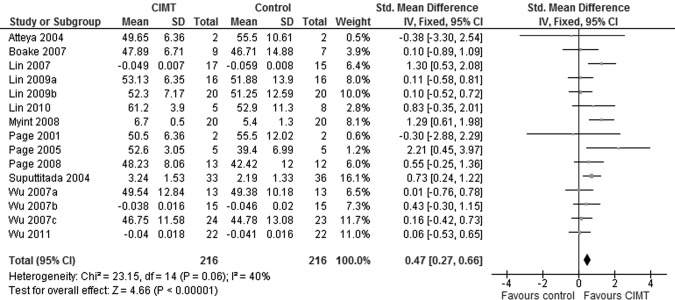
Nineteen trials included a measure of UL motor capacity. Despite attempts to contact the primary authors, we were unable to obtain outcomes from two trials43,44 in the format we required (group means and standard deviations). Another trial33 was excluded because it used a measure of UL motor capacity to describe the participants rather than using it to evaluate change over time. Finally, one trial37 was excluded because of differences in baseline scores. We therefore used 15 trials in our meta-analysis. A significant effect favouring the CIMT groups was found (Figure 2; SMD=0.47, 95% CI, 0.27–0.66). The heterogeneity among trials was acceptable (χ2=23.15, df=14, p=0.06; I2=40%).
Figure 2.
Forest plot of findings from studies comparing CIMT to dose-matched control intervention for upper-limb motor capacity outcomes.
The Fugl–Meyer Assessment (FMA) was the most commonly used instrument. Meta-analysis of the 10 trials for which FMA data were available showed a statistically significant effect favouring CIMT over the dose-matched control groups (MD=4.01, 95% CI, 1.34–6.69) without substantial heterogeneity among the trials (χ2=13.22, df=9, p=0.15; I2=32%). The other commonly used indicator of UL capacity—used in four trials—was derived from kinematic analysis of a reach task. Movement time was a common variable among the four trials; a smaller value indicates more efficient movement and, therefore, greater motor capacity. Since the same reaching task was not studied in all the trials, we used the SMD for meta-analysis of results; we found a significant effect favouring CIMT (SMD=−0.44, 95% CI, −0.76 to −0.11). Again, the indicators of heterogeneity did not reach the threshold for sub-group analysis (χ2=6.56, df=3, p=0.09; I2=54%). Three trials32,33,40 used technologies to describe neurophysiologic mechanisms underlying production of UL movement, but since each trial used a different technology, we were unable to identify a common variable that could plausibly be pooled, and therefore did not attempt a meta-analysis. Only two trials32,41 reported follow-up data on UL motor capacity; meta-analysis of these data was therefore not attempted.
Upper-limb ability
Sixteen trials gathered data on UL ability. Since we were again unable to obtain data from the same two trials,43,44 14 were left for meta-analysis. Three trials used more than one measure of UL ability (see Table 4); the Action Research Arm Test (ARAT) findings from these trials were used for analysis. As suggested by the Cochrane Handbook,28 we multiplied findings by −1 wherever necessary to account for the differences in test structures (e.g., desirable scores are low for the Wolf Motor Function Test [WMFT] but high for the ARAT and Grooved Pegboard Test). Meta-analysis showed a significant effect in favour of the CIMT groups (see Figure 3; SMD=0.80, 95% CI, 0.57–1.02) without substantial heterogeneity among groups (χ2=19.98, df=13, p=0.10; I2=35%). Meta-analysis of the 10 trials reporting ARAT scores revealed a significant effect favouring the CIMT groups (MD=8.91, 95% CI, 7.15–10.68).
Table 4.
Description of Instruments Used in the Included Trials
| Study | UL motor capacity | UL ability | Comprehensive function | Self-report measures |
|---|---|---|---|---|
| Atteya (2004)31 | FMA | ARAT WMFT |
— | MAL |
| Boake et al. (2007)32 | FMA TMS |
Grooved Pegboard test | — | MAL |
| Dromerick et al. (2009)33 | NIHSS MRI |
ARAT FIM-UE |
— | SIS |
| Dromerick et al. (2000)34 | — | ARAT | FIM Barthel |
— |
| Hayner et al. (2010)35 | — | WMFT | — | COPM |
| Lin et al. (2007)36 | Kinematics of a unilateral reaching task | — | FIM | MAL |
| Lin et al. (2008)37 | FMA | NEADL | FIM | MAL |
| Lin et al. (2009a)38 | FMA | NEADL | FIM | MAL SIS |
| Lin et al. (2009b)39 | FMA | — | FIM | MAL SIS |
| Lin et al. (2010)40 | FMA fMRI |
— | — | MAL |
| Myint et al. (2008)41 | Functional test | ARAT 9-Hole Peg test |
Modified Barthel | MAL |
| Page et al. (2001)42 | FMA | WMFT ARAT |
— | MAL |
| Page et al. (2002)43 | FMA | ARAT | — | MAL |
| Page et al. (2004)44 | FMA | ARAT | — | MAL |
| Page et al. (2005)45 | FMA | ARAT | — | MAL |
| Page et al. (2008)46 | FMA | ARAT | — | MAL |
| Suputtitada et al. (2004)47 | Pinch/grip strength | ARAT | — | — |
| Wang et al. (2011)48 | — | WMFT | — | — |
| Wu et al. (2007a)49 | FMA | — | FIM | MAL SIS Patient perception VAS |
| Wu et al. (2007b)50 | Kinematics of uni- and bilateral reaching tasks | — | FIM | MAL |
| Wu et al. (2007c)51 | FMA Kinematics of a unilateral reaching task |
— | — | MAL |
| Wu et al. (2011)52 | Kinematics of uni- and bilateral reaching tasks | WMFT | MAL |
UL=upper limb; FMA=upper extremity section of the Fugl–Meyer Assessment of Motor Function; ARAT=Action Research Arm Test; WMFT=Wolf Motor Function Test; MAL=Motor Activity Log; TMS=transcranial magnetic stimulation; NIHSS=National Institutes of Health Stroke Scale; FIM-UE=upper extremity sub-scale of the Functional Independence Measure; SIS=Stroke Impact Scale; MRI=magnetic resonance imaging; FIM=Functional Independence Measure; COPM=Canadian Occupational Performance Measure; NEADL=New England Activities of Daily Living Scale; VAS=visual analogue scale.
Figure 3.
Forest plot of findings from studies comparing the effect of CIMT and dose-matched control intervention on indicators of upper-limb ability.
Four trials32,33,35,41 reassessed UL ability 3–6 months post intervention. Because different instruments were used in these trials, our meta-analysis used the SMD. The meta-analysis showed a significant effect favouring CIMT (SMD=0.54, 95% CI, 0.14–0.94) without substantial heterogeneity among the trials (χ2=1.25, df=3, p=0.74; I2=0%).
Meta-analyses of secondary outcomes
Comprehensive function
Seven of the eight trials investigating the effect of CIMT on comprehensive function used the Functional Independence Measure (FIM); the other41 employed the modified Barthel Index (BI). One trial34 that used both BI and FIM scores reported change scores only, and we therefore could not include the data in our analysis. Limiting our analysis to the six trials with available FIM data, we found a significant effect favouring CIMT (see Figure 4; MD=5.05, 95% CI, 2.23–7.87) without substantial heterogeneity among trials (χ2=1.10, df=5, p=0.95; I2=0%). Since only one trial41 reported follow-up, meta-analysis was not possible.
Figure 4.
Forest plot summarizing findings from studies comparing CIMT to dose-matched control intervention for Functional Independence Measure scores immediately after completion of the intervention.
Self-report measures
Seventeen trials reported MAL scores; no other trial used an instrument measuring an attribute comparable to either the AoU or the QoM sub-scale. Five trials31,42–44,46 did not report results as group means and standard deviations, and we were unable to obtain the results in this format from the studies' authors. The MAL-QoM findings from another trial37 were excluded because the baseline CIMT and control scores were not comparable. This left 12 trials for meta-analysis of the MAL-AoU findings and 11 for the MAL-QoM. We found a significant effect in favour of CIMT for both sub-scales (AoU: see Figure 5; MD=1.05, 95% CI, 0.85–1.24; QoM: see Figure 6; MD=0.89, 95% CI, 0.69–1.08). The indicators of heterogeneity among trials prompted sub-group analyses for the AoU sub-scale (χ2=90.62, df=11, p<0.001; I2=88%) but not for the QoM sub-scale (χ2=18.47, df=10, p=0.05; I2=46%). When the meta-analysis of AoU scores was repeated with a random-effects model, we again found a preferential effect for CIMT (MD=0.75, 95% CI, 0.16–1.33). Follow-up MAL scores were reported by only two trials, and meta-analyses were therefore not attempted.
Figure 5.
Forest plot of findings from trials comparing the effects of CIMT and dose-matched control intervention on performance on the Amount of Use sub-scale of the Motor Activity Log.
Figure 6.
Forest plot of findings from trials comparing the effects of CIMT and dose-matched control intervention on performance on the Quality of Movement sub-scale of the Motor Activity Log.
The clinical factors available for investigating the heterogeneity among trials reporting MAL-AoU scores related to attributes of the participants (time since stroke onset; inclusion/exclusion criteria related to presence of learned non-use and UL spasticity) and features of the CIMT regimen (total number of training hours, total weeks of the regimen). The methodological factors available related to the quality of the trial (total sample size of the trial and total PEDro score). A summary of the results of the sub-group analyses is shown in Table 5. Trials that used inclusion criteria consistent with identifying participants with learned non-use do not seem to have produced different findings from those that did not. We found significant differences in effect when results were compared on the basis of participants' acuity status, whether the trial excluded people with spasticity, total sample size of the trial, and total PEDro scores. We did not perform sub-group analyses on the basis of aspects of the CIMT protocol, which was similar among trials (only two trials with available data used more than 30 h of training, and only one administered the training over 10 wk).
Table 5.
Summary of Sub-group Analyses Exploring Heterogeneity among Trials Reporting Motor Activity Log–Amount of Use Sub-scale Findings
| Sub-group comparison | No. of studies (n) | Effect estimate (95% CI) | Test for difference | p-value |
|---|---|---|---|---|
| Criteria for learned non-use used? | ||||
| Yes | 9 (283) | 1.01 (0.81–1.22) | χ2=0.83 | 0.36 |
| No | 3 (69) | 1.26 (0.77–1.75) | ||
| Time post stroke | ||||
| <6 mo | 3 (66) | 1.88 (1.58–2.17) | χ2=33.41 | 0.001 |
| ≥6 mo | 5 (178) | 0.62 (0.31–0.93) | ||
| Exclude participants with spasticity? | ||||
| Yes | 7 (180) | 1.61 (1.41–1.82) | χ2=23.9 | 0.001 |
| No | 5 (138) | 0.61 (0.26–0.95) | ||
| Sample size of trial | ||||
| ≥30 participants | 7 (265) | 0.84 (0.59–1.09) | χ2=6.63 | 0.01 |
| <30 participants | 5 (87) | 1.34 (1.05–1.64) | ||
| Total PEDro score | ||||
| ≥7 | 9 (313) | 0.61 (0.38–0.84) | χ2=46.34 | 0.001 |
| <7 | 3 (39) | 2.03 (1.69–2.38) |
Sensitivity analyses
We identified three assumptions for testing with sensitivity analysis. First, we assumed that findings could be pooled for analysis without regard to participants' time since stroke onset. To test this assumption, we compared UL motor capacity, UL ability, and comprehensive function findings from trials that included participants >6 months post stroke to those that included participants <6 months post stroke. Five trials37,40,48,49,51 recruited participants who spanned the categories, and therefore could not be used in the analysis. We found no significant differences (see Table 6), which suggests that trials produced similar findings regardless of time since stroke onset (<6 mo vs. >6 mo); therefore, this first assumption appears to be valid.
Table 6.
Summary of Findings for Sensitivity Analyses Testing Validity of Assumptions
| UL motor capacity | UL ability | Comprehensive function | |
|---|---|---|---|
| Acuity of participants | |||
| ≥6 mo post event | 7 trials n=270 SMD=0.52 95% CI, 0.27–0.77 |
6 trials n=180 SMD=0.74 95% CI, 0.43–1.04 |
4 trials n=134 SMD=0.59 95% CI, 0.24–0.93 |
| <6 mo post event | 5 trials n=74 SMD=0.93 95% CI, 0.41–1.45 |
8 trials n=150 SMD=1.00 95% CI, 0.65–1.36 |
2 trials n=63 SMD=0.61 95% CI, 0.10–1.12 |
| Test for sub-group difference | χ2=1.96; p=0.16 | χ2=1.21; p=0.27 | χ2=0.01; p=0.94 |
| Total weeks of CIMT regimen | |||
| 10 | 4 trials n=43 SMD=0.69 95% CI, 0.01–1.38 |
4 trials n=43 SMD=1.18 95% CI, 0.46–1.89 |
Unable to analyze |
| 2 or 3 | 12 trials n=411 SMD=0.39 95% CI, 0.19–0.59 |
7 trials n=235 SMD=0.92 95% CI, 0.65–1.19 |
|
| Test for sub-group difference | χ2=0.71; p=0.40 | χ2=0.44; p=0.51 |
UL=upper limb; SMD=standardized mean difference; CIMT=constraint-induced movement therapy.
Second, we assumed that the findings of trials administering different CIMT regimens could be pooled. To test this assumption, we compared UL motor capacity and UL ability findings from trials that used a 10-week regimen to those that used either a 2- or a 3-week regimen. (We were unable to compare the trials reporting comprehensive function outcomes because all used 2- or 3-week regimens.) We found no significant differences in effect, which suggests that our second assumption is also valid (see Table 6). Comparing outcomes on the basis of total number of training hours was not possible because of the lack of variability on this point among trials.
Finally, we assumed that all control interventions were equally likely to produce a positive effect in the UL function of the control subjects, and thus provided a consistent contrast for the CIMT experimental groups. A testable exception to this may be the two trials37,38 whose control interventions included a restraint component, which, by forcing use of the control patients' more-affected limb outside training sessions, may have reduced the contrast between CIMT and control groups. To test this assumption, we repeated the meta-analyses of the primary outcomes without these trials and found a significant effect in favour of CIMT for both of the primary outcomes (UL motor capacity: SMD=0.47, 95% CI, 0.29–0.70; UL ability: SMD=0.91, 95% CI, 0.67–1.16). Because the 95% CIs overlapped with those from the original analyses, we conclude that including these two trials was valid.
DISCUSSION
The main finding of this systematic review is that the existing literature shows CIMT to be superior to dose-matched control interventions in effecting positive change in performance-based measures of UL motor capacity, UL ability, comprehensive function (FIM), and a self-report measure of UL use and quality of movement (MAL) in adult survivors of stroke. Furthermore, the review demonstrates CIMT's superiority to dose-matched control interventions for performance on specific instruments (i.e., FMA, ARAT, and FIM). This superiority is documented only immediately after the intervention period, however, as few existing trials have included follow-up assessments. The effect is seen in survivors of stroke who have sufficient residual motor capacity to move their upper limb volitionally.
Although this finding largely replicates those of previous systematic reviews, the present review permits a stronger conclusion regarding the efficacy of CIMT for UL deficits in adult stroke survivors. As previous reviews5–7 noted, the variability of the control interventions among identified trials made it difficult to be conclusive as to the relative value of CIMT. Because we were able to identify sufficient trials that used dose-matched control groups, we can draw a more robust conclusion that the efficacy of CIMT does not seem to be a function of increased time spent in therapy. Another factor identified by early reviews as a barrier to synthesizing the findings of CIMT trials with meta-analysis related to the quality and diversity of instruments used to measure outcomes.3,5 The widespread use of instruments such as FMA, ARAT, FIM, and MAL would seem to indicate that this is a problem of the past.
Our strategy of limiting our review to trials designed with a dose-matched control group produced a set of trials consistent in other important clinical and methodological respects. Notably, 15 of the 22 included trials used a CIMT regimen 20 or 30 hours in duration. It is possible that the frequently discussed impracticality of applying the so-called signature CIMT regimen (totalling 60 h of training over 2 wk, with restraint of the less-affected arm for a goal of 90% of the individual's waking hours) in the clinical setting55–57 similarly applies to research settings and that, therefore, an RCT featuring a dose-matched control group would be more likely to administer a less resource-intensive CIMT regimen. This seems to be the experience of Shi and colleagues,8 whose search for trials examining specific CIMT regimens (training sessions of 0.5–3 h/d) revealed multiple trials designed with a dose-matched control group.
The meta-analyses showed acceptable levels of heterogeneity among the trials' results for all outcomes except the MAL-AoU. Sub-group analyses identified four factors that potentially explain the inconsistent findings (acuity of patients, possible presence of spasticity, sample size of the trial, and overall quality of the design). Another factor that might have contributed to the heterogeneity relates to the MAL's ability to provide dependable scores. The current state of knowledge on the measurement properties of the MAL sub-scales seems to be limited relative to that of other instruments used by the included trials (FMA, ARAT, and FIM). We are aware of three published studies that investigated the reliability of different versions of the MAL;27,58,59 all three examined reliability as a secondary concern, each used a different version of the instrument, and all examined a limited range of the scales (all patients scored ≤2.5 on the MAL-AoU) and reported modest results: Uswatte and colleagues27 reported a test–retest intra-class correlation coefficient (ICC 3,1) of 0.79 for a 28-item version of the MAL-AoU; van der Lee and colleagues58 calculated the inherent measurement error to be as much as 15% of the score from a 26-item version; and Uswatte and colleagues59 reported a Pearson correlation coefficient of 0.44 as an indication of test–retest reliability for the 14-item version). Since meta-analysis combines results from different trials and, therefore, different assessors, the lack of available evidence for the between-assessor reliability of the MAL is cause for concern. The observed heterogeneity in MAL-AoU findings among the included trials may therefore be attributable to the instrument's failing to provide robust data. This is a concern not only because the MAL is widely used to evaluate change over time but also because it has been used as an inclusion/exclusion criterion. Interestingly, the review by Shi and colleagues8 also found heterogeneity among trials for the MAL-AoU, but not for motor capacity, functional ability, or comprehensive function.
The literature search component of our project seems consistent with those of previous reviews, as we identified the same trials. An additional eight trials published since the last comprehensive search7 were suitable for this review; two additional trials53,54 not identified by previous reviews could have been included but, because we were unable to obtain the full publications, were not. According to the abstract, one trial53 describes a study comparing a CIMT intervention to a control intervention with 16 participants with “subacute and chronic stroke” and reports no difference in FMA, ARAT, or MAL outcomes; it is not clear whether the control intervention was dose-matched to the CIMT regimen. The second abstract54 reports that 20 participants were randomly allocated to receive either CIMT or a dose-matched control intervention, and that the CIMT group showed superior outcomes on the MAL but not on the WMFT. We were unable to obtain outcomes in the required format for two trials43,44 for the FMA and ARAT comparisons and for five31,42–44,46 trials for the MAL comparison. All five of the latter reported findings favouring the CIMT intervention over the dose-matched control intervention; therefore, the conservative scenario accounting for all missing data would have a “no difference” finding from a single study53 and positive findings for CIMT from the remaining six trials.31,42–44,46,54 It is likely that including these findings would not change the results of our meta-analyses.
The legitimacy of the three assumptions made in our meta-analyses is supported by sensitivity analysis. The finding that acuity of the patients studied did not influence UL motor capacity, UL ability, or comprehensive function outcomes is interesting in light of previous reports suggesting that time since stroke onset may be a significant factor. A large, methodologically rigorous, multi-centre RCT examining the efficacy of CIMT showed that adults who received the signature regimen between 3 and 9 months after stroke onset had superior outcomes to those whose CIMT intervention was delayed until 15–21 months post stroke.60 Further analysis demonstrated that stroke acuity was a significant predictor of a positive outcome when examined with univariate methods, but did not achieve significance when multivariate methods were used.61 Other attempts to identify predictors of CIMT outcomes have not consistently identified time since stroke as significantly influencing effectiveness of CIMT.62–64 These findings suggest that time since stroke is either a relatively weak predictor of outcome or has a substantial interaction effect with some other variable, such as initial motor capacity.
Testing our assumption about the legitimacy of combining outcomes from trials that administered different CIMT regimens produced a finding that warrants elaboration. Although it is tempting to use this analysis to rationalize the use of shorter-duration CIMT regimens, what the analysis actually shows is that the trials examining a 10-week regimen of CIMT and those examining a 2- or 3-week regimen showed comparable differences from their respective dose-matched control groups and that, therefore, we were justified in pooling these trials' results. This component of the sensitivity analysis should not be interpreted as indicating that the two CIMT regimens produced similar outcomes. The best way to determine the relative merits of different CIMT regimens is a dose-comparison trial; we are aware of only two such trials.33,65
Testing the assumption that all control interventions were equally likely to produce positive effects was difficult because of the limited descriptions of the control interventions. In particular, whereas the shaping process that provides general principles for selecting and progressing activities is well understood, we do not know what (if any) guiding principles were used in progressing the control interventions. The only analysis we were able to formulate was an examination of the trials that included a restraint component in their control interventions. Our finding that these trials did not influence the outcomes of the meta-analysis is consistent with those of studies demonstrating a lack of impact of restraint strategies on UL function in survivors of stroke.66,67
LIMITATIONS
The conclusions of this or any other systematic review are dictated by the methodological quality of the included studies and the thoroughness of their reporting. One factor pertinent to this review is the lack of in-depth descriptions of content for both control and CIMT interventions. While we contend that considering trials that matched groups for time in therapy allows for a stronger conclusion than would be possible if trials with non-matched groups were included, we acknowledge that other important aspects of the interventions may not have been equivalent between groups. For example, we could not account for use of motor learning principles such as provision of feedback, scheduling of practice/rest, and selection of tasks, which have been discussed as key considerations for stroke rehabilitation, in either CIMT or control interventions.67,68Similarly, we could not determine whether either the nature or the degree of challenge of the activities performed by participants was matched across groups. A large majority of the included trials indicated that the supervised training programme performed by the CIMT groups was guided by principles of shaping, a central tenet of which is that task difficulty is adjusted to each participant's sensorimotor ability so as to present him or her with tasks that are challenging but achievable;1 however, we were unable to glean any such guiding principles from the description of the various control interventions. It is noteworthy that the CIMT literature appears to be maturing to the point that trials have been designed with specific control interventions such as bilateral arm training.39,52
Eight of the included studies had small total sample sizes (<20 participants) and, consequently, give effect estimates associated with wide CIs. When the findings from underpowered studies are combined with meta-analysis, the observed effect is likely to be inflated; it is possible, therefore, that our estimate of effect is overestimated.
CONCLUSIONS
Meta-analyses show that CIMT appears to be more effective than dose-matched interventions in producing positive changes in indicators of UL motor capacity, UL ability, comprehensive function (FIM), and MAL scores in adult survivors of stroke who have residual movement in their affected upper limb but make limited use of it in everyday activities. With the exception of the MAL-AoU, the findings appear consistent across the available trials. Because few trials gathered follow-up outcomes, it is not known whether this effect persists over time.
This systematic review adds to and increases the strength of the evidence supporting the use of CIMT to address UL dysfunction in adult survivors of stroke. Ongoing questions about how best to translate these findings into clinical practice remain. The major implication of this review for researchers is the demonstration that the apparent effectiveness of the CIMT package cannot simply be attributed to increased time in therapy. Further research initiatives to clarify the retention of the CIMT effect, identify people most likely to respond well to CIMT, and articulate the optimal CIMT treatment package are required.
KEY MESSAGES
What is already known on this topic
To date, summaries of CIMT's effectiveness in treating upper limb (UL) dysfunction in adult survivors of stroke have noted that the methodological inconsistencies among trials prevent unqualified conclusions.
What this study adds
This systematic review indicates that the body of literature examining the impact of CIMT on UL dysfunction has matured sufficiently that the quantity and quality of trials permit meaningful synthesis of findings using meta-analysis. Specifically, meta-analyses show CIMT to be more effective than equivalent amounts of control interventions in improving UL function in adult survivors of stroke who have some volitional movement of the affected limb.
Appendix: Search strategy for CINAHL
(MH “Constraint-Induced Therapy”) or TX Constraint-Induced or TX constrained induced or TX constrain induced
(MH “Cerebrovascular Disorders+”) or (MH “Stroke Patients”) or (MH “Brain Injuries+”) or (MH “Hemiplegia”) or (MH “Dystonia+”) or AB (stroke or cerebrovascular or cerebral vascular or hemipleg* or paresis or pareses or hemipares* or parapares* or paretic or hemiparetic or paraparetic or dystoni*) or TI (stroke or cerebrovascular or cerebral vascular or hemipleg* or paresis or pareses or hemipares* or parapares* or paretic or hemiparetic or paraparetic or dystoni*)
(MH “Upper Extremity+”) or AB (upperextremit* or upper limb* or hand or hands or arm or arms or finger or fingers or shoulder*) or TI (upper extremit* or upper limb* or hand or hands or arm or arms or finger or fingers or shoulder*)
#1 and #2 and #3
(MH “Adult+”)
#5 and #4
Physiotherapy Canada 2012; 64(4);397–413; doi:10.3138/ptc.2011-24
REFERENCES
- 1.Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterizing the intervention protocol. Eura Medicophys. 2006;42(3):257–68. Medline:17039224. [PubMed] [Google Scholar]
- 2.Taub E, Crago JE, Burgio LD, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61(2):281–93. doi: 10.1901/jeab.1994.61-281. http://dx.doi.org/10.1901/jeab.1994.61-281. Medline:8169577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakkennes S, Keating JL. Constraint-induced movement therapy following stroke: a systematic review of randomised controlled trials. Aust J Physiother. 2005;51(4):221–31. doi: 10.1016/s0004-9514(05)70003-9. http://dx.doi.org/10.1016/S0004-9514(05)70003-9. Medline:16321129. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund A, Fecht A. The effectiveness of constraint-induced therapy as a stroke intervention: a meta-analysis. Occup Ther Health Care. 2006;20(2):31–49. doi: 10.1080/J003v20n02_03. http://dx.doi.org/10.1300/J003v20n02_03. [DOI] [PubMed] [Google Scholar]
- 5.Bonaiuti D, Rebasti L, Sioli P. The constraint induced movement therapy: a systematic review of randomised controlled trials on the adult stroke patients. Eura Medicophys. 2007;43(2):139–46. Medline:17525700. [PubMed] [Google Scholar]
- 6.French B, Thomas LH, Leathley MJ, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2007;4(4):CD006073. doi: 10.1002/14651858.CD006073.pub2. http://dx.doi.org/10.1002/14651858.CD006073.pub2. Medline:17943883. [DOI] [PubMed] [Google Scholar]
- 7.Sirtori V, Corbetta D, Moja L, et al. Constraint-induced movement therapy for upper extremities in stroke patients. Cochrane Database Syst Rev. 2009;4(4):CD004433. doi: 10.1002/14651858.CD004433.pub2. http://dx.doi.org/10.1002/14651858.CD004433.pub2. Medline:19821326. [DOI] [PubMed] [Google Scholar]
- 8.Shi YX, Tian JH, Yang KH, et al. Modified constraint-induced movement therapy versus traditional rehabilitation in patients with upper-extremity dysfunction after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2011;92(6):972–82. doi: 10.1016/j.apmr.2010.12.036. http://dx.doi.org/10.1016/j.apmr.2010.12.036. Medline:21621674. [DOI] [PubMed] [Google Scholar]
- 9.Ada L, Dean CM, Mackey FH. Increasing the amount of physical activity undertaken after stroke. Phys Ther Rev. 2006;11(2):91–100. http://dx.doi.org/10.1179/108331906X98994. [Google Scholar]
- 10.Moseley AM, Herbert RD, Sherrington C, et al. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48(1):43–9. doi: 10.1016/s0004-9514(14)60281-6. Medline:11869164. [DOI] [PubMed] [Google Scholar]
- 11.Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21. Medline:12882612. [PubMed] [Google Scholar]
- 12.Bhogal SK, Teasell RW, Foley NC, et al. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J Clin Epidemiol. 2005;58(7):668–73. doi: 10.1016/j.jclinepi.2005.01.002. http://dx.doi.org/10.1016/j.jclinepi.2005.01.002. Medline:15939217. [DOI] [PubMed] [Google Scholar]
- 13.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–33. doi: 10.1016/s0004-9514(09)70043-1. http://dx.doi.org/10.1016/S0004-9514(09)70043-1. Medline:19463084. [DOI] [PubMed] [Google Scholar]
- 14.Barbaric M, Brooks E, Moore L, et al. Effects of physical activity on cancer survival: a systematic review. Physiother Can. 2010;62(1):25–34. doi: 10.3138/physio.62.1.25. http://dx.doi.org/10.3138/physio.62.1.25. Medline:21197176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valkenet K, van de Port IG, Dronkers JJ, et al. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil. 2011;25(2):99–111. doi: 10.1177/0269215510380830. http://dx.doi.org/10.1177/0269215510380830. Medline:21059667. [DOI] [PubMed] [Google Scholar]
- 16.Hayward K, Barker R, Brauer S. Interventions to promote upper limb recovery in stroke survivors with severe paresis: a systematic review. Disabil Rehabil. 2010;32(24):1973–86. doi: 10.3109/09638288.2010.481027. http://dx.doi.org/10.3109/09638288.2010.481027. Medline:20964563. [DOI] [PubMed] [Google Scholar]
- 17.Batchelor F, Hill K, Mackintosh S, et al. What works in falls prevention after stroke?: a systematic review and meta-analysis. Stroke. 2010;41(8):1715–22. doi: 10.1161/STROKEAHA.109.570390. http://dx.doi.org/10.1161/STROKEAHA.109.570390. Medline:20616328. [DOI] [PubMed] [Google Scholar]
- 18.Macedo LG, Maher CG, Latimer J, et al. Motor control exercise for persistent, nonspecific low back pain: a systematic review. Phys Ther. 2009;89(1):9–25. doi: 10.2522/ptj.20080103. http://dx.doi.org/10.2522/ptj.20080103. Medline:19056854. [DOI] [PubMed] [Google Scholar]
- 19.Bovend'Eerdt TJ, Newman M, Barker K, et al. BovendEerdt. The effects of stretching in spasticity: a systematic review. Arch Phys Med Rehabil. 2008;89(7):1395–406. doi: 10.1016/j.apmr.2008.02.015. http://dx.doi.org/10.1016/j.apmr.2008.02.015. Medline:18534551. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. International classification of functioning, disability and health (ICF) Geneva: The Organization; 2001. [Google Scholar]
- 21.Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. Medline:1135616. [PubMed] [Google Scholar]
- 22.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4(4):483–92. doi: 10.1097/00004356-198112000-00001. http://dx.doi.org/10.1097/00004356-198112000-00001. Medline:7333761. [DOI] [PubMed] [Google Scholar]
- 23.Kellor M, Frost J, Silberberg N, et al. Hand strength and dexterity. Am J Occup Ther. 1971;25(2):77–83. Medline:5551515. [PubMed] [Google Scholar]
- 24.Morris DM, Uswatte G, Crago JE, et al. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82(6):750–5. doi: 10.1053/apmr.2001.23183. http://dx.doi.org/10.1053/apmr.2001.23183. Medline:11387578. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton BB, Laughlin JA, Fiedler RC, et al. Interrater reliability of the 7-level functional independence measure (FIM) Scand J Rehabil Med. 1994;26(3):115–9. Medline:7801060. [PubMed] [Google Scholar]
- 26.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–5. Medline:14258950. [PubMed] [Google Scholar]
- 27.Uswatte G, Taub E, Morris D, et al. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67(7):1189–94. doi: 10.1212/01.wnl.0000238164.90657.c2. http://dx.doi.org/10.1212/01.wnl.0000238164.90657.c2. Medline:17030751. [DOI] [PubMed] [Google Scholar]
- 28.Duncan PW, Wallace D, Studenski S, et al. Conceptualization of a new stroke-specific outcome measure: the stroke impact scale. Top Stroke Rehabil. 2001;8(2):19–33. doi: 10.1310/BRHX-PKTA-0TUJ-UYWT. http://dx.doi.org/10.1310/BRHX-PKTA-0TUJ-UYWT. Medline:14523743. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.0.2 [updated 2009 Sep] The Cochrane Collaboration; 2009. [cited 2011 Nov 11]. Available from: www.cochrane-handbook.org. [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. Medline:19622512. [DOI] [PubMed] [Google Scholar]
- 31.Atteya A-AA. Effects of modified constraint induced therapy on upper limb function in subacute stroke patients. Neurosci. 2004;9:24–9. [PubMed] [Google Scholar]
- 32.Boake C, Noser EA, Ro T, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21(1):14–24. doi: 10.1177/1545968306291858. http://dx.doi.org/10.1177/1545968306291858. Medline:17172550. [DOI] [PubMed] [Google Scholar]
- 33.Dromerick AW, Lang CE, Birkenmeier RL, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology. 2009;73(3):195–201. doi: 10.1212/WNL.0b013e3181ab2b27. http://dx.doi.org/10.1212/WNL.0b013e3181ab2b27. Medline:19458319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000;31(12):2984–8. doi: 10.1161/01.str.31.12.2984. http://dx.doi.org/10.1161/01.STR.31.12.2984. Medline:11108760. [DOI] [PubMed] [Google Scholar]
- 35.Hayner K, Gibson G, Giles GM. Comparison of constraint-induced movement therapy and bilateral treatment of equal intensity in people with chronic upper-extremity dysfunction after cerebrovascular accident. Am J Occup Ther. 2010;64(4):528–39. doi: 10.5014/ajot.2010.08027. http://dx.doi.org/10.5014/ajot.2010.08027. Medline:20825123. [DOI] [PubMed] [Google Scholar]
- 36.Lin K-C, Wu CY, Wei T-H, et al. Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: a randomized controlled study. Clin Rehabil. 2007;21(12):1075–86. doi: 10.1177/0269215507079843. http://dx.doi.org/10.1177/0269215507079843. Medline:18042603. [DOI] [PubMed] [Google Scholar]
- 37.Lin K-C, Wu C-Y, Liu J-S. A randomized controlled trial of constraint-induced movement therapy after stroke. Acta Neurochir Suppl (Wien) 2008;101:61–4. doi: 10.1007/978-3-211-78205-7_10. http://dx.doi.org/10.1007/978-3-211-78205-7_10. Medline:18642635. [DOI] [PubMed] [Google Scholar]
- 38.Lin K-C, Wu C-Y, Liu J-S, et al. Constraint-induced therapy versus dose-matched control intervention to improve motor ability, basic/extended daily functions, and quality of life in stroke. Neurorehabil Neural Repair. 2009;23(2):160–5. doi: 10.1177/1545968308320642. http://dx.doi.org/10.1177/1545968308320642. Medline:18981188. [DOI] [PubMed] [Google Scholar]
- 39.Lin K-C, Chang Y-F, Wu C-Y, et al. Effects of constraint-induced therapy versus bilateral arm training on motor performance, daily functions, and quality of life in stroke survivors. Neurorehabil Neural Repair. 2009;23(5):441–8. doi: 10.1177/1545968308328719. http://dx.doi.org/10.1177/1545968308328719. Medline:19118130. [DOI] [PubMed] [Google Scholar]
- 40.Lin K-C, Chung H-Y, Wu C-Y, et al. Constraint-induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. Am J Phys Med Rehabil. 2010;89(3):177–85. doi: 10.1097/PHM.0b013e3181cf1c78. http://dx.doi.org/10.1097/PHM.0b013e3181cf1c78. Medline:20173425. [DOI] [PubMed] [Google Scholar]
- 41.Myint JMWW, Yuen GFCY, Yu TKK, et al. A study of constraint-induced movement therapy in subacute stroke patients in Hong Kong. Clin Rehabil. 2008;22(2):112–24. doi: 10.1177/0269215507080141. http://dx.doi.org/10.1177/0269215507080141. Medline:18212033. [DOI] [PubMed] [Google Scholar]
- 42.Page SJ, Sisto SA, Levine P, et al. Modified constraint induced therapy: a randomized feasibility and efficacy study. J Rehabil Res Dev. 2001;38(5):583–90. Medline:11732835. [PubMed] [Google Scholar]
- 43.Page SJ, Sisto S, Johnston MV, et al. Modified constraint-induced therapy after subacute stroke: a preliminary study. Neurorehabil Neural Repair. 2002;16(3):290–5. doi: 10.1177/154596830201600307. Medline:12234091. [DOI] [PubMed] [Google Scholar]
- 44.Page SJ, Sisto S, Levine P, et al. Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004;85(1):14–8. doi: 10.1016/s0003-9993(03)00481-7. http://dx.doi.org/10.1016/S0003-9993(03)00481-7. Medline:14970962. [DOI] [PubMed] [Google Scholar]
- 45.Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19(1):27–32. doi: 10.1177/1545968304272701. http://dx.doi.org/10.1177/1545968304272701. Medline:15673841. [DOI] [PubMed] [Google Scholar]
- 46.Page SJ, Levine P, Leonard A, et al. Modified constraint-induced therapy in chronic stroke: results of a single-blinded randomized controlled trial. Phys Ther. 2008;88(3):333–40. doi: 10.2522/ptj.20060029. http://dx.doi.org/10.2522/ptj.20060029. Medline:18174447. [DOI] [PubMed] [Google Scholar]
- 47.Suputtitada A, Suwanwela NC, Tumvitee S. Effectiveness of constraint-induced movement therapy in chronic stroke patients. J Med Assoc Thai. 2004;87(12):1482–90. Medline:15822545. [PubMed] [Google Scholar]
- 48.Wang Q, Zhao J-L, Zhu Q-X, et al. Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. J Rehabil Med. 2011;43(7):619–25. doi: 10.2340/16501977-0819. http://dx.doi.org/10.2340/16501977-0819. Medline:21603848. [DOI] [PubMed] [Google Scholar]
- 49.Wu C-Y, Chen C-L, Tsai W-C, et al. A randomized controlled trial of modified constraint-induced movement therapy for elderly stroke survivors: changes in motor impairment, daily functioning, and quality of life. Arch Phys Med Rehabil. 2007;88(3):273–8. doi: 10.1016/j.apmr.2006.11.021. http://dx.doi.org/10.1016/j.apmr.2006.11.021. Medline:17321816. [DOI] [PubMed] [Google Scholar]
- 50.Wu CY, Lin KC, Chen HC, et al. Effects of modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke: a kinematic study of motor control mechanisms. Neurorehabil Neural Repair. 2007;21(5):460–6. doi: 10.1177/1545968307303411. http://dx.doi.org/10.1177/1545968307303411. Medline:17601803. [DOI] [PubMed] [Google Scholar]
- 51.Wu CY, Chen CL, Tang SF, et al. Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88(8):964–70. doi: 10.1016/j.apmr.2007.05.012. http://dx.doi.org/10.1016/j.apmr.2007.05.012. Medline:17678656. [DOI] [PubMed] [Google Scholar]
- 52.Wu CY, Chuang LL, Lin KC, et al. Randomized trial of distributed constraint-induced therapy versus bilateral arm training for the rehabilitation of upper-limb motor control and function after stroke. Neurorehabil Neural Repair. 2011;25(2):130–9. doi: 10.1177/1545968310380686. http://dx.doi.org/10.1177/1545968310380686. Medline:20947493. [DOI] [PubMed] [Google Scholar]
- 53.Dinçer Ü, Cakar E, Kiralp MZ, et al. The effect of modified constraint induced therapy on upper limb function in patient with subacute and chronic stroke. J Rheumatol Med Rehabil. 2008;19:125–31. [Google Scholar]
- 54.Li Z-L, Zhao J-X, Taub E. Recovery effect of constraint-induced movement therapy on upper extremity activity of stroke patients with hemiparesis. J Jilin Univers Med Ed. 2008;34:11–4. [Google Scholar]
- 55.Levine P, Page SJ. Modified constraint-induced therapy: a promising restorative outpatient therapy. Top Stroke Rehabil. 2004;11(4):1–10. doi: 10.1310/R4HN-51MW-JFYK-2JAN. http://dx.doi.org/10.1310/R4HN-51MW-JFYK-2JAN. Medline:15592985. [DOI] [PubMed] [Google Scholar]
- 56.Sterr A, Szameitat A, Shen S, et al. Application of the CIT concept in the clinical environment: hurdles, practicalities, and clinical benefits. Cogn Behav Neurol. 2006;19(1):48–54. doi: 10.1097/00146965-200603000-00006. http://dx.doi.org/10.1097/00146965-200603000-00006. Medline:16633019. [DOI] [PubMed] [Google Scholar]
- 57.Blanton S, Wilsey H, Wolf SL. Constraint-induced movement therapy in stroke rehabilitation: perspectives on future clinical applications. NeuroRehabilitation. 2008;23(1):15–28. Medline:18356586. [PubMed] [Google Scholar]
- 58.van der Lee JH, Beckerman H, Knol DL, et al. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35(6):1410–4. doi: 10.1161/01.STR.0000126900.24964.7e. http://dx.doi.org/10.1161/01.STR.0000126900.24964.7e. Medline:15087552. [DOI] [PubMed] [Google Scholar]
- 59.Uswatte G, Taub E, Morris D, et al. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke. 2005;36(11):2493–6. doi: 10.1161/01.STR.0000185928.90848.2e. http://dx.doi.org/10.1161/01.STR.0000185928.90848.2e. Medline:16224078. [DOI] [PubMed] [Google Scholar]
- 60.Wolf SL, Thompson PA, Winstein CJ, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41(10):2309–15. doi: 10.1161/STROKEAHA.110.588723. http://dx.doi.org/10.1161/STROKEAHA.110.588723. Medline:20814005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park S-W, Wolf SL, Blanton S, et al. The EXCITE trial: predicting a clinically meaningful motor activity log outcome. Neurorehabil Neural Repair. 2008;22(5):486–93. doi: 10.1177/1545968308316906. http://dx.doi.org/10.1177/1545968308316906. Medline:18780883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fritz SL, Light KE, Clifford SN, et al. Descriptive characteristics as potential predictors of outcomes following constraint-induced movement therapy for people after stroke. Phys Ther. 2006;86(6):825–32. Medline:16737408. [PubMed] [Google Scholar]
- 63.Lin KC, Huang YH, Hsieh YW, et al. Potential predictors of motor and functional outcomes after distributed constraint-induced therapy for patients with stroke. Neurorehabil Neural Repair. 2009;23(4):336–42. doi: 10.1177/1545968308321773. http://dx.doi.org/10.1177/1545968308321773. Medline:18984830. [DOI] [PubMed] [Google Scholar]
- 64.Huang YH, Wu CY, Hsieh YW, et al. Predictors of change in quality of life after distributed constraint-induced therapy in patients with chronic stroke. Neurorehabil Neural Repair. 2010;24(6):559–66. doi: 10.1177/1545968309358074. http://dx.doi.org/10.1177/1545968309358074. Medline:20439499. [DOI] [PubMed] [Google Scholar]
- 65.Sterr A, Elbert T, Berthold I, et al. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83(10):1374–7. doi: 10.1053/apmr.2002.35108. http://dx.doi.org/10.1053/apmr.2002.35108. Medline:12370871. [DOI] [PubMed] [Google Scholar]
- 66.Hammer AM, Lindmark B. Effects of forced use on arm function in the subacute phase after stroke: a randomized, clinical pilot study. Phys Ther. 2009;89(6):526–39. doi: 10.2522/ptj.20080017. http://dx.doi.org/10.2522/ptj.20080017. Medline:19372172. [DOI] [PubMed] [Google Scholar]
- 67.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. http://dx.doi.org/10.1097/01.wco.0000200544.29915.cc. Medline:16415682. [DOI] [PubMed] [Google Scholar]
- 68.Brogårdh C, Lexell J. A 1-year follow-up after shortened constraint-induced movement therapy with and without mitt poststroke. Arch Phys Med Rehabil. 2010;91(3):460–4. doi: 10.1016/j.apmr.2009.11.009. http://dx.doi.org/10.1016/j.apmr.2009.11.009. Medline:20298840. [DOI] [PubMed] [Google Scholar]