ABSTRACT
Purpose: To provide a comprehensive review of changes that occur in the muscle after stroke and how these changes influence the force-generating capacity of the muscle. Methods: A literature search of PubMed, CINAHL, MEDLINE, and Embase was conducted using the search terms stroke, hemiparesis, muscle structure, cross sectional area, atrophy, force, velocity, and torque. There were 27 articles included in this review. Results: Three changes occur in the muscle after stroke: a decrease in muscle mass, a decrease in fibre length, and a smaller pennation angle. In addition, the tendon is stretched and becomes more compliant. All of these factors reduce the affected muscle's ability to generate forces similar to controls or to non-paretic muscles. The result is a leftward shift in the length–tension curve, a downward shift in the torque–angle curve, and a downward shift in the force–velocity curve. Conclusion: Changes in muscle architecture contributing to weakness, such as muscle-fibre length, pennation angle, muscle atrophy, and tendon compliance, should be prevented or reversed by means of an appropriate rehabilitation programme.
Key Words: atrophy, muscles, paresis, stroke, torque
RÉSUMÉ
Objectif : Procéder à une étude exhaustive des changements qui surviennent dans les muscles après un accident vasculaire cérébral (AVC) et de la façon dont ces changements affectent la capacité des muscles à développer de la force. Méthode : Une recherche documentaire dans les bases de données PubMed, CINAHL, MEDLINE et Embase a été réalisée à l'aide des mots clés stroke (accident vasculaire cérébral), hemiparesis (hémiparésie), muscle structure (structure musculaire), cross sectional area (section transversale), atrophy (atrophie), force (force), velocity (vélocité) et torque (puissance). La recherche a permis de répertorier 27 articles. Résultats : À la suite d'un AVC, trois changements se produisent dans les muscles: une diminution de la masse musculaire, une diminution de la longueur des fibres musculaires et un angle de pennation. De plus, le tendon s'étire et devient plus lâche. Tous ces facteurs réduisent la capacité des muscles affectés à générer une force nécessaire pour contrôler ou similaire à celle des muscles qui ne sont pas paralysés. Le tout occasionne un glissement vers la gauche de la longueur de la courbe de tension, une poussée vers le bas de l'angle de la courbe de puissance et un abaissement similaire de la courbe de la vélocité de la force. Conclusion : À l'aide d'un programme de réadaptation approprié, il est possible de prévenir et de mettre fin aux changements dans l'architecture musculaire qui contribuent à l'affaiblissement, notamment dans la longueur des fibres musculaires et dans l'angle de pennation, de même qu'à l'atrophie musculaire et au relâchement des tendons.
Mots clés : accident vasculaire cérébral, atrophie, contraction, muscles squelettiques, paralysie
Hemiparesis, commonly found in people with stroke, can persist for years and limits functional performance. Some factors thought to explain, at least in part, the inability of muscles to generate the appropriate forces following a stroke are an increase in stretch reflex excitability,1–3 an increase in antagonist muscle coactivation4–8, a decrease in motor-unit firing rates,9–11 and force deficits dependent on muscle length.12,13 Considerable research has investigated neuroplasticity and the reorganization of neural pathways during recovery from stroke (for recent reviews, see14,15), but less is known about muscular factors that influence hemiparesis or muscle weakness.
The mechanical properties of muscle play an integral role in force generation; that is, a command from the nervous system to activate a muscle will generate varying levels of force depending on the state of that muscle. The influence of muscle length and velocity of shortening on force production are characterized in the length–tension relationship and the force–velocity relationship of muscle. The length–tension relationship (or the angle–torque relationship across a joint where multiple muscles act to produce a net torque) reveals that muscles produce less force in shortened positions than in lengthened positions.16 The force–velocity relationship reveals that the faster the shortening contraction, the lower the force generated, whereas the impact of increasing velocity of lengthening contractions on force generation is relatively small.17
Sarcopenia (loss of skeletal muscle mass and increase in fat mass) occurs in ageing muscles.18 Inactivity or immobilization is thought to accelerate sarcopenia; with 2 weeks of inactivity, muscle mass decreases, along with muscle strength.19 After a stroke, the time spent in bed during the day is greater than 50%.20 Thus, damage caused by the stroke and decreased mobility would combine to produce a decline in muscle mass in the paretic muscles and, to a lesser extent, in the non-paretic muscles. Since declines in muscle mass parallel deficits in force in studies on ageing, we would expect similar findings after stroke. Indeed, a recent systematic review and meta-analysis performed by English and colleagues21 demonstrated that lean tissue mass of paretic muscle was significantly less than that of non-paretic muscle, in both upper and lower extremities, in people at least 6 months post stroke. Longitudinal studies of changes in muscle mass over time could not be pooled for meta-analysis because of differences in outcome measures, characteristics, and timing of measurements.21 Our review extends the findings of English and colleagues21 by considering the interrelationship of stroke and age-related sarcopenia and by examining other important architectural components, including changes in pennation angle, fibre length, and tendon length, that occur after stroke and commenting on how these changes affect force generation. Specifically, the purpose of this review is to examine post-stroke changes in muscle size, muscle fibre length and orientation, and tendon length and to understand how these changes in muscle architecture may influence the angle–torque and force–velocity relationships for the generation of force, which is important in performing functional activities of daily living (ADL).
METHODS
We searched PubMed (1947–January 2010), CINAHL (1982–January 2010), MEDLINE (1946–January 2010), and Embase (1980–January 2010) using the following keywords: stroke, hemiparesis, muscle structure, cross sectional area, atrophy, force, velocity, and torque. The search was limited to studies published in English and involving humans. (An example search strategy is provided in Appendix 1.) The search yielded 2,083 articles, of which 1,514 remained after duplicates were removed. These 1,514 articles were screened first by title and abstract for the following inclusion criteria: (1) persons with hemiparesis as a result of a stroke; (2) studies examining the architecture of the muscle, such as pennation angle, atrophy, fibre length, and tendon changes; and (3) studies examining the influence of muscle properties on force production. Articles were excluded if (1) the study was examining validity or reliability, (2) treatment or an intervention was used, or (3) the investigation was a predictive study.
RESULTS
We identified 24 articles from the database search. The reference lists of these articles were reviewed for any additionally missed articles; this review identified a total of 3 additional articles, for a total of 27. Of the 27 articles included in this review, 14 investigated architectural changes in muscles (summarized in Table 1), 14 examined changes in force generation (summarized in Table 2), and one studied both.
Table 1.
Summary of Studies Examining Architectural Changes in Muscle Size, Muscle Fibre Length, Pennation Angle, and Tendon Length
| Study | n | Months* Post Stroke |
Comparison | Level of Recovery | Measures | Muscle | Results |
|---|---|---|---|---|---|---|---|
| Iversen et al.27 | 15 | >6 | P vs. NP; cross-sectional |
NR | DEXA: lean mass | Leg; arm | Arm and leg: P<NP |
| Ramnemark et al.28 |
19 | 1, 4, 7, and 12 |
P vs. NP; longitudinal |
Inability to lift arm or leg at 1 mo |
DEXA: lean mass and fat mass |
Whole leg and arm; side of body |
Fat mass: P<NP side of body at 4, 7, and 12 mo; P=NP for lean and fat mass from inclusion until 12 mo |
| Sunnerhagen et al.32 |
9 | >6 | P vs. NP; cross-sectional |
Walking speed 1.07 m/s |
CT: muscle volume | Mid-thigh | P=NP |
| Svantesson et al.50 |
12 | >6 | P vs. NP; cross-sectional |
Fugl–Meyer score 28.6/34 |
Tendon and muscle stiffness; force |
Triceps surae | Muscle stiffness P>NP; tendon stiffness P<NP, force: P<NP |
| Jørgensen and Jacobson29 |
25 | 1 wk; 2, 7 and 12 mo |
P vs. NP; longitudinal |
NR | DEXA: lean mass | Mid-thigh | 1 wk: P=NP; 2 mo: P<NP; 7 and 12 mo: P=NP unless non-ambulatory at 2 mo, then P<NP at 7 and 12 mo |
| Ryan et al.35 | 60 | >6 | P vs. NP; cross-sectional |
NR | DEXA: lean mass; CT: muscle volume |
Mid-thigh; arm | Lean mass leg, arm: P<NP; muscle volume leg, arm: P<NP |
| Metoki et al.33 | 50 | >6 | P vs. NP; cross-sectional |
Barthel Index ≥65 y 75/100; <65 y 83/100 |
CT: muscle volume | Thigh | P<NP |
| Tsuji et al.34 | 83 | 3 and 6 | P vs. NP; longitudinal |
3 groups based on degree of CSA P:NP FIM motor score: Group 1: 56.6/91; Group 2: 65.1/91; Group 3: 65.5/91 |
CT: muscle area | Mid-thigh; paravertebral |
Thigh P<NP; paravertebral P>NP; no differences over time |
| Carin-Levy et al.30 |
12 | 3 wk and 6 mo |
P vs. NP; longitudinal |
Rivermead Mobility Index 12/14 and FIM motor score 121/126 (initial values) |
DEXA: lean mass and fat mass |
Mid-thigh; upper arm |
Fat mass leg: P<NP; lean mass arm, leg: P=NP; P=NP over time |
| Gao and Zhang39 | 10 | NR | P vs. C; cross-sectional |
NR | US: pennation angle, fascicle length |
Medial gastrocnemius |
Resting position shifted into plantarflexion; ↓ pennation angle; ↓ fibre length |
| Li et al.41 | 7 | >2 y | P vs. NP; cross-sectional |
Motor Assessment Scale score 2.3/4 |
US: pennation angle, fascicle length |
Brachialis | ↑ paretic pennation angle ≤40° elbow extension; ↑ fascicle length ≥30° elbow flexion |
| Celik et al.31 | 35 | >6 | P vs. NP; cross-sectional |
Brunnstrom stage 28%: 5 or 6/7; 72%: 1–3/7 |
DEXA: lean mass | Leg | P<NP |
| Gao et al.40 | 10 | >1 y | P vs. C; cross-sectional |
Motor Assessment Scale score 2.6/4 |
US: pennation angle, fascicle length |
Medial gastrocnemius |
↓ paretic pennation angle; ↓ paretic fascicle length |
| Zhao et al.51 | 10 | >1 y | P vs. NP; cross-sectional |
Modified Ashworth Scale score 1.6/3 |
US: tendon length and muscle CSA, stiffness |
Achilles tendon | Tendon length: P>NP; CSA: P=NP; mechanical hysteresis: P>NP |
Unless otherwise stated.
P=paretic; NP=non-paretic; C=control; NR=not reported; FIM=Functional Independence Measure; DEXA=dual-energy X-ray absorptiometry; CT=computed tomography; CSA=cross-sectional area; US=ultrasound; ↓=decrease; ↑=increase.
Table 2.
Summary of Studies Examining the Functional Properties of Skeletal Muscle and Type of Contractions
| Study | n | Months* Post Stroke |
Comparison | Mean Level of Recovery |
Measures | Muscle | Results |
|---|---|---|---|---|---|---|---|
| Bohannon64 | 27 | mean 45 (SD 36) d |
P vs. NP | Modified Ashworth Scale score 0 or 1/4 |
CON torque, velocity at 30°, 60°, 120°, 180° |
Knee extensors | Knee extensor torque P<NP as velocity↑ |
| Davies et al.63 | 12 | 3–42 | P vs. C; P vs. NP; SO vs. isokinetic |
Ashworth Scale score 0.9/4; 10-m walk 16.5 s |
Torque (CON, ISO), velocity at 30°/s, 100°/s, 200°/s, 300°/s |
Quadriceps; hamstrings |
ISO torque both muscles: P<C; no difference between C and NP; isokinetic torque quadriceps: P<C; P<NP at 30°/s, 100°/s; hamstrings: P<C; NP<C at 30°/s |
| Svantesson and Sunnerhagen72 |
12 | 6–24 | P vs. NP | NR | Torque: CON only and CON after ECC |
Gastrocnemius | CON and ECC torque: P<NP; CON after ECC: greater increases in P CON torque |
| Ada et al.12 | 15 | mean 4.4 y |
P vs. C | Motor Assessment Scale score 6/6 (median) |
ISO torque, angle at 30°, 60°, 90° |
Elbow flexors and extensors |
Flexor torque: P<C at 90°; extensor torque: P<C at 30° |
| Svantesson et al.50 | 12 | >6 | P vs. NP | Fugl–Meyer motor score 28.6/34 |
Torque: CON, ISO, and CON after ECC |
Triceps surae | CON torque: P<NP; ISO torque: P<NP; CON after ECC: greater increases in P CON torque |
| Ada et al.55 | 22 | 5 mo–6 y | P vs. C | Motor Assessment Scale score 5/6 (contracture); 3.5/6 (no contracture) |
ISO torque, angle at 7 positions 0°–120° |
Elbow flexors and extensors |
↓ torque deficits in elbow flexors and extensors when muscle shortened but relative preservation when muscle is lengthened |
| Koo et al.13 | 10 | >1 yr | P vs. C | Upper Arm Function Score 4.7/6 |
ISO torque, EMG at 8 angles 15–120° |
Biceps; triceps; brachioradialis |
No differences except ↓ brachioradialis P EMG |
| Lum et al.57 | 14 | >6 | P vs. NP | Fugl–Meyer motor control score 43.1/66 |
CON torque, velocity at 30°/s, 75°/s, 120°/s |
Biceps; brachioradialis; triceps |
Flexion and extension velocity torque deficits; P<NP as velocity ↑ |
| Hu et al.56 | 11 | >6 | P vs. NP | Fugl–Meyer wrist and hand score 14/24 |
ISO torque, angle at 8 positions 15° increments −45°-60°† |
Wrist flexors and extensors |
Wrist flexion and extension torque: P<NP at all angles Peak extension: P=0°, NP=−15°† Peak flexion: P=15°, NP=0° |
| Clark et al.65 | 17 | >18 | P vs. C | Fugl–Meyer leg score 21/34 |
Torque, velocity (ECC 30°/s–180°/s; CON 30°/s–240°/s) |
Quadriceps; hamstrings |
Extension torque CON: P<C Extension torque ECC: no difference Power: P<C |
| Gao and Zhang54 | 10 | mean 88 | P vs. C | Modified Ashworth Scale score 2.6/4 |
US: fascicle length, force |
Medial gastrocnemius |
Force–length curve shifted left for P; fascicle length: P<C |
| Lomaglio and Eng58 | 19 | >1 y | P vs. C; P vs. NP |
CMSA: leg 6/7; foot 4/7 |
ISO torque at 6 angles | Knee flexors and extensors |
Torque of knee extensors: P<C Extension 15° NP > C at 95° and 100° |
| Horstmann et al.84 | 14 | 3–5 | P vs. C | FAC median 4 (quartiles 2.25–4) |
ISO torque, angle at 30°, 60°, 90° |
Knee flexors and extensors |
7 stroke participants unable to perform knee flexion in all angles Extensor torque: P<NP, C at 30° Flexor torque: P<NP, C at 60° and 90° |
| Eng et al.66 | 18 | >1 y | P vs. C; P vs. NP |
CMSA: leg 6/7; foot 4/7 |
Torque: CON and ECC | Hip and knee extensors and flexors; dorsiflexors; plantarflexors |
ECC to CON torque ratios: P 2.0; NP 1.8; Control 1.6 in all 6 muscles |
P=paretic; NP=non-paretic; C=control; NR=not reported; CMSA=Chedoke–McMaster Stroke Assessment; FAC=Functional Ambulation Categories; CON=concentric; ISO=isometric; US=ultrasound; ECC=eccentric; ↑=increase; EMG=electromyographic
Unless otherwise stated
Negative values signifies wrist extension; positive values signifies wrist flexion.
Architectural Changes to Muscle after Stroke
Muscle Size
It is well known that with increasing age, muscle volume, number of type II fast-twitch muscle fibres, and muscle fibre size are reduced,22 and motor units are fewer and larger.23 After a stroke, similar changes in the motor unit occur. There is a decreased descending neural input to the alpha motor neurons, which leads to downstream degeneration of the muscle fibres; typically, however, viable adjacent motor neurons adopt some of the muscle fibres, creating fewer yet larger motor units. Reorganization of motor units occurs as early as 9 days post stroke, and the number and size of motor units are stabilized after 3 months.24 The motor units lost tend to be large alpha motor neurons innervating fast-twitch fibres, which are more susceptible to death; the result is a muscle composed predominantly of larger and slower motor units.25 The reduction in fibre size following stroke may be influenced by disuse or by other age-related processes (see below).
Muscle mass and volume, which often are estimated by anatomic cross-sectional area (CSA) of one portion of a muscle, are important factors strongly and positively related to the muscle's force-generating capacity.26 Two types of imaging are used to estimate muscle mass; five of the studies we reviewed used dual-energy X-ray absorptiometry (DEXA),27–31 three used computed tomography (CT),32–34 and one study used both methods35 (see Table 1). DEXA provides a measure of appendicular lean soft tissue based on an algorithm that uses bone mineral density and soft tissue mass to calculate lean mass. CT takes multiple cross-sectional slices to estimate CSA of the skeletal muscle at a particular level of the limb. CT is considered the gold standard for assessing skeletal muscle mass, whereas DEXA directly measures the bone mineral density and calculates muscle mass indirectly.
At 1 month post stroke, no change in lean muscle mass was found in the paretic leg or arm with DEXA imaging,28 but by 2–4 months a decrease in lean muscle mass was evident in the paretic leg and arm muscles with both DEXA and CT imaging.28,29,34 In the chronic phase (>6 months after stroke), a decrease in lean muscle mass on the paretic side relative to the non-paretic side was found with both DEXA and CT imaging,27,31,33,35 meaning that many people with stroke are living with long-term effects of decreased muscle mass in the paretic muscles. A decrease in lean muscle mass with an increase in non-contractile tissue, including fat mass, is common with ageing.18 After stroke, no changes in fat mass were found with DEXA imaging, despite a decline in paretic lean muscle mass.27,31,35 Two other studies found increased fat mass using DEXA imaging,28,30 but found no change in paretic lean muscle mass. Perhaps the DEXA was not sensitive enough in these last two studies to detect changes in lean muscle mass.35 While these data are not consistent with the notion that the loss of muscle mass post stroke is simply age-related sarcopenia, any reduction in muscle mass after stroke would be exacerbated by sarcopenic processes of ageing.
There are indications that the severity of the stroke influences the presence and extent of muscle atrophy. Jørgensen and Jacobsen29 found that those who were not walking by two months post-stroke showed a greater decrease in lean muscle mass in the mid-thigh of the paretic leg than their counterparts who were walking by two months. By contrast, Carin-Levy and colleagues30 followed patients with DEXA imaging from 1 week to 6 months post stroke and found no overall changes in the lean muscle mass of the paretic upper arm or mid-thigh relative to the non-paretic side. In their study, participants' Functional Independence Measure (FIM) scores were high at 1 week post stroke, which suggests that their strokes were milder than those of participants in Jørgensen and Jacobsen's study. Ramnemark and colleagues28 also found no differences in lean muscle mass from 1 month to 1 year. Little is known about the motor recovery of the group in this study, except that participants were unable to lift their leg and/or arm at 1 month post stroke. The researchers found an increase in fat mass, which may indicate an increase in overall body fat mass because of a reduction of activity after stroke. In cases where muscle atrophy occurred, in-patient rehabilitation was shown to be beneficial in reducing the effect of atrophy.34 That is, Tsuji and colleagues34 found that when atrophy was present 2.5 months post stroke, it could be reversed with a conventional rehabilitation programme (range of motion, strengthening, basic activity training, gait, and ADL) before discharge from a rehabilitation facility (approximately 5 months post-stroke).
Muscle Fibre Length
Muscle fibre length is important because a shorter muscle fibre has fewer sarcomeres in series, and therefore generates less force and moves through a smaller range of motion. Muscles that remain in a shortened position are predisposed to adapt to this length, which results in a reduction in the number sarcomeres along the muscle fibre.36,37 For example, weakness of the ankle dorsiflexors may result in relative shortening of the plantarflexor muscles and reduce the number of sarcomeres in series. Some muscles are particularly prone to contractures, such as the plantarflexors.38 Ultrasound makes it possible to look at human skeletal muscle fibres in vivo, but only three studies have looked at muscle fibre length39–41 (see Table 1). Reductions in muscle fibre length have been reported in both the gastrocnemius and the brachialis muscles following stroke.39–41
Pennation Angle
Generally, muscle fibres are organized in fascicles relative to the long axis of the tendon, and the specific architecture influences the force-generating capacity.42 Fibres are oriented either in a fusiform configuration (parallel to the long axis) or in a penniform configuration (at an angle to the long axis). The angle created, commonly referred to as the pennation angle, ranges from 0° to 30° in the upper and lower extremities.43,44 Larger pennation angles accommodate a larger number of shorter muscle fibres than smaller pennation angles.45–47 The pennation angle is not fixed but increases when the muscle fibres shorten, either from a reduction in joint angle or during isometric contractions. Thus pennation angle influences effective CSA, and, where possible, a measurement of pennation angle should be included to correct anatomic CSA, providing a measure of physiologic cross-sectional area (PCSA). The PCSA accounts for the combined influences of CSA, fibre length, and pennation angle on a muscle's force-generating capacity.
In vivo measurements of pennation angles are taken via ultrasound by positioning the joint at different angles to determine whether certain positions are more prone than others to changes in pennation angle following stroke. Ultrasound creates a two-dimensional image of highly reflective structures such as epimysium, perimysium, and deep and superficial aponeuroses, allowing researchers to make architectural measures directly from the image. Both gastrocnemius and brachialis muscles are pennated; at rest, the pennation angle of the brachialis muscle (9°) is smaller than that of the gastrocnemius muscle (16°).45,46 The three studies39–41 discussed above in relation to muscle fibre length are the only studies to examine human skeletal muscle pennation angles after stroke (see Table 1). In persons with stroke, pennation angles in paretic medial gastrocnemius and brachialis muscles increased when the joint angle decreased, similar to those of healthy controls or to the non-paretic side. At rest, however, the paretic medial gastrocnemius was smaller relative to controls,39,40 whereas in the brachialis muscle, the pennation angle was larger on the paretic than on the non-paretic side.41 Further studies are required to resolve this discrepancy, but it may result from differences in the architectures of the two muscles (the brachialis being less pennated than the gastrocnemius); the pattern of use of the muscles; or muscle composition, including amount of fat, non-contractile tissue, and water, which can affect pennation angles.
More important functionally is the change in pennation angle during active generation of force. As the force generated increases in isometric contractions, the pennation angle increases while fibre length decreases and CSA increases (which should assist force generation).46 The pennation angle has been measured with increases in voluntary isometric force of the paretic and non-paretic brachialis in only one study.41 Li and colleagues41 found an increase in pennation angle with increasing voluntary force production on both the paretic and the non-paretic side, but, curiously, the pennation angle was always smaller on the paretic side than on the non-paretic side (contrary to what was found with changing joint angle). More research is required to determine the influence of changes in pennation angle on force and power generation after stroke.
Tendon Length
Changes in the properties of the tendon are one more component to consider in the architecture of the muscle, because the muscle force is transmitted through the tendon. It is thought that tendon stiffness declines as people age, but there is conflicting evidence in both human and animal models.48 When the tendon is more compliant, more time is required to transmit the force to the bones, which results in a slower contractile speed and less force. When a muscle contracts, the tendon will lengthen before it shortens, and the recoil of the tendon enables elastic energy to be stored and released, increasing the efficiency of the contraction.49 Because the tendon is an extension of the muscle, tendon stiffness (the force required to change the length of the tendon) and hysteresis (the amount of energy lost after stretch and recoil) are important components for force transmission. Minimizing hysteresis improves the efficiency of movement, since more stored energy is used for the contraction and less is dissipated.43 After stroke, the Achilles tendon on the paretic side is longer (i.e., if the muscle shortens, the tendon may be lengthened in compensation) and more compliant, and hysteresis is increased.40,50,51 A more compliant tendon reduces stored energy, contractile speed, and amount of power generated. Thus, it is important to maintain the integrity of the tendon and minimize compliance to prevent over-shortening of the muscle fibres, which would place them in a disadvantaged position along the length–tension curve (see below).
Influences of Architectural Changes on Force Generation after Stroke
As we have seen, after a stroke muscle mass and muscle fibre length decrease, pennation angle changes, and the tendon is more compliant. The decreases in muscle mass and muscle fibre length reduce the number of contractile protein (actin and myosin) filaments in parallel and in series; this reduction in contractile proteins results in less force generation. The angle of the muscle fibres and the reduced stiffness of the tendon also contribute to the decrease in overall force. This section summarizes the fundamental functional properties of skeletal muscle—length–tension, angle–torque, and force–velocity—and how these functional architectural properties influence the force generated.
Length–Tension Relationship
Muscle fibres lengthen as the joint angle increases and shorten as the joint angle decreases. For each joint, there is a certain position that generates an optimal force, depending on the relative position of the tendon insertion on the bone and the internal and external forces.52,53 This position depends on the optimal number of cross-bridges being formed by the actin and myosin filaments.
When the fibre length is reduced, the reduction in actin and myosin in series reduces force production, and the functional range of the muscle decreases. Both the resting position of the muscle and the peak force shift to a position where the muscle is shorter to accommodate the new fibre length. This shift, which has been reported in the wrist flexors and extensors, elbow flexors and extensors, and plantarflexors54–56 (see Table 2), effectively reduces the range of motion.39 For example, if the ankle is positioned in plantarflexion for long periods, the plantarflexor muscle fibres adapt to the new, shorter length by reducing the number of in-series contractile filaments. The shorter plantarflexor muscle fibre length shifts the resting position of the ankle toward plantarflexion, which reduces the range of motion in dorsiflexion. These changes in fibre length contribute to muscle weakness and limit functional ability, since the peak force is now generated in a position that is no longer optimal for functional purposes.
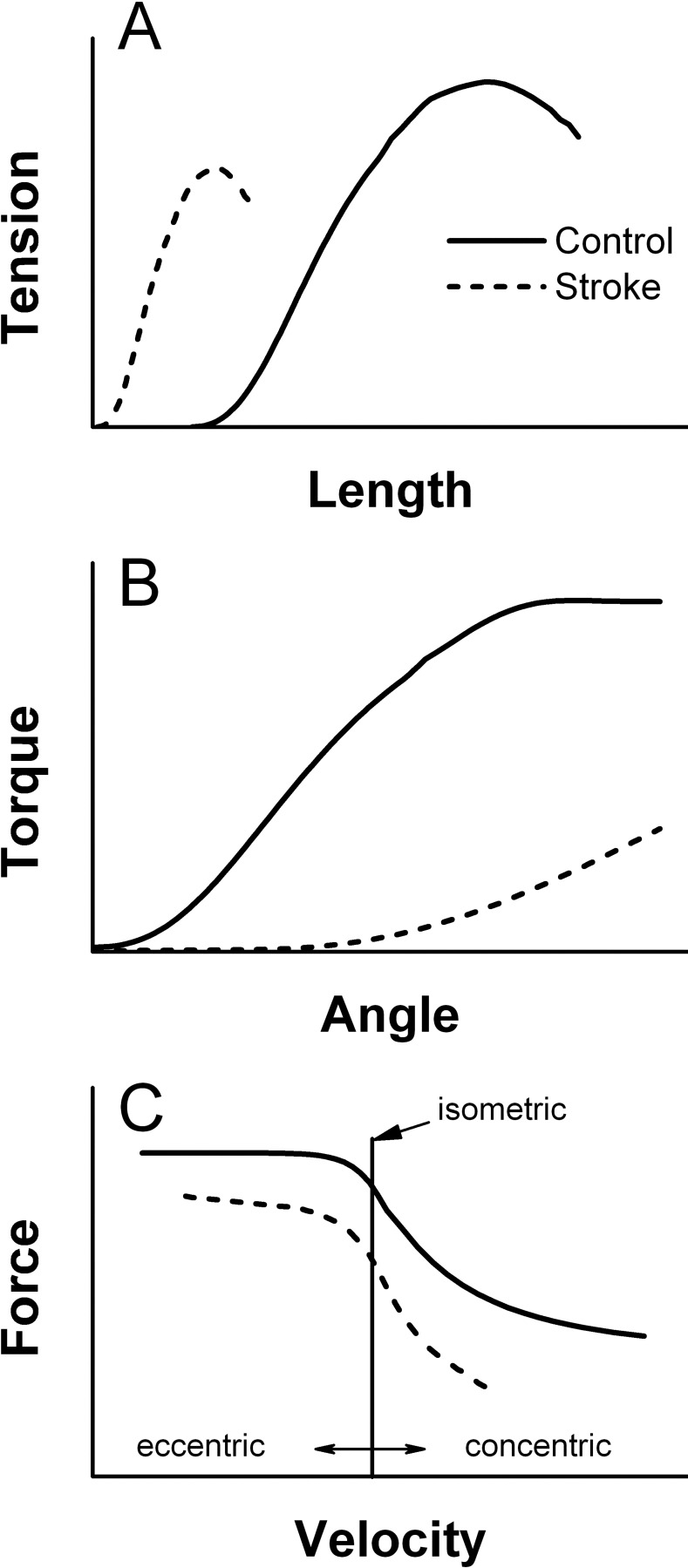
As Figure 1A shows, when the muscle lengthens, the force of the muscle increases, up to a plateau at which the maximum physiologic range of the muscle is reached. After this plateau, there is a decrease in muscle force generated, because the number of overlying sarcomeres decreases. Gao and Zhang54 found a much steeper, but narrower, length–tension relationship following stroke (dashed lines in Figure 1A). The force deficits in the shorter range may be a combination of less actin and myosin in the agonist muscle and increased stiffness in the antagonist muscle. As the agonist contracts and shortens, the antagonist lengthens, but resistance may increase because the shortened antagonist muscle fibres interfere with the agonist's ability to generate force.
Figure 1.
Schematic representation of the length–tension relationship (A), torque–angle relationship (B), and force–velocity relationship (C).
Angle–Torque Relationship
The length–tension relationship described in isolated preparations is referred to as the angle–torque relationship in human movement. The relationship between angle and torque has been studied using isometric contractions at different angles to determine which angles are more susceptible to deficits in force. Muscle weakness is characteristic of people following stroke, but greater force deficits are evident when the muscle fibres are shortened.12,56–58 Force deficits have been observed in a shortened position in the elbow flexors, elbow extensors, knee flexors, and knee extensors12,57 (see Table 2). It has been suggested that the reason for the deficits in a shortened position12,56–58 is a preference to use the muscle in the strongest position, halfway between extension and flexion, which results in relative disuse of the muscle in other positions. Similar to Ada and colleagues,12 Gao and colleagues39 found a drastically different angle–torque relationship in the medial gastrocnemius muscle after stroke, such that very little force was generated when the muscle was shortened and relatively greater force was preserved when the muscle was lengthened (see Figure 1B). By contrast, Koo and colleagues13 did not find differences in the angle–torque relationship between the paretic and control elbow flexors, apart from overall reduced torque deficit in the paretic elbow flexors. One plausible explanation for the differences between the findings of these two studies is the age of study participants following stroke: the group in Koo and colleagues' study13 were 20 years younger than those in the study by Ada and colleagues.12 The relatively young age of the former group may explain the lack of significant findings, as functional improvements are quicker to return in younger than in older people.59 Koo and colleagues' group with no deficits in force13 were more than 1 year post stroke, and the deficits in force production may have been recovered by 1 year.
One problem in measuring the angle–torque relationship after stroke is that people with stroke tend to have a reduced range of motion compared to controls. This makes it difficult to study the deficits in force at the extreme ranges. The limited range of motion may be a result of the decrease in length of muscle fibres, as discussed above, or of an inability to generate enough force when the muscle is shortened.
There seems to be a greater risk of deficits in force in muscles that are more prone to functional contractures, which may indicate that the deficits reported are related to changes in the length of the muscle fibre. This may suggest that the type of muscle function is not an indication of which muscle will demonstrate the greatest deficits in force and that the changes that occur in the architecture of the muscle may result from relative disuse, particularly in shortened positions.
Force–Velocity Relationship
The force generated by a muscle is a function of velocity. The amount of work performed by a muscle is less when muscle fibres are shorter, because work is a product of force and a change in fibre length. The time to develop the force as the fibre length is changing reflects the power that the muscle can generate. Because of changes in both the quantity and the quality of muscle, both work and power decrease with age.18
A concentric contraction involves the shortening of muscle fibres against a load; as velocity increases, less force is produced. As the myosin and actin filaments move past each other at higher velocities, the time for cross-bridge formation decreases, which reduces the force produced. The reverse is true of eccentric contractions: faster eccentric contractions develop more force than slow eccentric contractions.60–62 Based on the force–velocity relationship (see Figure 1C), eccentric contractions produce more force as lengthening velocity increases, while concentric contractions produce less force as shortening velocity increases. Thus, by comparison, eccentric contractions generate more force than isometric and isometric more than concentric contractions. Similar trends have been found after stroke, such that as velocity increases, less force is generated in the paretic muscles.57,63,64
After stroke, the studies reviewed found greater force deficits in concentric contractions65,66 than in isometric contractions,32,50,63 and smaller force deficits in eccentric contractions than in concentric and isometric contractions65,66 (see Table 2). There is an indication that less neural drive is used for eccentric contractions, which may explain why greater force was preserved; similar results have been found in older adults.18
At low velocity (30°/s), no force deficits were found in the paretic biceps or quadriceps muscles relative to the non-paretic side or controls, but force deficits were evident in the paretic hamstrings and triceps muscles.57,63–65 Similar decreases in force as velocity increased from 30°/s to 120°/s or 180°/s were found in the paretic and non-paretic quadriceps and triceps,57,64 indicating that the force–velocity relationship was maintained for these muscles or, at least, was maintained at these velocities. Although no difference in the force–velocity relationship was found in the elbow extensors, greater deficits as velocities increased were found in the elbow flexors.57 Lum and colleagues57 also found that when the elbow flexors were moved from extension to flexion, there were no deficits when the muscle was lengthened; torque deficits were found only late in flexion, when the elbow flexors were shortened.
In some cases, the extent of the impairment following stroke was so severe that movement velocities of 300°/s could not be produced, especially in knee flexion.63 Two possible reasons for the increased deficits in force at higher velocities are reduced muscle fibre length, as discussed above, and less time for the formation of cross bridges. In addition, as velocity increases, earlier recruitment of high-threshold motor units is necessary.67 Thus neural factors such as a reduced drive from the supraspinal centres after stroke (reflected in the decrease in motor-unit firing rate9–11) may compound the architectural changes in the muscle, producing force deficits, especially at higher velocities.
There is some indication that the velocity of force production is an important component of functional recovery. After a stroke, force deficits are evident, and at higher velocities there are greater impairments in producing movement. The product of force and velocity is the power that the muscle can generate. Among older adults, there is evidence that muscle power is a strong predictor of functional status in community dwellers.68 A recent study by Lee and colleagues69 indicated that strength can be trained in people after stroke in a 6-week exercise programme, but power (which was not trained) did not change with this programme. In an exercise programme combining strength training and speed of movement, power was improved in frail elderly adults.70 Because power (force and speed) is essential for many ADL (e.g., balance recovery), attention to retraining power through speed of movement, rather than strength alone, is likely to be important.
DISCUSSION
Our review identified 27 articles investigating architectural changes in muscles and how these changes influence force generation, 14 of which examined architectural change and 14 of which studied changes in force generation (1 article studied both). Although only a small number of articles were identified, we have nevertheless presented preliminary conclusions on how changes in the architecture of the muscle may influence the ability to generate force after stroke.
Architectural Changes in Muscle after Stroke
Muscle fibres undergo several alterations after stroke: a change in pennation angle,39–41 a decrease in fibre length,39–41 and a decrease in lean muscle mass.27,29,31,33–35 The studies reviewed found that the change in pennation angle differed for the muscles of the upper and lower extremities, increasing in the former and decreasing in the latter.39–41 Only three studies were identified that examined changes in pennation angle, which makes it difficult to draw conclusions as to whether the differences in pennation angle are related to the function of the muscles (differences in patterns of use between gastrocnemius in the leg and brachialis in the arm) or to their structural composition. Similarly, not all studies examining muscle atrophy reported a reduction in lean muscle mass. Differences in study findings were not related to whether the upper or the lower extremity was being investigated: both showed a significant loss in paretic lean muscle mass relative the non-paretic muscle. The lack of significant findings in muscle atrophy28,30,32 may relate to the patients' having higher levels of function after stroke, but not all studies reported participants' recovery, which makes it difficult to know whether the differences found were related to the severity of stroke. In addition, the studies used different techniques to assess muscle atrophy, which precluded meaningful comparisons. Using CT scans, Ryan and colleagues35 found that the lean tissue mass of the non-paretic leg was 20% larger than that of the paretic leg; using DEXA, they found the non-paretic leg to be only 4% larger. This suggests that CT scans are more sensitive than DEXA to changes in muscle mass.
One common theme throughout the studies reviewed was the effect of decreased muscle fibre length on a muscle's ability to generate force. Initially, after a stroke, neural inputs are reduced, resulting in muscle weakness, and the risk of muscle contractures increases. If the muscles are not moved regularly through their full range, the muscle fibres may adapt to the shortened position by decreasing muscle fibre length. The decreased length of the muscle fibres has an impact on many factors that influence the ability of muscles to generate force, producing a shift in peak force to a shorter muscle position, a decrease in peak muscle force, reduced tendon stiffness, and an increase in tendon length.
Influences of Architectural Changes on Force Generation after Stroke
Our review illustrates that greater torque deficits are found in both the upper and lower extremity when fibres are shortened and moving toward the inner range, particularly as velocity increases. In addition, there is relative preservation of force during eccentric contractions, compared to isometric and concentric contractions, after stroke.
The reason for the relative preservation of the eccentric contraction is not known, but eccentric strength is also better preserved in ageing than isometric and concentric strength. One possible reason for this is a change in the visco-elastic properties of the muscle and tendon as the muscle is lengthened; another explanation may be that changes in the neural inputs to the muscle, such as hyperactive stretch reflex, facilitate the contraction.71 Interestingly, Eng and colleagues66 found that preservation of force in eccentric contractions was not related to a person's level of physical activity, whereas concentric contraction force was correlated with physical activity. The key to understanding force preservation may be in the stretch shortening cycle, in which a larger force is generated when a concentric action follows an eccentric action than when the concentric action is performed alone. This phenomenon has been observed in people after stroke, and the percentage increase in the concentric action following an eccentric action was greater in paretic than in non-paretic plantarflexors.50 If an isometric action precedes a concentric action, on the other hand, there is no difference in the paretic or control muscle force generated.72 Thus, this phenomenon may relate only to the mechanics of the eccentric contraction. More research is needed to understand the elastic energy in the eccentric contraction and why it is less affected than other movements following stroke.
Implications for Rehabilitation
Rehabilitation is important in reducing the effects of muscle atrophy. Strength training is one modality that may be used to reduce the effects of muscle atrophy after stroke.69,73–77 Several types of contractions to improve strength are used in a rehabilitation setting: concentric, eccentric, and isometric. In many activities, such as running or jumping, all three types of muscle actions are required to perform a smooth, coordinated movement. In strength training with older adults, eccentric contractions can be beneficial in gaining strength with less energy cost than concentric or isometric contractions.18 After stroke, improvements have been found with eccentric compared to concentric strength training.78 In addition, eccentric contractions followed by a concentric contraction to generate more force may be a strategy to use in rehabilitation to reduce deficits in the concentric contraction.50 More research is needed to determine an optimal combination of concentric, eccentric, and isometric training to improve the strength of a muscle after stroke.
In addition to preventing muscle atrophy, maintaining the length of muscle fibres and tendons could improve the force-generating capacity of the muscle. Furthermore, minimizing changes in tendon compliance is important, since a longer and more compliant tendon may not be able to store as much elastic energy.79,80 More research is needed in this area to determine how this energy can be harnessed to promote the recovery of muscle weakness.
Power, which incorporates both strength and speed of movement, may be an important parameter to consider in rehabilitation. Indeed, deficits at higher velocities are a key component to address in a rehabilitation programme, because increased deficits with quicker movements could place an individual at risk of falling.81 Strength can be retrained in paretic muscles, but Lee and colleagues69 showed that strengthening exercises do not retrain power. In older adults, power is affected more by the reduction in speed of movement than by force deficits,18 and thus research is needed to investigate retraining of speed of movement and power after stroke.
The final clinical implication for rehabilitation is related to the clinical evaluation of muscle strength. The angle–torque relationship will influence the assessment of muscle weakness after stroke; therefore, to ensure that force generation is evaluated consistently, it is important to use the same test position over time. For example, larger forces can be generated by the knee extensors in sitting than in supine position.82 The force generated also varies with the type of muscle contraction, and therefore care must be taken when assessing muscles following stroke, since manual muscle testing is an isometric action whereas functional ADL rarely involve isometric actions alone. In a study by Knapik and colleagues, although concentric movements at lower velocities (30°/s) correlated with isometric contractions, there were no similarities with higher velocities.83 Therefore, an assessment of isometric muscle strength may overestimate the force-generating ability a muscle has in typical concentric contractions.
This review has several limitations that should be considered. The main limitation is related to the small number of studies that met the inclusion criteria. For example, only three studies were identified that examined pennation angle, which made it difficult to draw conclusions as to whether differences in pennation angle are related to the function of the muscles or to structural composition. Different techniques (CT, DEXA, and US) were used to examine muscle atrophy, and the small number of studies made it difficult to compare findings. Not all articles reported participants' level of recovery, making it difficult to know whether the differences in results, such as muscle atrophy, were related to the severity of stroke.
CONCLUSION
We have presented evidence from the literature that changes in the architecture of the muscle contribute to muscle weakness. A rehabilitation programme should endeavour to prevent or reverse many of the changes implicated in muscle weakness, such as decreased fibre length, altered pennation angle, muscle atrophy, and increased tendon compliance. There are areas where more research is needed to determine the impact that particular changes have on people with stroke, such as changes in pennation angle and preservation of force in eccentric actions. Further research into the mechanism of the short stretched cycle may be beneficial if elastic energy can be harnessed to enhance force in other types of contractions.
KEY MESSAGES
What Is Already Known on This Topic
Muscle weakness is common after stroke. Considerable research has examined the reorganization of neural pathways and the contribution of neuroplasticity to muscle weakness.
What This Study Adds
We reviewed studies exploring changes in the architecture of the muscle after stroke and their influence on muscle weakness. An understanding of the changes at the level of the muscle will help physiotherapists to understand what changes occur and how these changes affect muscle weakness.
Appendix 1: Example Search Strategies
Stroke.mp
Hemiparesis.mp
Muscle.mp
Velocity.mp
Torque.mp
Force.mp
Atrophy.mp
Cross sectional area.mp
4 or 5 or 6 or 7 or 8
1 or 2
3 and 10
9 and 11
Remove duplicates from 12
Physiotherapy Canada 2012; 64(4);415–426; doi:10.3138/ptc.2011-03
References
- 1.Corcos DM, Gottlieb GL, Penn RD, et al. Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain. 1986;109(5):1043–58. doi: 10.1093/brain/109.5.1043. doi: 10.1093/brain/109.5.1043. Medline:3779370. [DOI] [PubMed] [Google Scholar]
- 2.Fellows SJ, Kaus C, Thilmann AF. Voluntary movement at the elbow in spastic hemiparesis. Ann Neurol. 1994;36(3):397–407. doi: 10.1002/ana.410360311. doi: 10.1002/ana.410360311. Medline:8080247. [DOI] [PubMed] [Google Scholar]
- 3.Levin MF, Selles RW, Verheul MH, et al. Deficits in the coordination of agonist and antagonist muscles in stroke patients: implications for normal motor control. Brain Res. 2000;853(2):352–69. doi: 10.1016/s0006-8993(99)02298-2. doi: 10.1016/S0006-8993(99)02298-2. Medline:10640634. [DOI] [PubMed] [Google Scholar]
- 4.Levin MF, Hui-Chan C. Ankle spasticity is inversely correlated with antagonist voluntary contraction in hemiparetic subjects. Electromyogr Clin Neurophysiol. 1994;34(7):415–25. Medline:7859670. [PubMed] [Google Scholar]
- 5.Fellows SJ, Kaus C, Ross HF, et al. Agonist and antagonist EMG activation during isometric torque development at the elbow in spastic hemiparesis. Electroencephalogr Clin Neurophysiol. 1994;93(2):106–12. doi: 10.1016/0168-5597(94)90073-6. doi: 10.1016/0168-5597(94)90073-6. Medline:7512916. [DOI] [PubMed] [Google Scholar]
- 6.Dewald JP, Pope PS, Given JD, et al. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(2):495–510. doi: 10.1093/brain/118.2.495. doi: 10.1093/brain/118.2.495. Medline:7735890. [DOI] [PubMed] [Google Scholar]
- 7.el-Abd MA, Ibrahim IK, Dietz V. Impaired activation pattern in antagonistic elbow muscles of patients with spastic hemiparesis: contribution to movement disorder. Electromyogr Clin Neurophysiol. 1993;33(4):247–55. Medline:8359130. [PubMed] [Google Scholar]
- 8.Newham DJ, Hsiao S-F. Knee muscle isometric strength, voluntary activation and antagonist co-contraction in the first six months after stroke. Disabil Rehabil. 2001;23(9):379–86. doi: 10.1080/0963828001006656. doi: 10.1080/0963828001006656. Medline:11394588. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson F, Grimby L, Edström L. Motoneuron activity and muscle fibre type composition in hemiparesis. Scand J Rehabil Med. 1992;24(3):115–9. Medline:1411356. [PubMed] [Google Scholar]
- 10.Gemperline JJ, Allen S, Walk D, et al. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18(10):1101–14. doi: 10.1002/mus.880181006. doi: 10.1002/mus.880181006. Medline:7659104. [DOI] [PubMed] [Google Scholar]
- 11.Frontera WR, Grimby L, Larsson L. Firing rate of the lower motoneuron and contractile properties of its muscle fibers after upper motoneuron lesion in man. Muscle Nerve. 1997;20(8):938–47. doi: 10.1002/(sici)1097-4598(199708)20:8<938::aid-mus2>3.0.co;2-7. doi: 10.1002/(SICI)1097-4598(199708)20:8<938::AID-MUS2>3.0.CO;2-7. Medline:9236783. [DOI] [PubMed] [Google Scholar]
- 12.Ada L, Canning C, Dwyer T. Effect of muscle length on strength and dexterity after stroke. Clin Rehabil. 2000;14(1):55–61. doi: 10.1191/026921500671430626. doi: 10.1191/026921500671430626. Medline:10688345. [DOI] [PubMed] [Google Scholar]
- 13.Koo TK, Mak AF, Hung LK, et al. Joint position dependence of weakness during maximum isometric voluntary contractions in subjects with hemiparesis. Arch Phys Med Rehabil. 2003;84(9):1380–6. doi: 10.1016/s0003-9993(03)00238-7. doi: 10.1016/S0003-9993(03)00238-7. Medline:13680578. [DOI] [PubMed] [Google Scholar]
- 14.Cheatwood JL, Emerick AJ, Kartje GL. Neuronal plasticity and functional recovery after ischemic stroke. Top Stroke Rehabil. 2008;15(1):42–50. doi: 10.1310/tsr1501-42. doi: 10.1310/tsr1501-42. Medline:18250073. [DOI] [PubMed] [Google Scholar]
- 15.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38(2 Suppl):840–5. doi: 10.1161/01.STR.0000247943.12887.d2. doi: 10.1161/01.STR.0000247943.12887.d2. Medline:17261749. [DOI] [PubMed] [Google Scholar]
- 16.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184(1):170–92. doi: 10.1113/jphysiol.1966.sp007909. Medline:5921536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickiewicz TL, Roy RR, Powell PL, et al. Muscle architecture and force-velocity relationships in humans. J Appl Physiol. 1984;57(2):435–43. doi: 10.1152/jappl.1984.57.2.435. Medline:6469814. [DOI] [PubMed] [Google Scholar]
- 18.Paterson DH, Jones GR, Rice CL. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Can J Public Health. 2007;98(Suppl 2):S69–108. doi: 10.1139/1-H07-111. Medline:18213941. [PubMed] [Google Scholar]
- 19.Christensen B, Dyrberg E, Aagaard P, et al. Short-term immobilization and recovery affect skeletal muscle but not collagen tissue turnover in humans. J Appl Physiol. 2008;105(6):1845–51. doi: 10.1152/japplphysiol.90445.2008. doi: 10.1152/japplphysiol.90445.2008. Medline:18927270. [DOI] [PubMed] [Google Scholar]
- 20.Bernhardt J, Dewey H, Thrift A, et al. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35(4):1005–9. doi: 10.1161/01.STR.0000120727.40792.40. doi: 10.1161/01.STR.0000120727.40792.40. Medline:14988574. [DOI] [PubMed] [Google Scholar]
- 21.English C, McLennan H, Thoirs K, et al. Loss of skeletal muscle mass after stroke: a systematic review. Int J Stroke. 2010;5(5):395–402. doi: 10.1111/j.1747-4949.2010.00467.x. doi: 10.1111/j.1747-4949.2010.00467.x. Medline:20854624. [DOI] [PubMed] [Google Scholar]
- 22.Lexell J. Ageing and human muscle: observations from Sweden. Can J Appl Physiol. 1993;18(1):2–18. doi: 10.1139/h93-002. doi: 10.1139/h93-002. Medline:8471991. [DOI] [PubMed] [Google Scholar]
- 23.McNeil CJ, Doherty TJ, Stashuk DW, et al. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31(4):461–7. doi: 10.1002/mus.20276. doi: 10.1002/mus.20276. Medline:15685623. [DOI] [PubMed] [Google Scholar]
- 24.Hara Y, Masakado Y, Chino N. The physiological functional loss of single thenar motor units in the stroke patients: when does it occur? Does it progress? Clin Neurophysiol. 2004;115(1):97–103. doi: 10.1016/j.clinph.2003.08.002. doi: 10.1016/j.clinph.2003.08.002. Medline:14706475. [DOI] [PubMed] [Google Scholar]
- 25.Dattola R, Girlanda P, Vita G, et al. Muscle rearrangement in patients with hemiparesis after stroke: an electrophysiological and morphological study. Eur Neurol. 1993;33(2):109–14. doi: 10.1159/000116915. doi: 10.1159/000116915. Medline:8467816. [DOI] [PubMed] [Google Scholar]
- 26.Fukunaga T, Miyatani M, Tachi M, et al. Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand. 2001;172(4):249–55. doi: 10.1046/j.1365-201x.2001.00867.x. doi: 10.1046/j.1365-201x.2001.00867.x. Medline:11531646. [DOI] [PubMed] [Google Scholar]
- 27.Iversen E, Hassager C, Christiansen C. The effect of hemiplegia on bone mass and soft tissue body composition. Acta Neurol Scand. 1989;79(2):155–9. doi: 10.1111/j.1600-0404.1989.tb03729.x. doi: 10.1111/j.1600-0404.1989.tb03729.x. Medline:2711822. [DOI] [PubMed] [Google Scholar]
- 28.Ramnemark A, Nyberg L, Lorentzon R, et al. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke. 1999;30(4):755–60. doi: 10.1161/01.str.30.4.755. doi: 10.1161/01.STR.30.4.755. Medline:10187874. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone. 2001;28(6):655–9. doi: 10.1016/s8756-3282(01)00434-3. doi: 10.1016/S8756-3282(01)00434-3. Medline:11425655. [DOI] [PubMed] [Google Scholar]
- 30.Carin-Levy G, Greig C, Young A, et al. Longitudinal changes in muscle strength and mass after acute stroke. Cerebrovasc Dis. 2006;21(3):201–7. doi: 10.1159/000090792. doi: 10.1159/000090792. Medline:16401884. [DOI] [PubMed] [Google Scholar]
- 31.Celik B, Ones K, Ince N. Body composition after stroke. Int J Rehabil Res. 2008;31(1):93–6. doi: 10.1097/MRR.0b013e3282f7521a. doi: 10.1097/MRR.0b013e3282f7521a. Medline:18277212. [DOI] [PubMed] [Google Scholar]
- 32.Sunnerhagen KS, Svantesson U, Lönn L, et al. Upper motor neuron lesions: their effect on muscle performance and appearance in stroke patients with minor motor impairment. Arch Phys Med Rehabil. 1999;80(2):155–61. doi: 10.1016/s0003-9993(99)90113-2. doi: 10.1016/S0003-9993(99)90113-2. Medline:10025489. [DOI] [PubMed] [Google Scholar]
- 33.Metoki N, Sato Y, Satoh K, et al. Muscular atrophy in the hemiplegic thigh in patients after stroke. Am J Phys Med Rehabil. 2003;82(11):862–5. doi: 10.1097/01.PHM.0000091988.20916.EF. doi: 10.1097/01.PHM.0000091988.20916.EF. Medline:14566154. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji T, Liu M, Hase K, et al. Trunk muscles in persons with hemiparetic stroke evaluated with computed tomography. J Rehabil Med. 2003;35(4):184–8. doi: 10.1080/16501970306126. doi: 10.1080/16501970306126. Medline:12892245. [DOI] [PubMed] [Google Scholar]
- 35.Ryan AS, Dobrovolny CL, Smith GV, et al. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83(12):1703–7. doi: 10.1053/apmr.2002.36399. doi: 10.1053/apmr.2002.36399. Medline:12474173. [DOI] [PubMed] [Google Scholar]
- 36.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973;116(Pt 1):45–55. Medline:4798240. [PMC free article] [PubMed] [Google Scholar]
- 37.Williams PE, Catanese T, Lucey EG, et al. The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. J Anat. 1988;158:109–14. Medline:3225214. [PMC free article] [PubMed] [Google Scholar]
- 38.O'Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119(5):1737–49. doi: 10.1093/brain/119.5.1737. doi: 10.1093/brain/119.5.1737. Medline:8931594. [DOI] [PubMed] [Google Scholar]
- 39.Gao F, Zhang LQ. In vivo biomechanical evaluations of the medial gastrocnemius: changes in muscle properties in stroke survivors. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2083–6. doi: 10.1109/IEMBS.2006.260089. doi: 10.1109/IEMBS.2006.260089. Medline:17946936. [DOI] [PubMed] [Google Scholar]
- 40.Gao F, Grant TH, Roth EJ, et al. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil. 2009;90(5):819–26. doi: 10.1016/j.apmr.2008.11.004. doi: 10.1016/j.apmr.2008.11.004. Medline:19406302. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Tong KY, Hu XL. The effect of poststroke impairments on brachialis muscle architecture as measured by ultrasound. Arch Phys Med Rehabil. 2007;88(2):243–50. doi: 10.1016/j.apmr.2006.11.013. doi: 10.1016/j.apmr.2006.11.013. Medline:17270524. [DOI] [PubMed] [Google Scholar]
- 42.Lieber RL, Lieber RL. Skeletal muscle structure, function & plasticity the physiological basis of rehabilitation. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 43.Lieber RL, Steinman S, Barash IA, et al. Structural and functional changes in spastic skeletal muscle. Muscle Nerve. 2004;29(5):615–27. doi: 10.1002/mus.20059. doi: 10.1002/mus.20059. Medline:15116365. [DOI] [PubMed] [Google Scholar]
- 44.Wickiewicz TL, Roy RR, Powell PL, et al. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983;179:275–83. Medline:6617027. [PubMed] [Google Scholar]
- 45.Herbert RD, Gandevia SC. Changes in pennation with joint angle and muscle torque: in vivo measurements in human brachialis muscle. J Physiol. 1995;484(Pt 2):523–32. doi: 10.1113/jphysiol.1995.sp020683. Medline:7602542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narici MV, Binzoni T, Hiltbrand E, et al. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol. 1996;496(Pt 1):287–97. doi: 10.1113/jphysiol.1996.sp021685. Medline:8910216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binzoni T, Bianchi S, Hanquinet S, et al. Human gastrocnemius medialis pennation angle as a function of age: from newborn to the elderly. J Physiol Anthropol Appl Human Sci. 2001;20(5):293–8. doi: 10.2114/jpa.20.293. doi: 10.2114/jpa.20.293. Medline:11759268. [DOI] [PubMed] [Google Scholar]
- 48.Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disabil Rehabil. 2008;30(20-22):1548–54. doi: 10.1080/09638280701831058. doi: 10.1080/09638280701831058. Medline:18608375. [DOI] [PubMed] [Google Scholar]
- 49.Magnusson SP, Narici MV, Maganaris CN, et al. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008;586(1):71–81. doi: 10.1113/jphysiol.2007.139105. doi: 10.1113/jphysiol.2007.139105. Medline:17855761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svantesson U, Takahashi H, Carlsson U, et al. Muscle and tendon stiffness in patients with upper motor neuron lesion following a stroke. Eur J Appl Physiol. 2000;82(4):275–9. doi: 10.1007/s004210000216. doi: 10.1007/s004210000216. Medline:10958369. [DOI] [PubMed] [Google Scholar]
- 51.Zhao H, Ren YP, Wu YN, et al. Ultrasonic evaluations of Achilles tendon mechanical properties poststroke. J Appl Physiol. 2009;106(3):843–9. doi: 10.1152/japplphysiol.91212.2008. doi: 10.1152/japplphysiol.91212.2008. Medline:19118156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huijing PA, Baan GC. Myofascial force transmission: muscle relative position and length determine agonist and synergist muscle force. J Appl Physiol. 2003;94(3):1092–107. doi: 10.1152/japplphysiol.00173.2002. doi: 10.1152/japplphysiol.00173.2002. Medline:12571138. [DOI] [PubMed] [Google Scholar]
- 53.Maas H, Sandercock TG. Force transmission between synergistic skeletal muscles through connective tissue linkages. J Biomed Biotechnol. 2010;2010:575672. doi: 10.1155/2010/575672. doi: 10.1155/2010/575672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao F, Zhang LQ. Altered contractile properties of the gastrocnemius muscle poststroke. J Appl Physiol. 2008;105(6):1802–8. doi: 10.1152/japplphysiol.90930.2008. doi: 10.1152/japplphysiol.90930.2008. Medline:18948443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ada L, Canning CG, Low SL. Stroke patients have selective muscle weakness in shortened range. Brain. 2003;126(3):724–31. doi: 10.1093/brain/awg066. doi: 10.1093/brain/awg066. Medline:12566292. [DOI] [PubMed] [Google Scholar]
- 56.Hu XL, Tong KY, Tsang VS, et al. Joint-angle-dependent neuromuscular dysfunctions at the wrist in persons after stroke. Arch Phys Med Rehabil. 2006;87(5):671–9. doi: 10.1016/j.apmr.2006.02.003. doi: 10.1016/j.apmr.2006.02.003. Medline:16635630. [DOI] [PubMed] [Google Scholar]
- 57.Lum PS, Patten C, Kothari D, et al. Effects of velocity on maximal torque production in poststroke hemiparesis. Muscle Nerve. 2004;30(6):732–42. doi: 10.1002/mus.20157. doi: 10.1002/mus.20157. Medline:15468340. [DOI] [PubMed] [Google Scholar]
- 58.Lomaglio MJ, Eng JJ. Nonuniform weakness in the paretic knee and compensatory strength gains in the nonparetic knee occurs after stroke. Cerebrovasc Dis. 2008;26(6):584–91. doi: 10.1159/000165111. doi: 10.1159/000165111. Medline:18946213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kugler C, Altenhöner T, Lochner P, et al. the Hessian Stroke Data Bank Study Group ASH. Does age influence early recovery from ischemic stroke? A study from the Hessian Stroke Data Bank. J Neurol. 2003;250(6):676–81. doi: 10.1007/s00415-003-1054-8. doi: 10.1007/s00415-003-1054-8. Medline:12796828. [DOI] [PubMed] [Google Scholar]
- 60.Trew M, Everett T. Human movement an introductory text. 5th ed. Edinburgh: Elsevier/Churchill Livingstone; 2005. [Google Scholar]
- 61.Drury DG, Stuempfle KJ, Mason CW, et al. The effects of isokinetic contraction velocity on concentric and eccentric strength of the biceps brachii. J Strength Cond Res. 2006;20(2):390–5. doi: 10.1519/R-16154.1. Medline:16686569. [DOI] [PubMed] [Google Scholar]
- 62.Westing SH, Seger JY. Eccentric and concentric torque-velocity characteristics, torque output comparisons, and gravity effect torque corrections for the quadriceps and hamstring muscles in females. Int J Sports Med. 1989;10(3):175–80. doi: 10.1055/s-2007-1024896. doi: 10.1055/s-2007-1024896. Medline:2777436. [DOI] [PubMed] [Google Scholar]
- 63.Davies JM, Mayston MJ, Newham DJ. Electrical and mechanical output of the knee muscles during isometric and isokinetic activity in stroke and healthy adults. Disabil Rehabil. 1996;18(2):83–90. doi: 10.3109/09638289609166022. doi: 10.3109/09638289609166022. Medline:8869510. [DOI] [PubMed] [Google Scholar]
- 64.Bohannon RW. Relative decreases in knee extension torque with increased knee extension velocities in stroke patients with hemiparesis. Phys Ther. 1987;67(8):1218–20. doi: 10.1093/ptj/67.8.1218. Medline:3615590. [DOI] [PubMed] [Google Scholar]
- 65.Clark DJ, Condliffe EG, Patten C. Activation impairment alters muscle torque-velocity in the knee extensors of persons with post-stroke hemiparesis. Clin Neurophysiol. 2006;117(10):2328–37. doi: 10.1016/j.clinph.2006.07.131. doi: 10.1016/j.clinph.2006.07.131. Medline:16926111. [DOI] [PubMed] [Google Scholar]
- 66.Eng JJ, Lomaglio MJ, Macintyre DL. Muscle torque preservation and physical activity in individuals with stroke. Med Sci Sports Exerc. 2009;41(7):1353–60. doi: 10.1249/MSS.0b013e31819aaad1. doi: 10.1249/MSS.0b013e31819aaad1. Medline:19516167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wakeling JM, Uehli K, Rozitis AI. Muscle fibre recruitment can respond to the mechanics of the muscle contraction. J R Soc Interface. 2006;3(9):533–44. doi: 10.1098/rsif.2006.0113. doi: 10.1098/rsif.2006.0113. Medline:16849250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55(4):M192–9. doi: 10.1093/gerona/55.4.m192. doi: 10.1093/gerona/55.4.M192. Medline:10811148. [DOI] [PubMed] [Google Scholar]
- 69.Lee MJ, Kilbreath SL, Singh MF, et al. Effect of progressive resistance training on muscle performance after chronic stroke. Med Sci Sports Exerc. 2010;42(1):23–34. doi: 10.1249/MSS.0b013e3181b07a31. doi: 10.1249/MSS.0b013e3181b07a31. Medline:20010133. [DOI] [PubMed] [Google Scholar]
- 70.Hruda KV, Hicks AL, McCartney N. Training for muscle power in older adults: effects on functional abilities. Can J Appl Physiol. 2003;28(2):178–89. doi: 10.1139/h03-014. doi: 10.1139/h03-014. Medline:12825328. [DOI] [PubMed] [Google Scholar]
- 71.Knutsson E, Mårtensson A, Gransberg L. Influences of muscle stretch reflexes on voluntary, velocity-controlled movements in spastic paraparesis. Brain. 1997;120(9):1621–33. doi: 10.1093/brain/120.9.1621. doi: 10.1093/brain/120.9.1621. Medline:9313644. [DOI] [PubMed] [Google Scholar]
- 72.Svantesson U, Sunnerhagen KS. Stretch-shortening cycle in patients with upper motor neuron lesions due to stroke. Eur J Appl Physiol Occup Physiol. 1997;75(4):312–8. doi: 10.1007/s004210050166. doi: 10.1007/s004210050166. Medline:9134362. [DOI] [PubMed] [Google Scholar]
- 73.Teixeira-Salmela LF, Olney SJ, Nadeau S, et al. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch Phys Med Rehabil. 1999;80(10):1211–8. doi: 10.1016/s0003-9993(99)90018-7. doi: 10.1016/S0003-9993(99)90018-7. Medline:10527076. [DOI] [PubMed] [Google Scholar]
- 74.Cramp MC, Greenwood RJ, Gill M, et al. Low intensity strength training for ambulatory stroke patients. Disabil Rehabil. 2006;28(13-14):883–9. doi: 10.1080/09638280500535157. doi: 10.1080/09638280500535157. Medline:16777776. [DOI] [PubMed] [Google Scholar]
- 75.Ouellette MM, LeBrasseur NK, Bean JF, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke. 2004;35(6):1404–9. doi: 10.1161/01.STR.0000127785.73065.34. doi: 10.1161/01.STR.0000127785.73065.34. Medline:15105515. [DOI] [PubMed] [Google Scholar]
- 76.Weiss A, Suzuki T, Bean J, et al. High intensity strength training improves strength and functional performance after stroke. Am J Phys Med Rehabil. 2000;79(4):369–76. doi: 10.1097/00002060-200007000-00009. quiz 391–4. doi: 10.1097/00002060-200007000-00009. Medline:10892623. [DOI] [PubMed] [Google Scholar]
- 77.Sharp SA, Brouwer BJ. Isokinetic strength training of the hemiparetic knee: effects on function and spasticity. Arch Phys Med Rehabil. 1997;78(11):1231–6. doi: 10.1016/s0003-9993(97)90337-3. doi: 10.1016/S0003-9993(97)90337-3. Medline:9365354. [DOI] [PubMed] [Google Scholar]
- 78.Engardt M, Knutsson E, Jonsson M, et al. Dynamic muscle strength training in stroke patients: effects on knee extension torque, electromyographic activity, and motor function. Arch Phys Med Rehabil. 1995;76(5):419–25. doi: 10.1016/s0003-9993(95)80570-2. doi: 10.1016/S0003-9993(95)80570-2. Medline:7741611. [DOI] [PubMed] [Google Scholar]
- 79.Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. J Biomech. 2002;35(12):1639–46. doi: 10.1016/s0021-9290(02)00240-3. doi: 10.1016/S0021-9290(02)00240-3. Medline:12445617. [DOI] [PubMed] [Google Scholar]
- 80.Kinugasa R, Hodgson JA, Edgerton VR, et al. Reduction in tendon elasticity from unloading is unrelated to its hypertrophy. J Appl Physiol. 2010;109(3):870–7. doi: 10.1152/japplphysiol.00384.2010. doi: 10.1152/japplphysiol.00384.2010. Medline:20616227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31(2):119–25. doi: 10.1093/ageing/31.2.119. doi: 10.1093/ageing/31.2.119. Medline:11937474. [DOI] [PubMed] [Google Scholar]
- 82.Bohannon RW. Decreased isometric knee flexion torque with hip extension in hemiparetic patients. Phys Ther. 1986;66(4):521–3. doi: 10.1093/ptj/66.4.521. Medline:3960978. [DOI] [PubMed] [Google Scholar]
- 83.Knapik JJ, Wright JE, Mawdsley RH, et al. Isokinetic, isometric and isotonic strength relationships. Arch Phys Med Rehabil. 1983;64(2):77–80. Medline:6824423. [PubMed] [Google Scholar]
- 84.Horstman A, Gerrits K, Beltman M, et al. Muscle function of knee extensors and flexors after stroke is selectively impaired at shorter muscle lengths. J Rehabil Med. 2009;41(5):317–21. doi: 10.2340/16501977-0331. doi: 10.2340/16501977-0331. Medline:19363562. [DOI] [PubMed] [Google Scholar]