Abstract
Selective decontamination of the digestive tract has been proven to prevent severe infections and to reduce mortality in critically ill patients. Historical arguments against its use, like the development of bacterial resistance and the absence of influence on mortality, have not been confirmed. Recent clinical trials designed to evaluate these variables and meta-analyses showed remarkable reductions in the incidence of resistant bacteria and a significant beneficial effect on survival. This review will update the evidence on the efficacy of selective decontamination of the digestive tract, and the issue of emergence of resistance, using data from randomized controlled trials and meta-analyses.
Keywords: selective decontamination, pneumonia, ventilator-associated pneumonia, bloodstream infection, infection
Introduction
The first clinical experience on selective decontamination of the digestive tract (SDD) goes back to 1983 [1], whilst the first randomized trial was published in 1987 [2]. Subsequently, 66 randomized controlled trials (RCTs) [3, 4] and 11 meta-analyses of RCTs of SDD have been published [5,6,7,8,9,10,11,12,13,14,15].
They demonstrated that SDD reduces severe infections of the lower airways, of the bloodstream and mortality, with resistance being controlled. Surprisingly, although the evidence supporting the use of SDD in intensive care unit (ICU) is high, SDD is not widely used in clinical practice. SDD has been the subject of strong controversies among detractors and advocates of the manoeuvre. SDD opponents still rely on historical arguments against its use, such as the lack of any effect on mortality and the emergence of resistance.
This article will review the evidence on the efficacy of SDD and the issue of emergence of resistance, using data from RCTs and meta-analyses of SDD.
What is SDD
SDD is a prophylactic strategy designed to prevent or minimize endogenous and exogenous infections, and to reduce mortality in critically ill patients. The aim of SDD is to prevent or eradicate, if present, the oropharyngeal and intestinal abnormal carriage of potentially pathogenic microorganisms (PPMs), such as Gram-negative aerobic microorganisms (AGNB), methicillin-sensitive Staphylococcus aureus and yeasts [16,17,18]. The philosophy of SDD has some fundamentals [16,17,18]:
critical illness importantly impacts the body flora promoting a shift from normal to abnormal carriage and from low-grade carriage to high-grade carriage, i.e. overgrowth of normal and abnormal flora;
gut overgrowth (i.e. ≥105 colonies per ml of saliva or faeces), in particular due to abnormal AGNB, is a critical event which precedes endogenous infections, and is a risk factor for antimicrobial resistance;
a limited range of 15 PPMs is responsible for the majority of infections in ICU, and SDD mainly impacts these microorganims;
most ICU infections are primary endogenous infections, followed by secondary endogenous and exogenous infections;
the use of the full four components protocol of SDD is crucial.
SDD selectively targets both normal (e.g. methicillin-sensitive S. aureus, Streptococcus pneumoniae,Haemophilus influenzae, Moraxella catarrhalis, Escherichia coli, Candida albicans) and abnormal flora (e.g.Klebsiella, Enterobacter, Citrobacter, Serratia, Proteus, Morganella, Pseudomonas, Acinetobacter species, and methicillin-resistant S. aureus). By design SDD does not cover low-level pathogens, such as anaerobes, viridans streptococci, enterococci, and coagulase-negative staphylococci, which rarely cause infections during ICU stay.
The full protocol of SDD classically consists of four components [18]:
a short course (4 days) of parenteral antibiotics to control primary endogenous infections caused by PPMs present in the patient’s admission flora. Previously healthy subjects can be treated with a β-lactam antibiotic, such as cefotaxime 80-100 mg/kg/day, active against normal and the majority of microorganisms belonging to the abnormal flora. Patients with a chronic underlying disease, such as diabetes, chronic obstructive pulmonary disease, and patients transferred from other ICUs or wards, may carry both normal and abnormal flora in throat and gut, including Pseudomonas species. This group of patients requires combination therapy, or an anti-pseudomonal cephalosporin (e.g. ceftazidime);
enteral antimicrobials, i.e. polymyxin E, tobramycin, and amphotericin B (PTA), given throughout ICU stay to control secondary oropharyngeal and intestinal carriage, and subsequent secondary endogenous infections due to PPMs acquired in the ICU. Half a gram of paste or gel containing 2% PTA is applied in the oropharyngeal cavity with a glowed finger four times a day, combined with 10 ml of a suspension containing 100 mg of polymyxin E, 80 mg of tobramycin and 500 mg of amphotericin B and are administered into the gut through the nasogastric tube four times a day. In case of endemicity of methicillin-resistant S. aureus (MRSA), i.e. incidence of over one new case per month with a diagnostic sample positive for MRSA throughout a six-month period, oropharyngeal gel and/or intestinal solution of vancomycin can be added to the classical PTA regimen;
high levels of hygiene prevent exogenous infections, as well as the topical antimicrobials of PTA on the tracheostomy in tracheostomized patients to control exogenous lower airway infections;
surveillance cultures of throat and rectum on admission and, afterwards, twice weekly to monitor the effectiveness of SDD and to detect resistance at an early stage.
Unfortunately, variations from the original four-component protocol have been employed, such as use of solely intestinal antimicrobials, administration of enteral antimicrobials without the parenteral component, and selective oropharyngeal decontamination (SOD) in which the oropharyngeal components is used omitting both the parenteral and the intestinal components.
Additionally, antimicrobials different from polymyxin/tobramycin have been introduced: they were mainly quinolones or different aminoglycosides, such as gentamicin or neomycin.
The effectiveness of SDD is based on the ability of antimicrobials to clear oropharyngeal and gut carriage in overgrowth concentration of both normal and abnormal PPMs rather than to selectively remove aerobic bacteria leaving the anaerobic intestinal flora unaffected, as the name SDD misleadingly implies [19]. However, the term SDD is now so well established that to change it would cause too much confusion.
Effectiveness
After 25 years of clinical research SDD has been assessed in 66 RCTs [3,4] and in 11 meta-analyses of only RCTs [5,6,7,8,9,10,11,12,13,14,15] in approximately 15,000 patients. The full protocol using parenteral and enteral antimicrobials has been assessed in 22 RCTs.
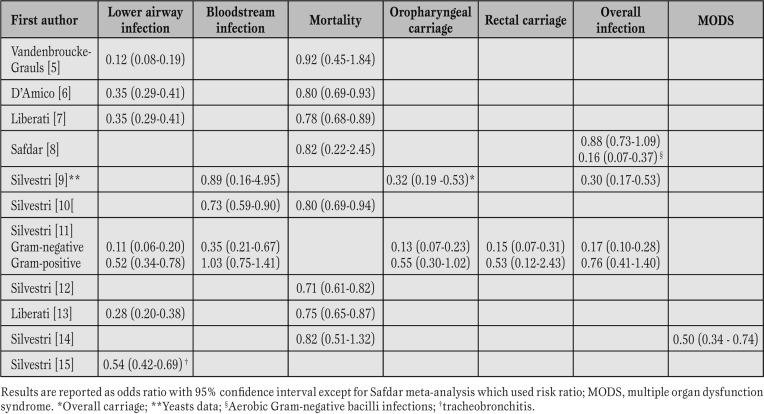
Amongst the 66 RCTs, 52 are from Europe and 14 from non-European countries. All meta-analyses, but one, are European (one from The Netherlands, and nine from Italy). Table 1 summarizes the results of the 11 meta-analyses.
Table 1.
Effectiveness of selective decontamination of the digestive tract assessed in 11 meta-analyses of randomized controlled trials.
SDD significantly reduces oropharyngeal and rectal carriage of Gram-negative microorganisms by 87% (OR [odds ratio] 0.13; 95% confidence interval [CI] 0.07-0.23) and 85% (OR 0.15; 95% CI 0.07-0.31), respectively [11]. Gram-positive carriage is also reduced, but not significantly [11]. Additionally, SDD significantly reduces yeast carriage (OR 0.32; 95% CI 0.19-0.53) [9].
All meta-analyses demonstrated an impact of SDD on lower respiratory tract infections. The use of the full protocol of enteral and parenteral antimicrobials of SDD reduces lower airway infections by 72% (OR 0.28; 95% CI 0.20-0.38) [13], due to both Gram-negative and Gram-positive bacteria [11]. Interestingly, the full protocol of SDD is more effective in reducing Gram-negative lower airway infections than solely enteral antimicrobials (OR 0.07; 95% CI 0.04-0.13, and OR; 0.28 95% CI 0.11-0.68, respectively) [11]. A recent meta-analysis explored the effectiveness of SDD on ventilator-associated tracheobronchitis (VAT), and showed a 46% VAT reduction in patients receiving SDD (OR 0.54; 95% CI 0.42-0.69) [15].
Polyenes, nystatin or amphotericin B, are part of the SDD antimicrobials. A meta-analysis, exploring the impact of SDD on fungal infections, showed a 70% significant reduction of fungal infections (OR 0.30, 95% CI 0.17-0.53), although fungaemia was not significantly reduced due to the low event rates in test and control group [9].
Fifty-one RCTs in 8065 critically ill patients were included in a systematic review on bloodstream infection [10]. SDD significantly reduced overall bloodstream infections by 27% (OR 0.73; 95% CI 0.59-0.90) and Gram-negative bloodstream infections (OR 0.39; 95% CI, 0.24-0.63), without any impact on Gram-positive bloodstream infections (OR 1.06; 95% CI, 0.77-1.47). Again, the effect on overall bloodstream infections and bloodstream infections due to Gramnegative bacteria was even larger when parenteral and enteral antimicrobials were used with ORs of 0.63 (95% CI, 0.46-0.87), and 0.30 (95% CI, 0.16-0.56), respectively. These findings have been substantiated by a recent multicentre trial which demonstrated that ICU-acquired bacteraemia due to enterobacteriaceae was significantly reduced in SDD vs standard care (OR 0.44, 95% CI 0.34-0.47), in SOD vs standard care (OR 0.68, 95% CI 0.53-0.86), and, remarkably, in SDD vs SOD (OR 0.65, 95% CI 0.49-0.85) [20].
A recent meta-analysis explored the efficacy of SDD in the prevention of multiple organ dysfunction syndrome (MODS) [14]. In seven RCTs including 1270 patients, SDD reduced MODS by 50% (OR 0.50, 95% CI 0.34-0.74).
Out of 11 meta-analyses, 8 explored the effect on mortality [5,6,7,8, 10, 12,13,14]. All meta-analyses assessing the impact of the full protocol and providing a large sample size (more than 2000 patients) showed a survival benefit of SDD [6, 7, 10, 12, 13]. In particular, an Italian meta-analysis demonstrated a mortality reduction of 29% (OR 0.71, 95% CI 0.61-0.82), reaching a 42% mortality reduction in studies where SDD eradicated the carrier state (OR 0.58, 95% CI 0.45-0.77) [12]. Eighteen patients should be treated with SDD to save one life. Remarkably, in meta-analyses with a small sample size the reduction in mortality was not significant [5, 8, 14]. There are two large Dutch RCTs with the endpoint of mortality [20, 21]. In the first the risk of mortality was reduced by 40% in the unit where SDD was administered to all patients [21]. The second RCT [20], the largest SDD study ever published, compared SDD and SOD vs standard care. Both SDD and SOD equally reduced mortality compared to standard care (OR 0.83, p=0.02; 0.86, p=0.045, respectively), although the mortality reduction was higher, albeit not significantly, in SDD group than in SOD group. A recent meta-analysis of SOD RCTs failed to demonstrate any significant impact on mortality (OR 0.93; 95% CI 0.81-1.07) [22].
SDD has been assessed in selected patient groups, such as burns [23], patients receiving oesophageal/gastrointestinal surgery [24], paediatric population [25], transplant patients [8]. The meta-analysis on mortality in burn patients demonstrated a significant reduction in the odds of death by 78% (OR 0.22, 95% CI 0.12-0.43) [23].
Three RCTs investigating gastroesophageal surgery were pooled together in a meta-analysis which showed that SDD reduced pneumonia rate by 64% (OR 0.36, 95% CI 0.19-0.69) [24]. A meta-analysis of SDD in transplant recipients found a significant reduction of infections due to AGNB and yeasts (OR 0.16, 95% CI 0.07-0.37) [8].
The reduction was not significant when all infections were analysed, owing to a substantial number of infections due to low-level pathogens, such as enterococci and coagulase-negative staphylococci, which are intrinsically not covered by SDD. The small sample size may explain why mortality was not significantly reduced. Four pediatric RCTs were included in a recent meta-analysis. Pneumonia was significantly reduced by SDD (OR 0.31, 95% CI 0.11-0.87), whilst overall mortality was not (OR 1.18, 95% CI 0.50-2.76) [25]. However, the results of all these analyses should be interpreted with caution due to the low number of studies and patients included.
Resistance
AGNB resistance was the endpoint of four RCTs [4, 20, 21, 26]. During an outbreak of K. pneumoniae producing extended spectrum beta-lactamase in a French ICU, SDD significantly reduced both carriage and infections due to this microorganism [26].
Similarly, SDD cleared gut overgrowth of carbapenem-resistant K. pneumoniae in the Beer-Sheva ICU, Israel [4]. A Dutch mono-centre RCT of about 1000 patients found that carriage of AGNB resistant to imipenem, ceftazidime, ciprofloxacin, tobramycin and polymyxins occurred in 16% of patients receiving parenteral and enteral antimicrobials, compared to 26% of control patients that received parenteral antibiotics only with a relative risk of 0.6 (95% CI 0.5-0.8) [21]. In the multi-center clustered RCT from The Netherlands the proportion of patients with AGNB in rectal swabs that were not susceptible to the marker antibiotics was lower with SDD than with standard care or SOD [20]. Remarkably, a post-hoc analysis of the same Dutch RCT, explored the incidence of bacteremia and lower respiratory tract colonization due to highly resistant micro-organisms (HRMOs), in particular AGNB [27]. Bacteremia due to HRMOs was significantly reduced by SDD compared with SOD (OR 0.37; 95% CI 0.16-0.85), and lower respiratory tract colonization due to HRMOs was less with SDD (OR 0.58, 95% CI 0.43-0.78) than SOD (OR 0.65, 95% CI 0.49-0.87) compared with standard care. In an ecological survey [28] undertaken during the study period of the Dutch RCT [20], an increase in resistance after SOD and SDD discontinuation was observed.
This seems to contradict the reduction in resistance demonstrated in the main study. However, that ecological study has some important limitations. First, the study used a point-prevalence method in which all patients in the unit, whether enrolled or not in the study, were included.
Second, the change in resistance could also be due to a simultaneous change of the resistance spectrum in patients admitted to the hospital, as admission cultures were included in the study. Finally, the survey showed that AGNB resistant to the marker antibiotics in the respiratory tract were significantly lower during SDD/SOD than during the pre- and post-intervention periods, and AGNB resistance to ciprofloxacin and tobramycin in rectal swabs was significantly lower during SDD than during standard care/SOD. These findings confirm that SDD does not increase the resistance problem, but actually reduces it, and that SDD was superior to SOD and standard care in controlling the emergence of resistance.
By design, SDD does not cover MRSA, and, therefore, may promote carriage and infection due to this PPM. There are seven RCT conducted in ICUs in which MRSA was endemic during the study and demonstrated a trend toward higher MRSA infection rates in patients receiving SDD [29]. Under these circumstances, the original SDD component requires the addition of oropharyngeal and/or intestinal vancomycin. Interestingly, three studies [30,31,32] using 2 g of 4% vancomycin gel or paste and/or 2 g of vancomycin solution added to the non-absorbable PTA component demonstrated that prevention and eradication of carriage and overgrowth of MRSA was followed by the control of MRSA infection, transmission and outbreaks. Severe infection, including MRSA pneumonia, were significantly reduced using enteral vancomycin [32, 33].
Although, studies on AGNB resistance have not led to an increased resistance problem, clinicians should be aware that highly resistant microorganisms may be selected during SDD, in particular in ICUs where these microorganisms are endemic. Therefore, surveillance samples of throat and rectum are mandatory to detect resistance at an early stage.
Costs
Costs were reduced in the majority of SDD RCTs, including cost per survivor [34]. In the recent Dutch trial, although the median number of defined daily doses of systemic antibiotics per patient-day did not significantly differ among SDD, SOD and standard care, the use of carbapenems, quinolones and lincosamides was reduced in SDD compared with SOD and standard care [21]. Van Nieuwenhoven et al [35], using a combination of crude cost analysis, decision model analysis, and bootstrap analysis, provided strong evidence that preventing ventilator-associated pneumonia by means of oropharyngeal decontamination was cost-effective. Also the Agency for Healthcare Research and Quality of the United States Department for Health and Human Services considers SDD to be a cheap manoeuvre [36]. The cost of less than 10 €/day is not an issue for an intervention that reduces lower airway and bloodstream infections, and mortality.
Why is SDD not widely used?
Despite the vast body of evidence showing a significant reduction in morbidity and mortality, with resistance being controlled, SDD is not widely applied in clinical practice.
The main reason for SDD not being widely used is the primacy of opinion over evidence. Two surveys revealed that SDD is used in only 5% and 30% of the British [37] and Dutch [38] intensive care units, respectively, mainly due to an insufficient evidence of efficacy and concerns about resistance. Additionally, SDD has been recently ranked the worst manoeuvre for prevention of ventilator-associated pneumonia by a panel of experts, although none of them has published on the topic [39]. The reason for this is multifactorial, but the longstanding disagreement among experts and opinion leaders has been an important factor which contributed to the confusion. For example, in early nineties the first meta-analysis on SDD by the Cochrane Italian Center in Milan had already demonstrated a significant impact of SDD on mortality [40], but, subsequently, a biased meta-analysis has been published in an influential American journal with opposite results [41]. Four further meta-analyses and two large RCTs have been needed before opinion leaders acknowledged that SDD significantly impacted mortality. More recently, also the National Institute for Health and Clinical Excellence, NICE, although admitting that SDD impacted morbidity and mortality, did not support SDD as “few studies had been undertaken in the UK, and therefore they did not reflect current National Health Service practice” [42]. History repeats itself! It seems that the evidence based medicine can not be applicable to SDD, similarly to Semmelweis’ findings, which were heavily opposed by Virchow, the expert pathologist and influential opinion leader of that time. Previous experience with thrombolytic drugs indicates a similar pattern, with an undesirable lag between the appearance of meta-analytic evidence and the recommendation of clinical experts [43].
Concerns expressed by experts about resistance are based on low level evidence, but have delayed the implementation of SDD. European and American experts stated that the most important objection to the widespread use of SDD is the unknown effects on antibiotic resistance in the long term. They invariably refer to their own review articles, e.g. the lowest level of evidence [44, 45].
SDD has never been promoted by pharmaceutical companies, probably because there is little profit in older agents such as cefotaxime, polymyxin E, tobramycin and amphotericin B, which are inexpensive and off-patent. Furthermore, SDD is not supported by authoritative-looking data sheets, and it is not marketed to clinicians in the traditional manner. Paste, gel or suspension are not readily available on the shelf. Hence, the application of SDD requires more effort in terms of commitment and monitoring from the ICU team, the pharmacist, and the microbiologist, than is the case with the mere systemic administration of the latest antibiotic on the market. The most recent example is the Surviving Sepsis Campaign sponsored by the industry that recommends all evidence-based medicine interventions [46], except SDD [47]. Moreover, intensivists are not familiar with surveillance cultures of throat and rectum and they are worried about an increased team workload [48]. However, ICUs using SDD experienced less workload due to a reduction in infection rates, less systemic antibiotics use, declining in the frequency of tracheal suctioning, and absence of resistant strains requiring patients’ cohorting or isolation.
Finally, there is an interaction between physicians and pharmaceutical industries. Most opinion leaders have links with the industry and receive grants for the evaluation of new antibiotics, both in vitro and in vivo. The same clinicians are invited to national and international meeting at which they report data often promoting these new drugs as first-line antibiotics. The traditionalists on the “circuit” have relied on the industry to develop new drugs at regular intervals, usually after publications of case reports of superinfections of the currently favoured antibiotics. The realisation that the pharmaceutical companies failed to provide new classes of antibiotics came as a severe blow. Industrialized countries have largely delegated the control of drug trials to the pharmaceutical companies, which place clear limitation on research [49]. However, economic interests seek the best financial return and establishing new potent antibiotics to treat rather than prevent pneumonia is more profitable.
Conclusions
SDD is the best-ever evaluated intervention in intensive care medicine, and has been assessed by 66 RCTs and several meta-analyses in approximately 15,000 patients, over a period of 25 years. The control of digestive tract overgrowth of normal and abnormal flora by achieving high antimicrobials concentrations is the mechanism of action of SDD.
The results of individual studies and meta-analyses indicate a strong protective effect of the full protocol of parenteral and enteral antimicrobials of SDD on pneumonia and bloodstream infections, and a reduction in mortality, with resistance being controlled.
Footnotes
Source of Support Nil.
Conflict of interest None declared.
Cite as: Silvestri L, van Saene H.K.F. Selective decontamination of the digestive tract: an update of the evidence. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2012; 4(1): 21-29
References
- Stoutenbeek CP, van Saene H.K., Miranda DR. et al. A new technique of infection prevention in the ICU by selective decontamination of the digestive tract. Acta Anaesth Belg. 1983;3:209–221. [PubMed] [Google Scholar]
- Unertl K, Ruckdeschel G, Selbmann H.K.. et al. Prevention of colonization and respiratory infections in long-term ventilated patients by local antimicrobial prophylaxis. Intensive Care Med. 1987;13:106–113. doi: 10.1007/BF00254795. [DOI] [PubMed] [Google Scholar]
- Anonymous . [Selective digestive decontamination: 65 SDD RCTs. available at: http://www.signavitae.com/sdd/sddrcts; accessed 2 March 2012.]
- Saidel-Odes L, Polachek H, Peled N. et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol. 2012;33:14–19. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke-Grauls CM, Vandenbroucke JP. Effect of selective decontamination of the digestive tract on respiratory tract infections and mortality in the intensive care unit. Lancet. 1991;338:859–862. doi: 10.1016/0140-6736(91)91510-2. [DOI] [PubMed] [Google Scholar]
- D\'Amico R, Pifferi S, Leonetti C. et al. Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. BMJ. 1998;316:1275–1285. doi: 10.1136/bmj.316.7140.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, D\'Amico R, Pifferi S. et al. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD000022.pub2. [DOI] [PubMed] [Google Scholar]
- Safdar N, Said A, Lucey MR. et al. The role of selective decontamination for reducing infection in patients undergoing liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2004;10:817–827. doi: 10.1002/lt.20108. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene H.K., Milanese M. et al. Impact of selective decontamination of the digestive tract on fungal carriage and infection: systematic review of randomized controlled trials. Intensive Care Med. 2005;31:898–910. doi: 10.1007/s00134-005-2654-9. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene H.K., Milanese M. et al. Selective decontamination of the digestive tract reduces bloodstream infections and mortality in critically ill patients: a systematic review of randomized controlled trials. J Hosp Infect. 2007;65:187–203. doi: 10.1016/j.jhin.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene H.K., Casarin A. et al. Impact of selective decontamination of the digestive tract on carriage and infection due to Gram-negative and Gram-positive bacteria. Systematic review of randomized controlled trials. Anaesth Intens Care. 2008;36:324–338. doi: 10.1177/0310057X0803600304. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene H.K., Weir I. et al. Survival benefit of the full selective digestive decontamination regimen. J Crit Care. 2009;24:474e7–414. doi: 10.1016/j.jcrc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Liberati A, D\'Amico R, Pifferi S. et al. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD000022.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L, van Saene H.K., Zandstra DF. et al. Impact of selective decontamination of the digestive tract on multiple organ dysfunction syndrome: Systematic review of randomized controlled trials. Crit Care Med. 2010;38:1370–1376. doi: 10.1097/CCM.0b013e3181d9db8c. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Milanese M, Taylor N. et al. Selective digestive decontamination reduces ventilator-associated tracheobronchitis. Respir Med. 2010;104:1953–1955. doi: 10.1016/j.rmed.2010.05.023. [DOI] [PubMed] [Google Scholar]
- van Saene H.K., Petros AJ, Ramsay G, Baxby D. All great truths are iconoclastic: selective decontamination of the digestive tract moves from heresy to level 1 truth. Intensive Care Med. 2003;29:677–690. doi: 10.1007/s00134-003-1722-2. [DOI] [PubMed] [Google Scholar]
- Zandstra DF, van Saene HK. Selective decontamination of the digestive tract as infection prevention in the critically ill. A level 1 evidence-based strategy. Minerva Anestesiol. 2011;77:212–219. [PubMed] [Google Scholar]
- Silvestri L, van Saene HK, Zandstra DF. Preventing infection using selective decontamination of the digestive tract. In HKF van Saene, L. Silvestri, MA de la Cal, A Gullo (eds) Infection control in the intensive care unit. Springer, Milan, 3rd edition. 2012:203–215.
- Zandstra DF, Petros AJ, Silvestri L. Selective decontamination of the digestive tract. Selectivity is not required. Intensive Care Med. 2010;36:1783–1784. doi: 10.1007/s00134-010-1945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet AM, Kluytmans JA, Cooper BS. et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- de Jonge E, Schultz MJ, Spanjaard L. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011–1016. doi: 10.1016/S0140-6736(03)14409-1. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene HK, Zandstra DF. et al. SDD, SOD or oropharyngeal chlorhexidine to prevent pneumonia and to reduce mortality in ventilated patients: which manoeuvre is evidence-based? Intensive Care Med. 2010;31:1436–1437. doi: 10.1007/s00134-010-1809-5. [DOI] [PubMed] [Google Scholar]
- Silvestri L, de la Cal MA, Taylor N. et al. Selective decontamination of the digestive tract in burn patients: an evidence-based maneuver that reduces mortality. J Burn Care Res. 2010;31:372–373. doi: 10.1097/BCR.0b013e3181d1b61f. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene HK. Selective digestive decontamination to prevent pneumonia after esophageal surgery. Ann Thorac Cardiovasc Surg. 2010;16:220–221. [PubMed] [Google Scholar]
- Petros AJ, Silvestri L, Booth R. et al. Selective decontamination of the digestive tract in critically ill children: systematic review and meta-analysis. Ped Crit Care Med. 2012 doi: 10.1097/PCC.0b013e3182417871. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C, Legrand P, Rauss A. et al. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med. 1989;110:873–881. doi: 10.7326/0003-4819-110-11-873. [DOI] [PubMed] [Google Scholar]
- de Smet AM, Kluytmans JA, Blok HE. et al. Selective digestive decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis. 2011;11:372–80. doi: 10.1016/S1473-3099(11)70035-4. [DOI] [PubMed] [Google Scholar]
- Oostdijk EA, de Smet AM, Blok HE. et al. Ecological effects of selective decontamination on resistant Gram-negative bacterial colonization. Am J Respir Crit Care Med. 2010;181:452–457. doi: 10.1164/rccm.200908-1210OC. [DOI] [PubMed] [Google Scholar]
- Zandstra DF, van Saene HK, van der Voort PH. Antimicrobial resistance during 20 years of clinical SDD research. In PHJ van der Voort, HKF van Saene (eds). Selective digestive tract decontamination in intensive care medicine. Springer, Milan. 2008:121–131. [Google Scholar]
- de la Cal MA, Cerda E, van Saene HK. et al. Effectiveness and safety of enteral vancomycin to control endemicity of methicillin-resistant Staphylococcus aureus in a medical/surgical intensive care unit. J Hosp Infect. 2004;56:175–183. doi: 10.1016/j.jhin.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Solidoro A, Milanese M. et al. Topical oropharyngeal vancomycin to control methicillin-resistant Staphylococcus aureus lower airway infection in ventilated patients. Minerva Anestesiol. 2010;73:193–202. [PubMed] [Google Scholar]
- Silvestri L, Milanese M, Oblach L. et al. Enteral vancomycin to control methicillin-resistant Staphylococcus aureus outbreak in mechanically ventilated patients. Am J Infect Control. 2002;30:391–399. doi: 10.1067/mic.2002.122255. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene HK, Milanese M. et al. Prevention of MRSA pneumonia by oral vancomycin decontamination: a randomised trial. Eur Respir J. 2004;23:921–926. doi: 10.1183/09031936.04.00109704. [DOI] [PubMed] [Google Scholar]
- Silvestri L, van Saene HK, de la Cal MA. et al. Prevention of ventilator associated pneumonia by selective decontamination of the digestive tract. Eur Respir J. 2008:241–243. doi: 10.1183/09031936.00023808. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhoven CA, Buskens E, Bergmans DC. et al. Oral decontamination is cost-saving in the prevention of ventilator-associated pneumonia in intensive care units. Crit Care Med. 2004;32:126–130. doi: 10.1097/01.CCM.0000104111.61317.4B. [DOI] [PubMed] [Google Scholar]
- Collard hr, Saint s. Preventative practices for ventilator-associated pneumonia. In KG Shojania, B Duncan, KM McDonald, RM Wachter (eds). Making health care safer: a critical analysis of patients safety practices. Evidence report/technology assessment N°43. Agency for Healthcare Research and Quality, publication 01-E058. 2001 [ Rockville, MD, 2001] [PMC free article] [PubMed] [Google Scholar]
- Bastin aj, Ryanna kb. Use of selective decontamination of the digestive tract in United Kingdom intensive care units. Anaesthesia. 2009;64:46–49. doi: 10.1111/j.1365-2044.2008.05676.x. [DOI] [PubMed] [Google Scholar]
- Barends H, Zandstra DF, van der Voort PH. Current state of affairs: SDD application in Dutch ICUs. Neth J Crit Care. 2008;12:109–112. [Google Scholar]
- Rello J, Lode H, Cornaglia G. et al. A European care bundle for prevention of ventilator-associated pneumonia. Intensive Care Med. 2010;36:773–780. doi: 10.1007/s00134-010-1841-5. [DOI] [PubMed] [Google Scholar]
- Collaborative Group . Selective Decontamination of the Digestive Tract Trialists\' Collaborative Group. Meta-analysis of randomised controlled trials of selective decontamination of the digestive tract. BMJ. 1993;307:525–532. doi: 10.1136/bmj.307.6903.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwenhoven CA, Buskens E, van Tiel FH. et al. Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA. 2001;286:335–340. doi: 10.1001/jama.286.3.335. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence . National Patient Safety Agency. www.nice.org.uk/nicemedia/live/12053/41684/41684.pdf. 2008 [Google Scholar]
- Antman EM, Lau J, Kupelnick B. et al. A comparison of results of meta-analyses of randomized controlled trials and recommendation of clinical experts. JAMA. 1992;268:240–248. [PubMed] [Google Scholar]
- Vincent JL. Selective digestive decontamination for everyone, everywhere? Lancet. 2003;362:1006–1007. doi: 10.1016/S0140-6736(03)14445-5. [DOI] [PubMed] [Google Scholar]
- Kollef MH. Selective digestive decontamination should not be routinely employed. Chest. 2003;123:464–468. doi: 10.1378/chest.123.5_suppl.464s. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM. et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L, van Saene HK, de la Cal MA. et al. Surviving Sepsis Campaign needed consensus to exclude selective decontamination of the digestive tract. Crit Care Med. 2008;36:2716–2717. doi: 10.1097/CCM.0b013e31818474a2. [DOI] [PubMed] [Google Scholar]
- Silvestri L, Petros AJ, de la Cal MA. et al. Selective digestive decontamination. Why are intensivists more "resistant" than microorganisms? Minerva Anestesiol. 2011;77:658–659. [PubMed] [Google Scholar]
- Garattini S, Bertelè V. Ethics in clinical research. J Hepatol. 2009;51:792–797. doi: 10.1016/j.jhep.2009.07.005. [DOI] [PubMed] [Google Scholar]