Abstract
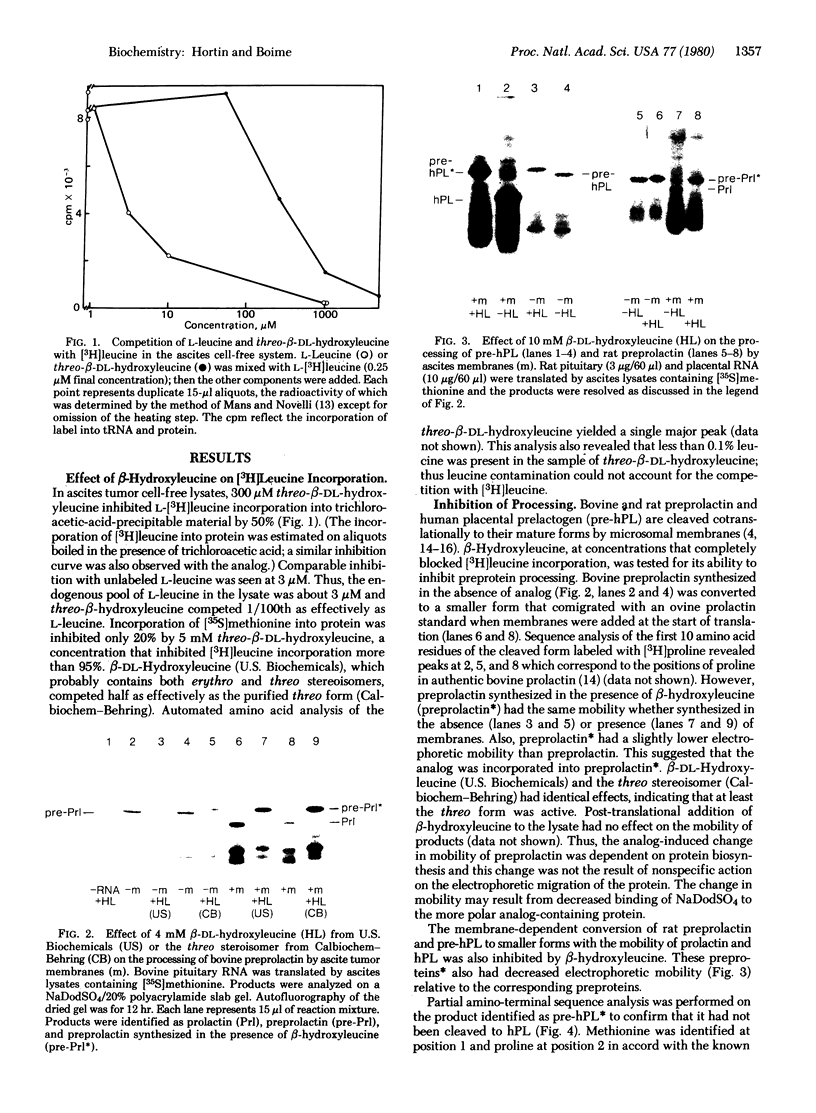
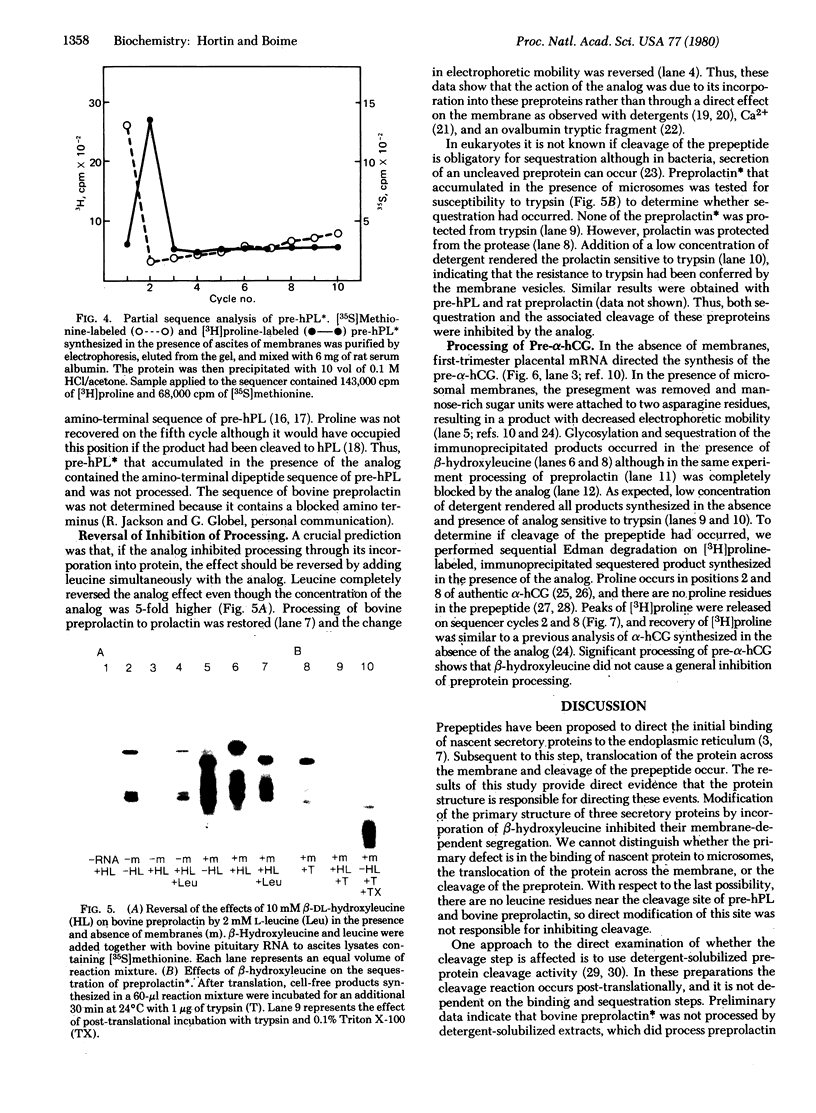
Leucine analogs were tested in the Krebs II ascites cell-free translation system for the ability to inhibit preprotein cleavage by replacing leucine in nascent chains of bovine preprolactin, rat preprolactin, human placental prelactogen (pre-hPL), and pre-α subunit of human chorionic gonadotropin (α-hCG). In the absence of analog, ascites microsomal membranes cleaved these preproteins to their mature forms and sequestered the processed products. Also, two asparagine residues in α-hCG were glycosylated. When 4 mM β-DL-hydroxyleucine was added to the lysate instead of L-leucine, cotranslational processing and sequestration of both species of preprolactin and pre-hPL were inhibited. Sequential Edman degradation confirmed that pre-hPL was not cleaved. The inhibition of processing by β-hydroxyleucine resulted from its incorporation into protein. This was shown by reversal of the effect by addition of leucine and by inhibition of [3H]leucine incorporation into protein. Of significance, the processing of pre-α-hCG was less sensitive to β-hydroxyleucine because its prepeptide contains only four scattered leucine residues, whereas the presegments of hPL and the prolactins contain six to eight clustered leucine residues. These experiments demonstrate that translocation and processing of secretory proteins require structural features determined by the primary amino acid sequence.
Keywords: secretory proteins, cell-free protein synthesis, preprotein cleavage, β-hydroxyleucine
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Bellisario R., Carlsen R. B., Bahl O. P. Human chorionic gonadotropin. Linear amino acid sequence of the alpha subunit. J Biol Chem. 1973 Oct 10;248(19):6796–6809. [PubMed] [Google Scholar]
- Bielinska M., Boime I. mRNA-dependent synthesis of a glycosylated subunit of human chorionic gonadotropin in cell-free extracts derived from ascites tumor cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1768–1772. doi: 10.1073/pnas.75.4.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M., Grant G. A., Boime I. Processing of placental peptide hormones synthesized in lysates containing membranes derived from tunicamycin-treated ascites tumor cells. J Biol Chem. 1978 Oct 25;253(20):7117–7119. [PubMed] [Google Scholar]
- Birken S., Fetherston J., Desmond J., Canfield R., Boime I. Partial amino acid sequence of the preprotein form of the alpha subunit of human choriogonadotropin and identification of the site of subsequent proteolytic cleavage. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1247–1253. doi: 10.1016/0006-291x(78)91137-3. [DOI] [PubMed] [Google Scholar]
- Birken S., Smith D. L., Canfield R. E., Boime I. Partial amino acid sequence of human placental lactogen precursor and its mature hormone form produced by membrane-associated enzyme activity. Biochem Biophys Res Commun. 1977 Jan 10;74(1):106–112. doi: 10.1016/0006-291x(77)91381-x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Szczesna E., Smith D. Membrane-dependent cleavage of the human placental lactogen precursor to its native form in ascites cell-free extracts. Eur J Biochem. 1977 Mar 1;73(2):515–520. doi: 10.1111/j.1432-1033.1977.tb11345.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Kemper B. Conversion of pre-proparathyroid hormone to proparathyroid hormone by dog pancreatic microsomes. Biochemistry. 1978 Dec 12;17(25):5550–5555. doi: 10.1021/bi00618a034. [DOI] [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. Isolation, cloning and sequence analysis of the cDNA for the alpha-subunit of human chorionic gonadotropin. Nature. 1979 Oct 4;281(5730):351–356. doi: 10.1038/281351a0. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Bradshaw R. A. D-3-Phosphoglycerate dehydrogenase from chicken liver. II. Chemical and physical properties. J Biol Chem. 1978 Apr 25;253(8):2727–2731. [PubMed] [Google Scholar]
- Habener J. F., Potts J. T., Jr Biosynthesis of parathyroid hormone (second of two parts). N Engl J Med. 1978 Sep 21;299(12):635–644. doi: 10.1056/NEJM197809212991205. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A., McKean D. J. Synthesis of preprolactin and conversion to prolactin in intact cells and a cell-free system. J Biol Chem. 1978 Sep 25;253(18):6315–6318. [PubMed] [Google Scholar]
- McKean D. J., Maurer R. A. Complete amino acid sequence of the precursor region of rat prolactin. Biochemistry. 1978 Nov 28;17(24):5215–5219. doi: 10.1021/bi00617a022. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Morgan F. J., Birken S., Canfield R. E. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975 Jul 10;250(13):5247–5258. [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Schechter I. Partial amino acid sequence of the precursor of immunoglobulin light chain programmed by messenger RNA in vitro. Science. 1975 Apr 11;188(4184):160–162. doi: 10.1126/science.803715. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M., Burstein Y., Schechter I. Primary structure of the NH2-terminal extra piece of the precursor to human placental lactogen. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3819–3823. doi: 10.1073/pnas.76.8.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood L. M., Handwerger S., McLaurin W. D., Lanner M. Amino-acid sequence of human placental lactogen. Nat New Biol. 1971 Sep 8;233(36):59–61. doi: 10.1038/newbio233059a0. [DOI] [PubMed] [Google Scholar]
- Smith D. L., Boime I. Reversible calcium inhibition of the membrane-dependent cleavage of pre-placental lactogen in ascites cell-free extracts. FEBS Lett. 1977 Dec 1;84(1):115–118. doi: 10.1016/0014-5793(77)81069-7. [DOI] [PubMed] [Google Scholar]
- Strauss A. W., Zimmerman M., Boime I., Ashe B., Mumford R. A., Alberts A. W. Characterization of an endopeptidase involved in pre-protein processing. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4225–4229. doi: 10.1073/pnas.76.9.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]