Abstract
Ever since the early days of homograft implantation in 1956, and the introduction into clinical practice by Ross and Barrat Boyes, homograft heart valves have proven to have many advantages. Its disadvantages became evident during long-term follow up. Factors, such as donor and recipient morbidity, tissue banking techniques, and the often complex surgical technique required to implant, are of great influence on the long term results. Because of European Directives, legally binding quality assurance regulations have been introduced in homograft banks. However, still not all processing methods have been scientifically sub-structured on their effects on the final product and its durability. The donor shortage has stimulated researchers and industries to develop and improve mechanical and biological valve substitutes such as the stentless bioprostheses. In general, candidates for homograft valve implantation include patients with: endocarditis, congenital defects and women who wish to become pregnant. For each category of patients different implantation techniques are required. The results of homograft banking and homograft transplantation in the German Heart Institute Berlin are satisfactory. Freedom of re-infection rate after homograft implantation is 91.9% +/- 3.6% after 15 years. Current developments show an increased interest in tissue engineered as well as in de- and re-cellularization of heart valve homografts. The advantages and disadvantages of the several processing techniques have not yet been proven in long term clinical results. For homograft bankers these developments pose as a challenge to join forces and to initiate cooperate projects aimed at scientific and organizational development.
Keywords: tissue banks, homografts, valves
Introduction
Heart valves are marvels of natural engineering. Most people’s last a lifetime. But when valves become diseased, they may need to be repaired or replaced. Many Europeans need surgery for valve diseases.
Most of them will get new, replacement valves made of mechanical or biological (homograft/animal) materials. Which type of valve they get is an important decision and is often not decided until the time of surgery. Up until now, the biological valve had one big disadvantage: it did not last very long. Recently, however, researchers at Deutsches Herzzentrum Berlin have turned that belief on its head. Biological valves turns out to be a lot tougher than many people thought: they last.
The first implantation of a homograft in the descending aorta was performed by Murray, in 1956 [1].
From then on, homografts, also known as human allograft heart valves, have been increasingly used for valve repair.
Their use has been clearly established since they were introduced into clinical practice by Ross [2] in 1962 and Barratt Boyes [3] in 1965. At that time, they were the only successful biological heart valve prosthesis beside the mechanical ones.
To date there is no ideal valve substitute; however, homograft valves offer many theoretical and proven advantages, such as restoration of normal flow in the aortic root, sinuses and coronary orifices; superior hemodynamic properties over mechanical valves, especially to those available in the early 1960s and 1970s, a low rate of thromboembolism, thus avoiding a lifetime of anticoagulation therapy, and resistance to infection. Its disadvantages became evident during long-term follow-up. Homograft implantation requires a slightly more complex technique of insertion. Its limited availability because of the shortage of human organ and tissue donors is a clear disadvantage.
The valves can be explanted either from recipients of heart transplantation, from organ donors whose hearts are not accepted for heart transplantation for reasons other than valve problems, and from patients who had died due to noncardiac reasons. Likewise, the possibility of explantation and use of the homografts depends also on the local rules governing organ and tissue donors.
The last factor (limited durability) is very important because it necessitates reoperation, with attendant risk, inconvenience and cost. In the first few years after their introduction into clinical practice, homografts were thought to have the same durability as a normal human valve. However, during mid- and long-term follow-up, it became evident that these homograft valves undergo structural degeneration as are other biological valve substitutes.
These valves undergo early calcification with slow progression. Thus, in 20 years, 30% to 40% of homografts implanted are still functional. Although the durability is higher compared with xenogeneic valves, it is still less than that of mechanical prostheses. Patients must be informed about the strong possibility of a repeat valve replacement surgery. Regarding the huge and still growing number of patients requiring valve replacement, it became clear that these valves could not be used in every case.
Methods
Effects of Sterilization, Preservation and Storage on Vitality of Homografts
Different techniques of sterilization, preservation and storage are described in literature. Although other preservation techniques [4, 5] have been reported, the procedures were modified and improved with time. Presently mainly two alternatives are applied, namely cryopreservation in the vapor phase of liquid nitrogen and fresh-wet storage at 4°C after antibiotic sterilization [6, 7].
Fresh wet storage results in more viable donor cells at implantation and therefore might increase the immunologic response of the recipient.
Cryopreservation has the advantage of the possibility of a long storage period. During the rewarming process, care must be taken to achieve a homogenous defrosting of the complete graft in order to avoid any interstitial ice formation.
Any crystallization of interstitial water can be the starting point of later degeneration. In both processes, great care must be taken to ensure complete sterile processing and storage; this must be controlled by repeated microbiological testing of the fluids used for storage, since any contamination of the homograft could be disastrous for the recipient.
In one large series of O’Brien and his group [7], there was an advantage for cryopreserved grafts during long-term follow-up.
The viability of the grafts stored at 4C could explain these findings.
Homograft Banking
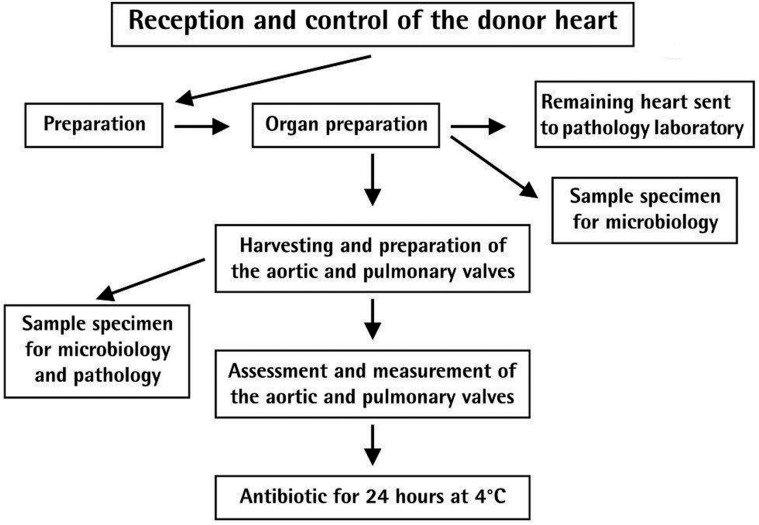
Preparation. Figure 1 shows the algorithm on how the homografts after harvesting are processed and sterilized on the first day.
Measurements of Homograft. The measurement criterion of the aortic valve homograft limits the subvalvular myocardial length to a minimum of 5 mm, and the length of the aortic wall from the commissure to a minimum of 5 mm.
The measurement criterion of the pulmonary homograft limits the subvalvular myocardial length to a minimum of 5 mm, and the pulmonary wall length to a minimum of 5 mm from the commissure.
Figure 1.
Homograft banking, first day processes.
Sterilization
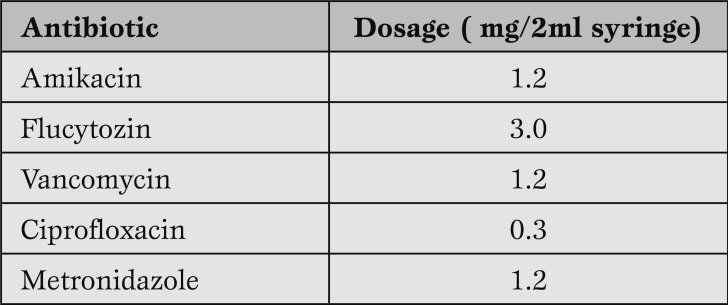
After measurements, the homograft is stored for a period of 18-24 hours in an antibiotic solution and medium at 4oC.
The components of the antibiotic solution is shown in Table 1. The composition of the antibiotic solution is chosen such that all non virulent pathogens are eliminated, while toxic influence on the cells of the homograft wall is being avoided. If any virulent microorganisms are found, the homograft is discarded.
Table 1.
Antibiotic solution for homograft sterilization.
Documentation protocol
All physical and processing data with respect to the homograft are documented and recorded on a homograft production form.
Labelling
Depending on the morphology of the homograft, different grafts may be produced such as: human aortic valve, human ascending aorta, human descending aorta, human pulmonary artery, human pericardium. These homografts are then labeled accordingly.
Packaging
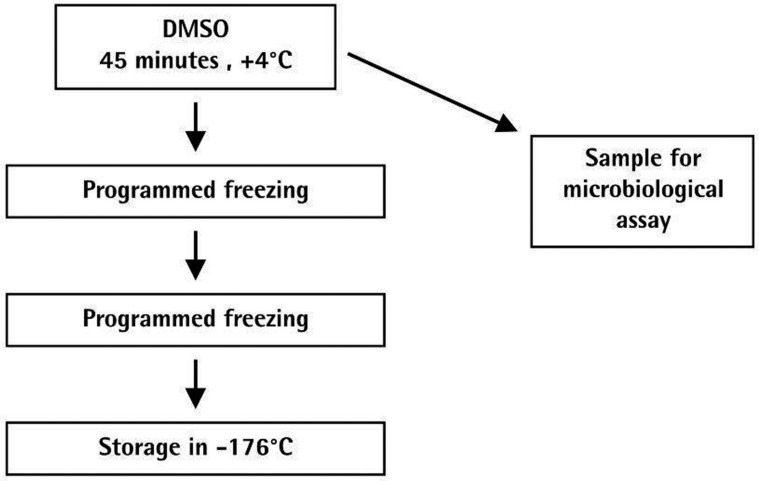
On the second day, provided all microbiological tests do have negative results, the homografts are packaged and, under cryo-protection of DMSO, stored in the vapor phase of liquid nitrogen at -176oC (Figure 2).
Figure 2.
Homograft banking, second day processes.
Quantitative results
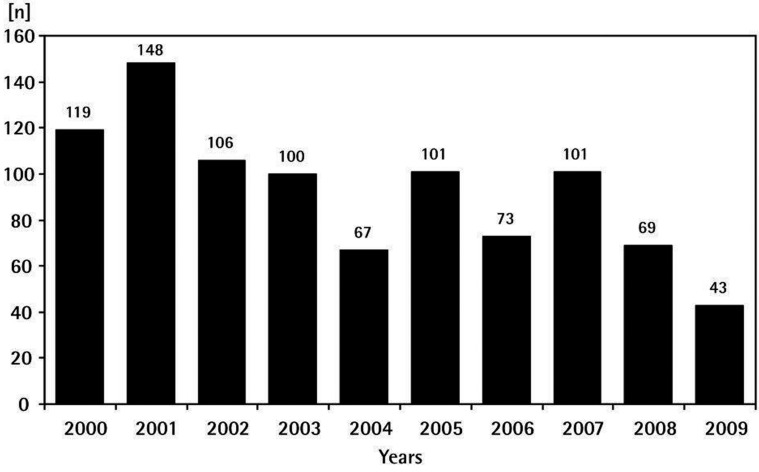
As shown in Figure 3, the availability of suitable donors is limited. While over a 10 year period 927 hearts were sent to the Berlin Homograft Bank an average of 92.7 per year, only 43 hearts were received in 2009.
While the donor source consists of recipients of heart transplants, organ donors whose hearts were not accepted, or donors who were autopsied and their relatives had agreed to using their tissues, the possibilities would be greater if hearts could be retrieved from non-organ donors with a cardiac arrest from up to 24 hours.
Figure 3.
Donor hearts received at the Deutsches Herzzentrum Berlin Homograft Bank: 2000-2009.
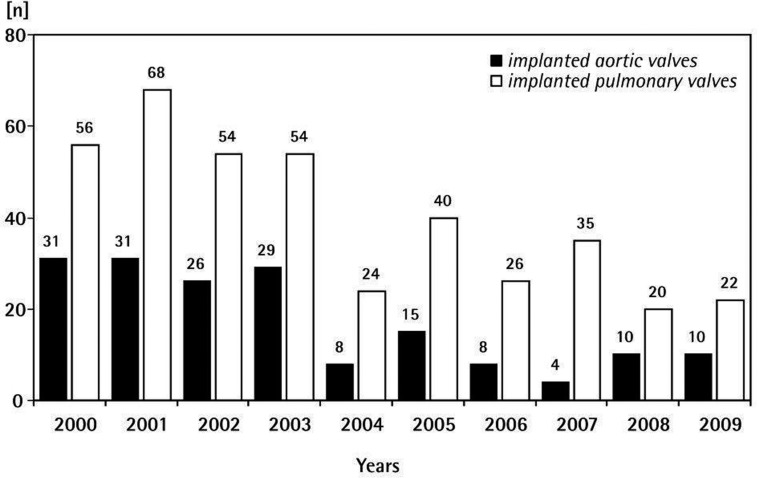
Given the limitations of the donor source in Germany, many homografts had to be imported over the years. Figure 4 shows the aortic and pulmonary homograft implantations in the German Heart Center Berlin from 2000-2009.
Figure 4.
Homograft valves implanted in the Deutsches Herzzentrum Berlin.: 2000-2009.
Indications for homograft implantation
Choice of homograft for valve replacement. Although most of the available prosthetic heart valves function remarkably well, the variety of available choices attests to the inability of any single one to fulfill the requirements of the ideal valve substitute. Review of several large comparative studies on valve performance reveals that the overall results with tissue and mechanical valves are about equal at the end of 10 years. The characteristics of each type of valve substitute dictate the selection of one prosthesis in preference to others for a particular patient.
Mechanical prostheses are recommended for patients without contraindications for anticoagulants. Tissue valves are reserved for patients over 65 years of age or for patients in whom anticoagulation is contraindicated.
Multiple other patient-related factors need to be considered in selecting the appropriate valve, including the psychosocial situation and patient preference. After the first two decades of constant improvements in valve prostheses, no major advance has occurred since the mid 1980s. Hence, valve replacement remained the exchange of one disease for another.
With minor and, for the most part, statistically nonsignificant variations, the spectrum of late valve-related complications remained unaltered and the few series published in the year under review brought no additional information of relevance. By contrast, in the past few years there has been a growing enthusiasm for the use of allografts, stentless porcine bioprostheses, and pulmonary autografts. Not only was there a surge of interest in the allografts as aortic valve substitutes, but in the past years there have also been several reports of use for whole or partial mitral or tricuspid valve replacement.
On the other hand, stentless bioprostheses are also gaining increasing acceptance, and all major manufacturers of heart valve prostheses have models for use in different situations and with different techniques. Finally, the Ross operation is now being performed around the world. Despite these advances, valve repair still merits the preference of many surgeons. Mitral valvuloplasty preserves left ventricular function much better than valve replacement. By contrast, the results of aortic valve repair look much less impressive. Recent reports focus on special aspects of surgery for native or prosthetic valve endocarditis, especially with the use of allografts or autografts, on the results of valve surgery in elderly patients, and on the controversial issues of anticoagulation in patients with artificial valves.
Indications for use of homograft
Although no clear indications exist about which a homograft must be used, these valves are a useful tool in heart valve surgery. Candidates for homograft valve implantation included the following.
Endocarditis
Adult patients with persistent native valve or prosthetic valve endocarditis involving the aortic valve usually require aortic valve replacement as part of their medical care.
Homograft aortic valve replacement has been shown to have a decreased incidence of recurrent bacterial endocarditis in patients with native valve or prosthetic valve endocarditis.
For this reason, many surgeons believe that in patients with aortic valve endocarditis the replacement of choice is the aortic homograft [8].
The reasons homograft are selected for patients with endocarditis, are:
homografts were thought to possess anti-infective properties;
homografts demonstrated that there were still living cells at the time of implantation: therefore, it was assumed that these living grafts could cope better with bacterial infection. These however are clinically not proven.
An advantage of homografts seems not to result from tissue or some specific properties but from the fact that, particularly when using the mini-root technique, they allow the replacement of annular tissue and even parts of the anterior mitral leaflet.
This allows the most radical excision of all infective tissue, which is an important surgical goal in all infections.
Congenital heart disease
A number of operations used for correction of complex congenital heart disease requires valved and nonvalved conduits.
In Norwood procedure or reconstruction of the ascending aorta and aortic arch for hypoplastic left heart syndrome, or reconstruction of the right ventricular outflow tract in Tetralogy of Fallot with pulmonary atresia (right ventricle to pulmonary artery conduits) homografts are used in such patients when available in the required size. However, a strong drawback is its poor durability, often requiring several replacements in a growing child. Research is underway to provide substitutes for children. Tissue engineering might be the most promising approach, but still far from clinical application.
For aortic valve replacement in children, homografts do not play a role anymore, homografts play a limited role, due to their poor durability and the higher risk of repeated valve replacements.
Mitral valve replacement
Gulbins et al. [9, 10] applied homografts for mitral valve replacement, mostly with endocarditis.
The maintenance of the “skeleton of the heart” stabilizes the left ventricle preventing it from dilation. Technique of implantation is very demanding and there is evidence of reduced durability [11]; hence its use is still experimental and far from clinical routine.
Ross procedure
The Ross procedure uses the patient’s pulmonary valve as a substitute for the aortic valve. The homograft is then implanted in the pulmonary position.
The hemodynamic situation thereafter is excellent with regards to the left ventricle and the degeneration rate of the new aortic valve is very low. Degeneration normally occurs at the site of the implanted homograft in the pulmonary position. This might be a problem as high gradients in the right ventricular outflow tract are reported after a short follow-up period. Homografts in the pulmonary position seems to be the subject to an inflammatory reaction or rejection., which leads to pulmonary stenosis during the first year of follow-up [12]. However, only 4% required reoperation during the first 5 years of follow-up. The Ross procedure seems to be most effective in young patients, avoids lifetime anticoagulation with excellent hemodynamics. New valves based on tissue-engineering could be used in the pulmonary position, thus reducing the frequency of reoperation.
Implantation technique
The implantation technique of heart valve homografts is more demanding compared with stent-mounted mechanical valves.
Three techniques are described:
a. The subcoronary technique
The walls of the sinuses are excised, and leaflet commissures are suspended into the recipient’s own aortic root. This ensures that the coronary ostia are not touched and the amount of foreign tissue is minimized. Small differences in the position of the leaflets can cause valve incompetence under physiologic conditions. Thus, applying the subcoronary technique, the precautions taken by surgeons of the German Heart Institute Berlin are the following:
Avoid any size mismatch between the implanted graft and the recipient’s aortic root as this would lead to graft incompetence and aortic regurgitation.
The integrity of the sino-tubular junction must be maintained.
Avoid any torsion or stretching of the structure.
Since degeneration starts at the free wall due to an immunologic reaction, as little foreign tissue as possible should be implanted.
Therefore interposition of a vascular prosthesis must be done when replacing the whole ascending aorta. In summary, the success depends on the morphology and diameter of the aortic root and accuracy of the implantation.
b. The mini root technique
This requires the implantation of the coronary arteries; however, this technique leaves the geometry of the leaflets, their commissures and the sinuses untouched.
As the complete valve with its own valvular apparatus is implanted, valvular incompetence due to implantation errors are rare. Its main disadvantage is the necessity for re-implantation of the coronary arteries and the larger amount of allogenic tissue implanted. Several authors [13,14,15] have reported that no significant differences between the subcoronary and mini root replacement techniques could be observed. In experienced hands, both techniques can be considered safe and efficient.
Results of homograft implantation at the German Heart Institute Berlin
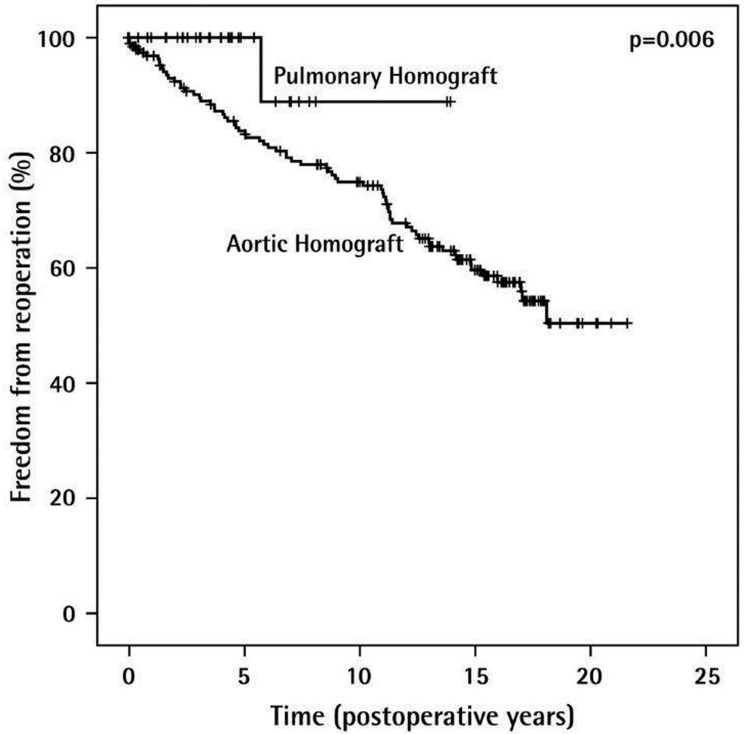
Musci and colleagues [16] reported satisfactory early and long-term results with significantly better survival in native valve endocarditis than prosthetic valve endocarditis. Freedom from re-infection rate after homograft implantation is 91.9±3.6% after 15 years. Despite the advantages of considerably decreasing the risk of recurrent endocarditis, structural valve deterioration, as shown in Figure 5 increases over time, especially in young patients, and reoperation remains a challenge.
Figure 5.
Freedom from reoperation after homograft implantation (n=276).
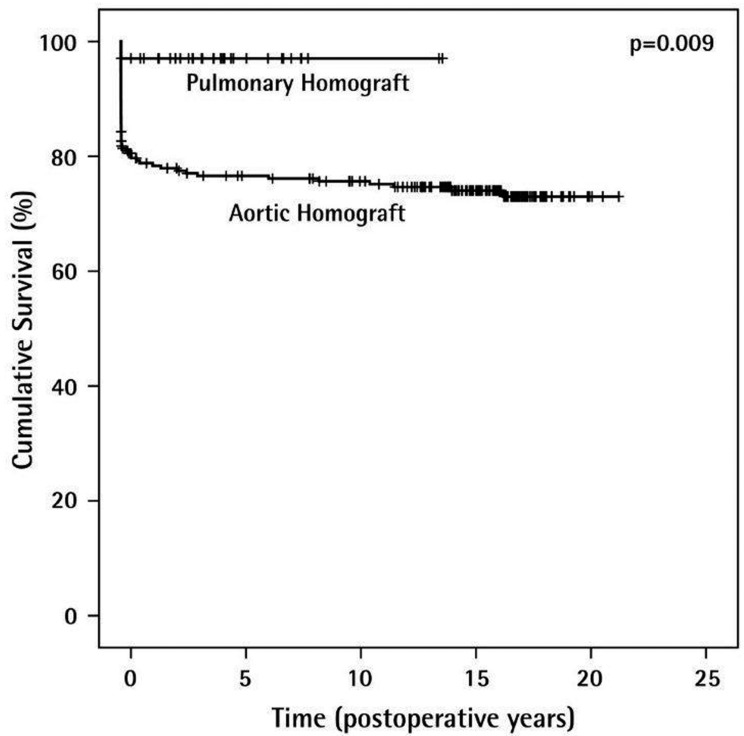
Overall, the long term function of the homograft, particularly in pulmonary position, is very satisfactory. The Kaplan Meier curve (Figure 6) shows a cumulative survival of 97% for pulmonary homografts after 20 years and 76.6% for aortic homografts over the same period of time.
Figure 6.
Long-term survival rates of homografts (n=276).
Discussion
Although homograft implantation remains the first choice for the treatment of specific valve lesions, it cannot be denied that the therapeutic possibilities of homografts do have limitations. The ideal replacement valve have the following characteristics:
an unlimited availability;
be easy to implant;
should have an antithrombotic surface;
an unlimited durability allowing physiologic flow.
To date however, no prostheses fulfill these requirements.
1. Limited availability. Over the past 25 years, the availability of these homograft valves was a major problem as suitable donors were recipients of heart transplants, organ donors whose hearts were not accepted, or donors who were autopsied and their relatives had agreed to using their tissues. The absence of an integrated structure for tissue donor recognition and subsequent recovery of donor hearts is one of the major causes for the shortage. When homografts have to be bought from other institutions or even companies, the costs exceed those of other heart valves. At the German Heart Institute Berlin we believe that availability, quality and costs of these grafts are under control if major cardiac surgery centers run their own homograft bank. Centers that perform fewer implants may be serviced by the homograft bank which is better equipped to respond the high quality standards that are required by the authorities and which are able to provide 24 hour tissue bank services. Thus, unnecessary discard of tissue grafts can be avoided. Reports of less favorable results showing that homografts are subject to early degeneration, annular dilatation and subsequent regurgitation are partly due to the limited availability of these grafts. This results, especially in non selective urgent operations, to a mismatch between the available valve and the necessary diameter of the recipient and in some cases, pulmonary homografts have been used for aortic valve replacement and vice-versa.
2. Complicated implantation techniques. Current implantation technique is somewhat more difficult compared with standardized procedures used for mechanical or stented biological valves. One can expect a more pronounced learning curve when starting to implant homografts. However, with proper techniques and experience, perioperative results are not worse when compared with stented valve substitutes.
3. Antithrombotic surface. As with other biological valves, homografts have a low rate of thromboembolic events after implantation without anticoagulation.
This might however, in part be a result of the younger age of most patients at the time of valve replacement, as the incidence of thromboembolism increases with age. Overall, the avoidance of lifetime anticoagulation is a big advantage, specifically for young children and women who wish to give birth.
4. Unlimited durability, allowing natural physiologic flow. Homografts were thought to have the same durability as a normal human valve. During follow-up, it has become evident that these grafts are also subject to degeneration. They undergo calcification rather early but progression is often slow. It proceeds over the leaflet commissures down to cusps. Moreover, the immunologic process is more accentuated in infants. For these reasons patients have to be well informed about the risks factors and of the likelihood of future re-intervention.
Therefore, after 20 years, 30-40% of the implanted grafts were still in place. Calcification starts at the free walls of the grafts, where immunologic cells have the best access by ingrowing vessels from the surrounding scar tissues.
Current developments
During the first decade of this century studies and subsequent publications concentrate on tissue engineering [17, 18] decellularization [19,20,21,22] and re-cellularization [23]. The advantage of a tissue- engineered homograft would be twofold.
First, such grafts could be modeled exactly according to the recipients’ anatomical morbidity, thus avoiding a mismatch in diameter of the valve as is now sometimes the case with homografts.
Second, the graft would be constructed such that the difficulties which are present when implanting a donor homograft are eliminated. As a consequence operative complications would be significantly reduced.
The removal of endothelial cells by means of decellularization is widely believed to eliminate the immune reaction of the homograft recipient after implantation. Although experience is still limited to small series, and long term success must be scientifically demonstrated, decellularization is likely to be the answer to early calcification caused by the immune reaction of the recipient.
Recellularization of previously decellularized homografts, either by pre-implantation tissue engineering, or by the natural process in the recipient may combine the best of both worlds.
Some decades ago, it was reported that at substituting a deteriorated donor graft some years after implantation, pathological examination showed the presence of recipient endothelium which had replaced the donor cells [1].
The re-seeding of endothelial cells on the valve has multiple advantages: Autologous cells reconstitute the natural situation of the valve structure resulting in a higher durability of the grafts, while avoiding the immunologic reaction incited by allogenic cells.
Conclusion
The future of homograft valve banking is actually a past revisited. From previous experiences and data gathered from homografts implantation over the years, perspectives on where else we can improve is a tremendous challenge. Over the past 25 different preservation processing and storage, as well as surgical techniques have evolved to optimize the therapeutic possibilities for patients indicated for implantation of a homograft. Although long term follow-up shows satisfactory results, it is hard to pinpoint the key success and failure factors in homograft implantation.
As implantation techniques are fairly well standardized, there is still room for improvement: development of the donor source is of great importance, not only to cover the demand, also to enhance the chance for a good size match of the valve diameter between donor and recipient. Decontamination techniques show a great variety between the different international homograft banks. In addition there is room for rationalization of processing procedures, definitions of graft quality and the thawing process. The two common storage methods each have their advantages and disadvantages. Effects of these should be further demonstrated. This will help to achieve a standardized, reproducible quality of the homografts used in clinical practice. Probably a scoring system: to allow all surgeons adapted to homograft implantation to know what they can expect from one graft with a given score.
Given the fact that many differences in applied homograft banking methods are unexplained, and their effect on long term survival and function of the grafts, homograft banks should strongly cooperate to scientifically substructure the methods and their clinical consequences. In addition, given the high quality demands imposed by European Directives [24], it is expected that the smaller homograft banks will have difficulty to generate a critical mass of expertise, necessary to respond to the many present and future challenges they are confronted with.
Close cooperation, or even mergers, with a division of tasks may be instrumental for the survival of their services.
Recent publications concerning the emerging bioengineering projects, de-cellularization and re-cellularization, as well as the improvement of mechanical and biological heart valves must be seen as a challenge for homograft bankers to join forces to optimize their credibility and performance.
Footnotes
Source of Support Nil.
Conflict of interest None declared.
Presented at the Meeting of the Foundation of European Tissue Banks, June 15-16, 2011, Berlin, Germany
Cite as: Delmo Walter EM, de By TMMH, Meyer R, Hetzer R. The future of heart valve banking and of homografts: perspective from the Deutsches Herzzentrum Berlin. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2012; 4 (2): 97-108
References
- Murray G. Homologous aortic valve segment transplants as surgical treatment for non-aortic and mitral insufficiency. Angiology. 1956;7:446–471. doi: 10.1177/000331975600700509. [DOI] [PubMed] [Google Scholar]
- Ross D N. Homograft replacement of the aortic valve. Lancet. 1962;2:487. doi: 10.1016/s0140-6736(62)90345-8. [DOI] [PubMed] [Google Scholar]
- Barratt-Boyes B G, Lowe J B, Cole D S, Kelly D T. Homograft replacement for aortic valve disease. Thorax. 1965;20:495–504. [PMC free article] [PubMed] [Google Scholar]
- Salles C A, Buffolo E, Andrade J C. et al. Mitral valve replacement with glutaraldehyde preserved aortic allografts. Eur J Cardiothorac Surg. 1998;13:135–143. doi: 10.1016/s1010-7940(97)00320-5. [DOI] [PubMed] [Google Scholar]
- Parker R, Randev R, Wain W H, Ross D N. Storage of heart valve allografts in glycerol with subsequent antibiotic sterilisation. Thorax. 1978;33:638–645. doi: 10.1136/thx.33.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D R, Campbell O N, Hayward A R, Bishop D A. Degeneration of aortic valve allograft in young recipients. J Thorac Cardiovasc Surg. 1993;105:934–942. [PubMed] [Google Scholar]
- O\'Brien M F, Stafford G, Gardner M. et al. The viable cryopreserved allograft aortic valve. Journal of Cardiac Surgery. 1987;2:153–167. doi: 10.1111/jocs.1987.2.1s.153. [DOI] [PubMed] [Google Scholar]
- Yankah A C, Pasic M, Klose H. et al. Homograft reconstruction of the aortic root for endocarditis with periannular abscess: a 17-year study. Eur J Cardiothorac Surg. 2005;28:69–75. doi: 10.1016/j.ejcts.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Gulbins H, Kreuzer E, Haushofer M. et al. Homografts for mitral valve replacement in patients with acute endocarditis - an alternative to prosthetic valve replacement. Z. Kardiol. 1999;88:363–368. doi: 10.1007/s003920050298. [DOI] [PubMed] [Google Scholar]
- Gulbins H, Kreuzer E, Uhlig A, Reichart B. Mitral valve surgery utilizing homografts; early results. J Heart Valve Dis. 2000;9:222–229. [PubMed] [Google Scholar]
- Gulbins H, Anderson I, Kilian E. et al. Five years experience with mitral valve homografts. Thorac Cardiovasc Surg. 2002;50:223–229. doi: 10.1055/s-2002-33094. [DOI] [PubMed] [Google Scholar]
- Carr-White G S, Kilner P J, Hon J K. et al. Incidence, location, pathology and significance of pulmonary homograft stenosis after the Ross operation. Circulation. 2001;104:I16–20. doi: 10.1161/hc37t1.094545. [DOI] [PubMed] [Google Scholar]
- Yankah C A, Yacoub M H, Hetzer R. Springer. 1997. Cardiac Valve Allografts, Science and Practice. [Darmstadt: Steinkopf, New York] [Google Scholar]
- Barratt-Boyes B G. Aortic allograft valve implantation: freehand or root replacement? J Card Surg. 1994;9:196–197. doi: 10.1111/j.1540-8191.1994.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Willems T P, van Herwerden L A, Steyerberg E W. et al. Subcoronary implantation or aortic root replacement for human tissue valves: sufficient data to prefer either technique? Ann Thorac Surg 1995; 60: 83-86. 1995;60:83–86. doi: 10.1016/0003-4975(95)00276-q. [DOI] [PubMed] [Google Scholar]
- Musci M, Weng Y, Amiri A. et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: 20-year single center experience. J Thorac Cardiovasc Surg. 2010;139:665–673. doi: 10.1016/j.jtcvs.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Simon P, Kasimir M T, Seebacher G. et al. Early failure of tissue engineered porcine heart valve Synergraft in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–1006. doi: 10.1016/s1010-7940(03)00094-0. [DOI] [PubMed] [Google Scholar]
- Cebotari S, Mertsching H, Kallenbach K. et al. Construction of autologous human heart valved based on an acellular allograft matrix. Circulation. 2002;106:I63–I68. [PubMed] [Google Scholar]
- Zehr K J, Yagubyan M, Connolly H M. et al. Aortic root replacement with a novel decellularized cryopreserved aortic homograft: Postoperative immunoreactivity and early results. J Thorac Cardiovasc Surg. 2005;130:1010–1015. doi: 10.1016/j.jtcvs.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Hopkins R A, Jones A L, Wolfinbarger L. et al. Decellularization reduces calcification while improving both durability and 1-year functional results of pulmonary homograft valves in juvenile sheep. J Thorac Cardiovasc Surg. 2009;137:907–913. doi: 10.1016/j.jtcvs.2008.12.009. [DOI] [PubMed] [Google Scholar]
- da Costa F D, Costa A C, Prestes R. et al. The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg. 2010;90:1854–1860. doi: 10.1016/j.athoracsur.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Brown J W, Ruzmetov M, Eltayeb O. et al. Performance of Synergraft decellularized pulmonary homograft in patients undergoing a Ross procedure. Ann Thorac Surgery. 2011;91:416–423. doi: 10.1016/j.athoracsur.2010.10.069. [DOI] [PubMed] [Google Scholar]
- Elkins R C, Goldstein S, Hewitt C W. et al. Recellularization of heart valve grafts by a process of adaptive remodeling. Semin Thorac Cardiovasc Surg. 2001;13:87–92. [PubMed] [Google Scholar]
- EU Directives 2004/23/EC, 2006/17/EC and 2006/84/EC.