Abstract
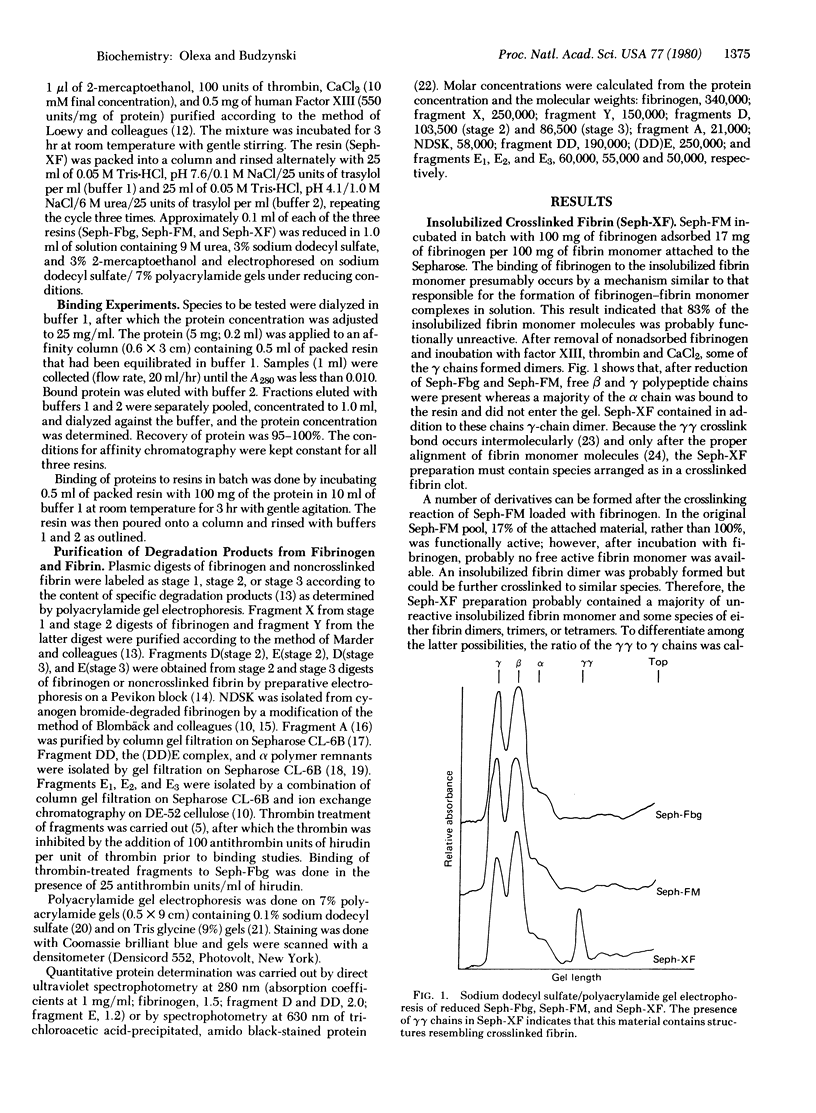
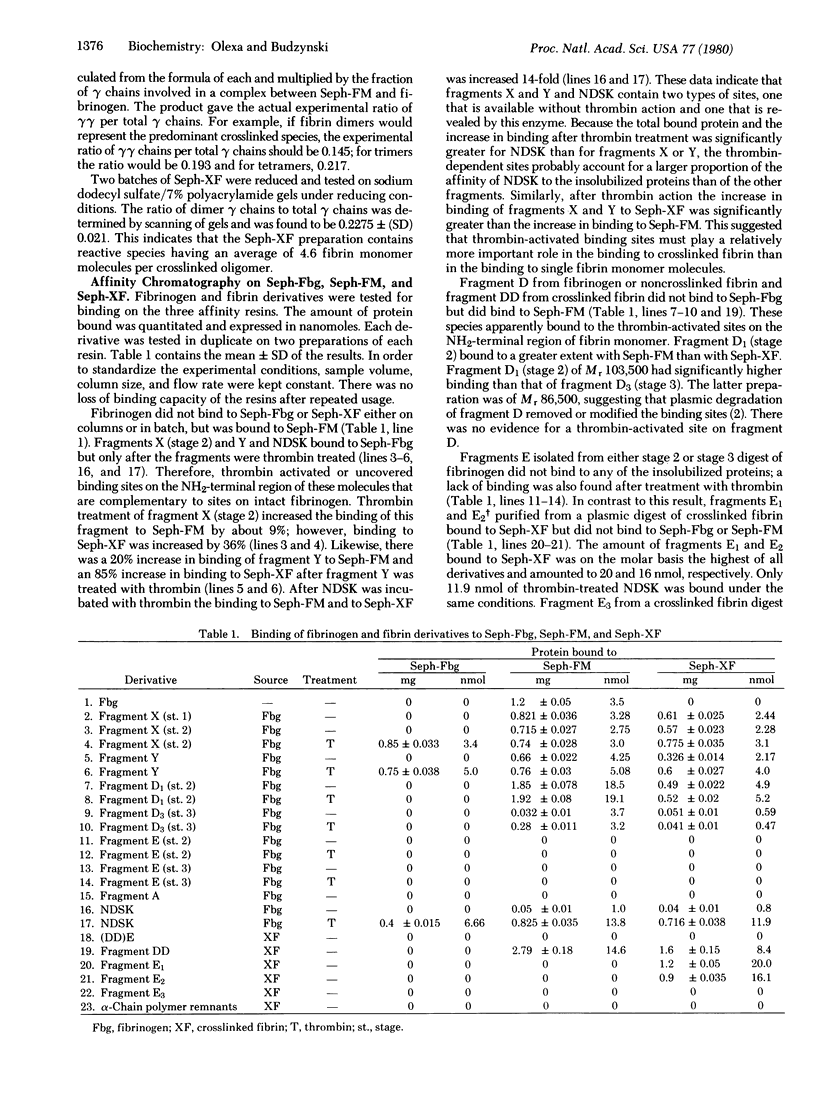
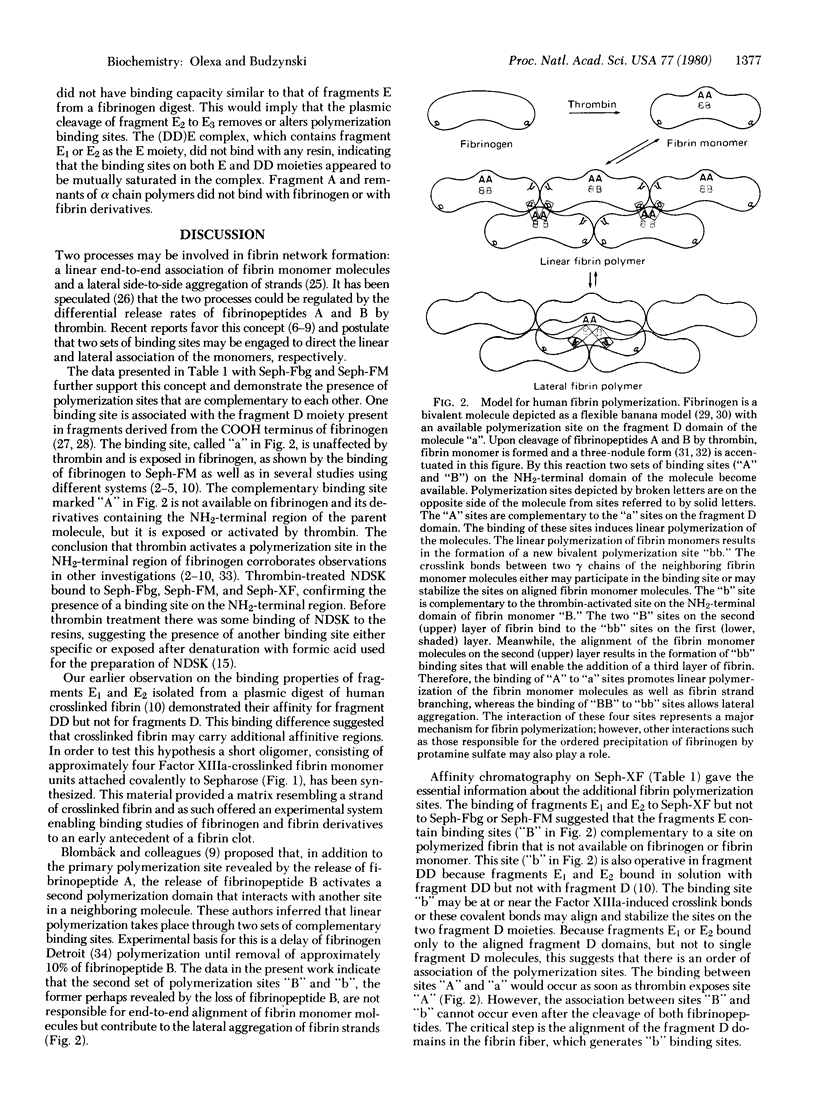
The mechanism of association and the organization of human fibrin were studied by using affinity chromatography. Insolubilized fibrinogen, fibrin monomer, and crosslinked fibrin were used to localize the binding sites on fibrinogen and fibrin derivatives. Four different polymerization sites have been distinguished. A binding site ("a"), available without thrombin action, is present on the fibrinogen fragment D domain. The complementary ("A") is inoperative in fibrinogen and requires thrombin for activation; it is located on the fibrinogen NH2-terminal domain. A third polymerization site ("b") appears to be formed by the alignment of the fragment D domains on two fibrin monomer molecules upon polymerization; this site functions without thrombin mediation and the alignment is stabilized by the Factor XIIIa-catalyzed crosslink bonds. The "b" site is complementary to another thrombin-activated site ("B") on the fibrinogen NH2-terminal domain. The two thrombin activable sites, "A" and "B", are distinguishable, although they are located in the same fibrinogen domain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blombäck B., Blombäck M., Henschen A., Hessel B., Iwanaga S., Woods K. R. N-terminal disulphide knot of human fibrinogen. Nature. 1968 Apr 13;218(5137):130–134. doi: 10.1038/218130a0. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Blombäck M., Blombäck B., Mammen E. F., Prasad A. S. Fibrinogen Detroit--a molecular defect in the N-terminal disulphide knot of human fibrinogen? Nature. 1968 Apr 13;218(5137):134–137. doi: 10.1038/218134a0. [DOI] [PubMed] [Google Scholar]
- Budzynski A. Z., Marder V. J., Ménaché D., Guillin M. C. Defect in the gamma polypeptide chain of a congenital abnormal fibrinogen (Paris I). Nature. 1974 Nov 1;252(5478):66–68. doi: 10.1038/252066a0. [DOI] [PubMed] [Google Scholar]
- Budzynski A. Z., Marder V. J., Shainoff J. R. Structure of plasmic degradation products of human fibrinogen. Fibrinopeptide and polypeptide chain analysis. J Biol Chem. 1974 Apr 10;249(7):2294–2302. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- HALL C. E., SLAYTER H. S. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959 Jan 25;5(1):11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryk B. J., Collen D., Woods K. R., Blombäck B. Evidence for localization of polymerization sites in fibrinogen. J Biol Chem. 1974 May 25;249(10):3322–3325. [PubMed] [Google Scholar]
- Kudryk B., Blombäck B., Blombäck M. Fibrinogen Detroit - an abnormal fibrinogen with non-functinal NH2-terminal polymerization domain. Thromb Res. 1976 Jul;9(1):25–36. doi: 10.1016/0049-3848(76)90146-8. [DOI] [PubMed] [Google Scholar]
- LOEWY A. G., DUNATHAN K., KRIEL R., WOLFINGER H. L., Jr Fibrinase. I. Purification of substrate and enzyme. J Biol Chem. 1961 Oct;236:2625–2633. [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder V. J., James H. L., Sherry S. The purification of fibrinogen degradation products by pevikon block electrophoresis. Thromb Diath Haemorrh. 1969 Nov 15;22(2):234–239. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Marguerie G., Stuhrmann H. B. A neutron small-angle scattering study of bovine fibrinogen. J Mol Biol. 1976 Mar 25;102(1):143–156. doi: 10.1016/0022-2836(76)90078-4. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z. Binding phenomena of isolated unique plasmic degradation products of human cross-linked fibrin. J Biol Chem. 1979 Jun 10;254(11):4925–4932. [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z., Marder V. J. Modification of high molecular weight plasmic degradation products of human crosslinked fibrin. Biochim Biophys Acta. 1979 Jan 25;576(1):39–50. doi: 10.1016/0005-2795(79)90482-3. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z. Primary soluble plasmic degradation product of human cross-linked fibrin. Isolation and stoichiometry of the (DD)E complex. Biochemistry. 1979 Mar 20;18(6):991–995. doi: 10.1021/bi00573a009. [DOI] [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrin. J Biol Chem. 1973 Jul 10;248(13):4574–4583. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Shainoff J. R., Dardik B. N. Fibrinopeptide B and aggregation of fibrinogen. Science. 1979 Apr 13;204(4389):200–202. doi: 10.1126/science.155308. [DOI] [PubMed] [Google Scholar]
- Takagi T., Doolittle R. F. Amino acid sequence studies on the alpha chain of human fibrinogen. Location of four plasmin attack points and a covalent cross-linking site. Biochemistry. 1975 Nov 18;14(23):5149–5156. doi: 10.1021/bi00694a020. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- York L. L., Blombäck B. Interaction of fragments of fibrinogen with insolubilized fibrin monomers (activated fibrinogen). Thromb Res. 1976 May;8(5):607–618. doi: 10.1016/0049-3848(76)90242-5. [DOI] [PubMed] [Google Scholar]



