We recently discovered that some cells in the pedunculopontine nucleus (PPN), the cholinergic arm of the reticular activating system (RAS) involved in waking and REM sleep, and in the subcoeruleus nucleus (SubC), a descending target of the PPN involved in REM sleep, were electrically coupled [1,2]. We had previously reported the presence of dye coupling and spikelets in some PPN and SubC neurons, as well as in the parafascicular nucleus (Pf), an ascending target of the PPN involved in thalamocortical oscillations. We established the presence of electrical coupling using patch-clamp recordings of pairs of neurons and blocking action potentials with tetrodotoxin (TTX) and fast synaptic transmission with excitatory amino acid and GABA receptor blockers. In these conditions, intracellular pulses delivered to one cell induced a response in the other cell and vice versa, suggesting the presence of gap junctions between these neurons (Figure 1). We also determined that the neuronal gap junction protein connexin 36 (Cx36) was present in these regions, and decreased in level along with the developmental decrease in REM sleep [1,2].
Figure 1. Electrical coupling and responses of SubC and PPN neurons.
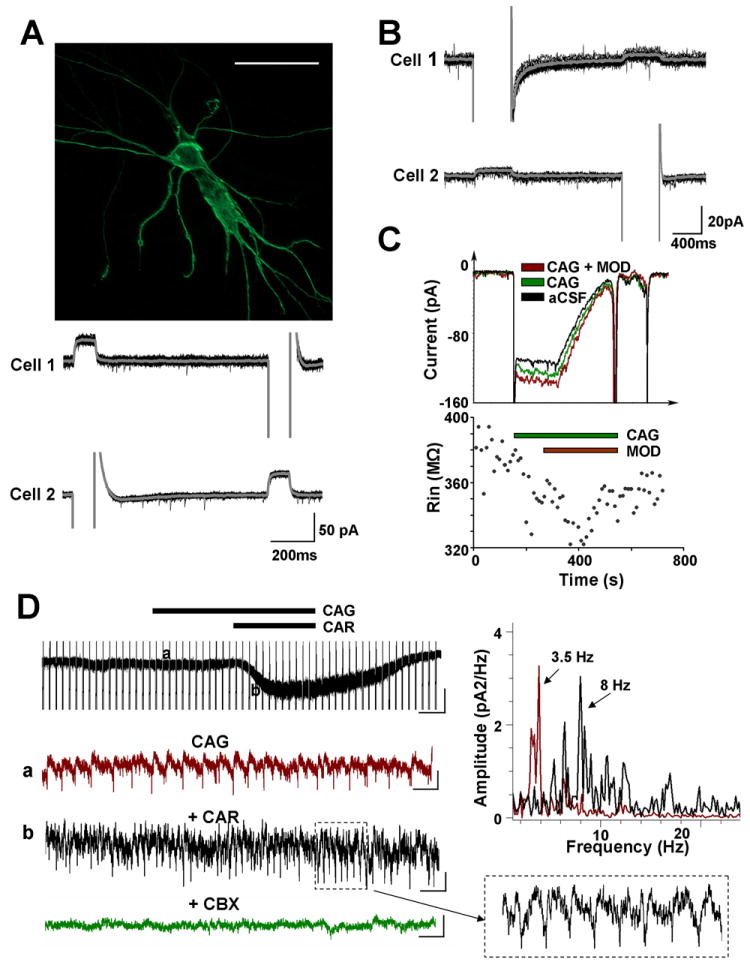
A. Electrical coupling in the SubC. Top: Two SubC neurons that were electrotonically coupled were imaged with neurobiotin (Cy2) immunofluorescence. The scale bar is 50μm. Bottom: Dual voltage clamp recordings (Holding Potential=-50mV) of the above neurons were conducted in the presence of TTX (1 μM). Hyperpolarizing pulses (-60 mV) injected into one cell induced an outward current in the neighboring cell, and vice versa. Gray line represents the average of 15 sweeps. The coupling ratio was calculated by dividing the outward current induced in one cell by the negative current injected into the neighboring cell. The coupling ratio of cell 1 to cell 2 was 8.13 ± 0.59 %, and of cell 2 to cell 1 was 6.25 ± 0.38 %. B. Whole-cell patch-clamp recordings from a pair of electrically coupled PPN neurons under voltage clamp. The same protocol as in A was applied, except that hyperpolarizing pulses were from -60 mV to -110 mV of 500 ms duration. The coupling ratio of cell 1 to cell 2 was 3.98 ± 0.55%, and of cell 2 to cell 1 was 4.08 ± 0.55%. C. An example of a PPN cell whose input resistance was decreased by fast synaptic blockers (CAG = 6-cyano-7-nitro-quinoxaline-2, 3-dione [CNQX] 10 μM, (±)-2-amino-5-phosphopentanoic acid [APV] 10 μM and gabazine 10 μM), then decreased further by the superfusion of modafinil (MOD, 150 μM). A 500 ms hyperpolarization step (from -60 mV to -105 mV) followed by a 1000 ms ramp (-105 mV to -35 mV) was applied in order to test the change of input resistance (Rin) and reversal potential of activated current. A higher current was required to compensate for the voltage change in the presence of modafinil, indicating a decrease in Rin (top record in red compared to control in black and in fast synaptic blockers in green). Rin changes during 12 min recording are shown on the bottom. D. Cholinergic modulation of spontaneous spikelets, indicative of electrical coupling. Left: In the presence of fast synaptic blockers (CAG), non-selective cholinergic receptor agonist, carbachol (CAR), induced 60 pA inward current in this PPN neuron with spontaneous oscillations (top, vertical bar 50 pA, horizontal bar 4 sec). Enlarged records from points (a) and (b) are shown below. Recording (a) showed spontaneous oscillations in the presence of CAG. Recording (b) demonstrated that CAR increased the frequency of oscillations. The box on the bottom right shows enlarged 1 sec record (b). Carbenoxolone, a gap junction blocker, completely blocked the oscillations (bottom). Blockade did not affect membrane potential. This suggested that the oscillations were modulated by electrical coupling. Scale bars for the enlarged records are vertical 10 pA and horizontal 500 ms. Top Right: Power spectrum histogram of the oscillations in (a) and (b). Each histogram was obtained from a 30 sec recording. The frequency of spontaneous oscillations was 3.5 Hz, which increased to 8 Hz following the application of CAR.
A landmark study recently showed that modafinil, an agent approved for use in treating excessive sleepiness in narcolepsy, sleepiness in obstructive sleep apnea, and for shift work sleep disorder, increased electrical coupling between cortical, reticular thalamic and inferior olive neurons [3]. We then showed that modafinil decreased input resistance in electrically coupled PPN and SubC neurons in the presence of TTX and fast synaptic blockers, an effect that could be reversed by the gap junction blockers mefloquine or carbenoxolone [2, Figure 1]. Modafinil and these blockers produced their effects without changing membrane potential or affecting other conductances [1,2,3], an important issue since mefloquine and carbenoxolone are thought to have a number of unspecific actions, although on differing mechanisms.
Several studies showed that while gross motor activity patterns appeared normal in the Cx36 knockout (KO) mouse, detailed analysis of motor patterns showed a 10-20 msec degradation in coordination [4], and a delay >20 msec in the optokinetic reflex [5]. These differences appear vital to survival. In terms of consciousness and sleep, two laboratories found that cortical gamma oscillations in vitro were impaired in Cx36 KO mice [6,7]. A later study from this lab on Cx36 KO mice showed that Cx36 gap junctions contributed to gamma oscillations [8], while others showed that gap junctions may play a role in learning and memory [9]. All of these studies taken together suggest that gap junctions confer an advantage in timing, probably due to their ability to promote coherence in brain rhythms for optimal performance. The unique ability of coupled cells to maintain synchrony across a wide range of membrane potentials [10] probably allows brain rhythms to persist for longer periods without waning.
What is the role of electrical coupling? We hypothesize that the role of such coupling may be to enhance ensemble rhythmic activity across populations of cells within each nucleus. While some individual neurons manifest intrinsic rhythmic firing properties, it is the coherence of activity across the population that would lead to the propagation of rhythms such as are involved in changes in arousal state, e.g. in the transition to waking or REM sleep. Such coherence may be provided by electrical coupling, and we are examining how such coupling is organized at the cellular level, how it is enhanced or reduced, how it interacts with known transmitter systems, and which cell types are involved in these processes. We propose that electrical coupling is not involved in generating oscillations in individual neurons, which appear to be due to intrinsic properties and neurochemically modulated interactions, rather, gap junctions promote ensemble activation of cell populations [11]. An audience clapping in synchrony is louder than one in which each member is clapping out of rhythm.
The implications for sleep-wake control are considerable. Most electrically coupled neurons appear to be GABAergic, which exhibit high input resistance and can be induced to fire by minimal input. If electrical coupling is increased, input resistance is shunted and these cells decrease firing, thus disinhibiting their targets, presumably the mechanism by which the stimulant modafinil promotes alertness. Agents that block gap junctions include halothane, propofol, oleamide and anadamide, all of which promote sleep or anesthesia [reviewed in 11]. The gap junction blockers carbenoxolone and mefloquine are both somnogenic. One possibility arising from this research is that a mechanism behind anesthesia is gap junction blockade, especially in the RAS.
Clinically, disturbances in electrical coupling can be expected to have a wide range of effects beginning with a decrement in synchronization, especially of fast rhythms such as gamma oscillations, leading to decreased alertness, such as is present in narcolepsy and other conditions inducing daytime sleepiness. Upregulation of electrical coupling can be expected to lead to increased vigilance and increased REM sleep drive, such as is evident in schizophrenia, anxiety disorders, depression, and other arousal-related symptoms. In REM sleep behavior disorder, the atonia of REM sleep is absent, so that it would be interesting to determine if modafinil affects this disturbance. Similarly, restless legs syndrome may be modulated by this agent since it appears to smooth out motor dysregulation in Parkinson’s disease patients, presumably by affecting coupling in the inferior olive [3]. We recently found that modafinil may affect electrical coupling in spinal cord neurons since oral treatment normalized excessive reflexes induced by spinal cord transection, suggesting that this agent may useful for the treatment of hyperreflexia and spasticity [12].
Electrical coupling introduces another layer of control to the manifestation of sleep-wake cycles, and may represent the first major breakthrough in sleep research since the discovery of orexin ten years ago. Hopefully, this finding will generate an equal amount of attention due to its multiple implications for understanding, and avenues for novel therapeutic strategies for, a number of sleep, movement and psychiatric disorders.
Acknowledgments
Supported by USPHS grants NS20246 and RR20146.
Literature Cited
- 1.Heister DS, Hayar A, Charlesworth A, Yates C, Zhou Y, Garcia-Rill E. Evidence for electrical coupling in the SubCoeruleus (SubC) nucleus. J Neurophysiol. 2007;97:3142–3147. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urbano FJ, Leznik E, Llinas R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Placatonakis DG, Bukovsky AA, Zeng XH, Kiem HP, Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc Nat Acad Sci. 2004;101:7164–7169. doi: 10.1073/pnas.0400322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kistler WM, De Jeu MT, Elgersma Y, et al. Analysis of Cx36 knockout does not support tenet that olivary gap junctions are required for complex spike synchronization and normal motor performance. Ann NY Acad Sci. 2002;978:391–404. doi: 10.1111/j.1749-6632.2002.tb07582.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Michelson HB. Gap junctions synchronize the firing of inhibitory interneurons in guinea pig hippocampus. Brain Res. 2001;907:139–143. doi: 10.1016/s0006-8993(01)02582-3. [DOI] [PubMed] [Google Scholar]
- 7.Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 8.Buhl DL, Harris KD, Hormudzi SG, Monyer H, Buszaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisch C, De Souza-Silva MA, Sohl G, et al. Stimulus complexity dependent memory impairment and changes in motor performance after deletion of the neuronal gap junction protein connexin36 mice. Behav Brain Res. 2005;157:177–185. doi: 10.1016/j.bbr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Llinas R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurons and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. doi: 10.1093/sleep/31.5.673. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yates C, Reese N, Kiser T, Skinner RD, Garcia-Rill E. Modafinil (MOD) normalizes hyperreflexia induced by spinal cord transection in the rat. Neurosci Abst. 2007;33:405.21. [Google Scholar]


