Abstract
Background
Carnitine palmitoyltransferase 1(CPT1) is a rate-limiting step of mitochondrial β-oxidation by controlling the mitochondrial uptake of long-chain acyl-CoAs. The muscle isoform, CPT1b, is the predominant isoform expressed in the heart. It has been suggested that inhibiting CPT-1 activity by specific CPT-1 inhibitors exerts protective effects against cardiac hypertrophy and heart failure. However, clinical and animal studies have shown mixed results, thereby posting concerns on the safety of this class of drugs. Preclinical studies using genetically modified animal models should provide a better understanding of targeting CPT1 in order to evaluate it as a safe and effective therapeutic approach.
Methods and Results
Heterozygous CPT1b knockout mice (CPT1b+/−) were subjected to transverse aorta constriction (TAC)-induced pressure-overload. These mice showed overtly normal cardiac structure/function under the basal condition. Under a severe pressure-overload condition induced by two weeks of transverse aorta constriction (TAC), CPT1b+/− mice were susceptible to premature death with congestive heart failure. Under a milder pressure-overload condition, CPT1b+/− mice exhibited exacerbated cardiac hypertrophy and remodeling compared with that in wild-type littermates. There were more pronounced impairments of cardiac contraction with greater eccentric cardiac hypertrophy in CPT1b+/− than in controlled mice. Moreover, the CPT1b+/− heart exhibited exacerbated mitochondrial abnormalities and myocardial lipid accumulation with elevated triglycerides and ceramide content, leading to greater cardiomyocytes apoptosis.
Conclusions
We conclude that CPT1b deficiency can cause lipotoxicity in the heart under pathological stress, leading to exacerbation of cardiac pathology. Therefore, caution should be applied in the clinical use of CPT-1 inhibitors.
Keywords: CPT1, cardiac hypertrophy, heart failure, lipotoxicity
The heart is an energy-demanding organ relying on fatty acid and glucose oxidation. Long-chain fatty acids contribute up to 70% of the energy required by an adult heart to function under normal physiological conditions (see review1, 2). The remaining energy needs are derived mainly from glucose. A failing heart usually shows impaired transcription of key enzymes involved in fatty acid metabolism.1–5 Consequently, the heart switches to utilize glucose as the main fuel. Nevertheless, whether this substrate utilization shift is adaptive or maladaptive remains controversial. Several preclinical and clinical studies showed beneficial effects by inhibiting various steps of fatty acid oxidation (FAO) in animal and human subjects with cardiac hypertrophy and heart failure.6–10 Meanwhile, others reported adverse effects of drugs that exert inhibiting effects on FAO in animal studies,11–13 raising the safety concerns of this class of drugs. Nevertheless, it remains unclear whether the adverse effects are derived from attenuated FAO in the heart or from non-specific confounding effects of a particular compound.
Carnitine palmitoyltransferase 1 (CPT1) is located within the mitochondrial outer membrane as a rate-limiting enzyme of mitochondrial β-oxidation by controlling mitochondrial entry of long chain fatty acids. CPT1b is one of the three CPT1 isoforms (CPT1a, b and c). It is expressed mainly in skeletal muscle, heart, brown adipose tissue, and testis.14, 15 In adult cardiomyocytes, CPT1b is the predominant isoform and contributes about 98% of total cardiac CPT1 activity.16, 17 CPT1 is a major target for metabolic therapies aiming to improve cardiac performance in patients with cardiac hypertrophy and heart failure by suppressing FAO. Small-scale clinical trials reported beneficial effects of CPT1 inhibitors in treating heart failure patients. Specific CPT-1 inhibitors, such as etomoxir, have been actively pursued as a therapeutic agent.18, 19 On the other hand, animal studies showed mixed results, from beneficial, unchanged or harmful effects on pressure-overload induced cardiac dysfunction, hypertrophy and progression to end-stage failure.11, 20–22 A major hurdle is to differentiate the specific CPT1 inhibition effects from non-specific off target effects. Studies on animal models with genetic manipulation should help identify the CPT1 effect without potential confounding effects from a compound. The homozygous CPT1b knockout mice exhibit embryonic lethality; whereas the heterozygous CPT1b deficient mice show no apparent phenotypic change.23 The present study was designed to determine whether partial CPT1b deficiency in the heterozygous CPT1b knockout mice is beneficial or detrimental to cardiac structure/function under physiological and pathological conditions.
Methods
An expanded Methods section is available in the online-only Data Supplement.
Experiment animals
Heterozygous CPT1b+/− knockout mice were generated as described previously.23 The wild-type (WT) littermates with CPT1b+/+ genotyping were used as controls. Both male and female mice were used. Mice were kept on a 12 h/12 h light/dark cycle in temperature-controlled rooms and had ad libitum access to water and standard rodent diet. All experimental procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
CPT1 activity assay
A modified mitochondrial CPT1 assay was employed by measuring the rate of formation of palmitoylcarnitine from palmitoyl-CoA plus carnitine according to the Methods section mostly described in online-only data supplement.
Echocardiography measurement
Echocardiographic measurement was performed with the high resolution echocardiography analysis system for small animals (Vevo 770™ imaging system, Visual sonics). Mice were anesthetized by inhalation with isoflurane and O2. A two-dimensional short-axis view and M-mode tracings of the left ventricle were obtained with a 30 MHz transducer.
Isolated Working Mouse Heart Perfusions
Myocardial contractile function and metabolism under basal physiological conditions were determined ex vivo through isolated working heart perfusions as previously described.24 All hearts were perfused in the working mode in a non-recirculating manner with a preload of 8.0 mm Hg and an after-load of 55 mm Hg. Radiolabeled tracers were utilized to monitor glucose oxidation and palmitate oxidation, as specified in individual experiments as described previously.24 For hypertrophied hearts subjected to pressure-overload induced by TAC, the isolated mitochondria were used to assay FAO rate and heart homogenates were used to assay glucose oxidation rate. The detailed methods about TAC procedure, palmitate and glucose oxidation assay were described in online-only data supplement.
Heart ceramide assay
Ceramide species were quantified by ESI-MS/MS (Applied Biosystems/MDS sciex,Canada) as described in online-only data supplement.
Mitochondrial DNA copy number analysis
Total genomic DNA was isolated from left ventricles, processed by standard procedures using a DNA extraction kit (Wizard), and subjected to real-time qPCR analysis. Cytochrome b (Cyto b) was employed as a mitochondrial DNA (mtDNA) marker and the regulator of calcineurin 1 (Rcan1) as a nuclear DNA (nDNA) marker. The relative amount of mtDNA copy number was assessed as an amplicon within a mitochondrial gene (Cyto b) to that of a nuclear gene (Rcan 1).
Quantitative Real-Time RT-PCR Analyses
Quantitative real-time RT-PCR analyses were carried out using Step one real-time PCR system (Applied Biosystems). Results from each gene/primer pair were normalized to β-actin, and compared across conditions. The sequences of the primers are listed in “On line Table 1”.
Western blot analyses
Western blots were conducted using commercially available antibodies. The immunoblotting images were captured using KODAK image Station 4000R (Carestream Health Inc.) by developing the membranes in Supersignal West substrates (Thermo Scientific), and analyzed with KODAK IM software (Ver 4.5.1).
Statistical Analyses
Data for two-group comparison were analyzed using Nonparametric Student's t-test, otherwise, the data were analyzed by one factor or mixed, two-factor analysis of Variance (ANOVA) using GraphPad Prism software (GraphPad Software Inc.). Survival data was analyzed with the Kaplan-Meier method using GraphPad Prism software. Values of quantitative results were expressed as mean±SEM. Differences between groups and treatments were regarded as significant at the P<0.05 probability level.
Results
Mice with heterozygous CPT1b KO (CPT1b+/−) exhibit no cardiac phenotype under basal condition
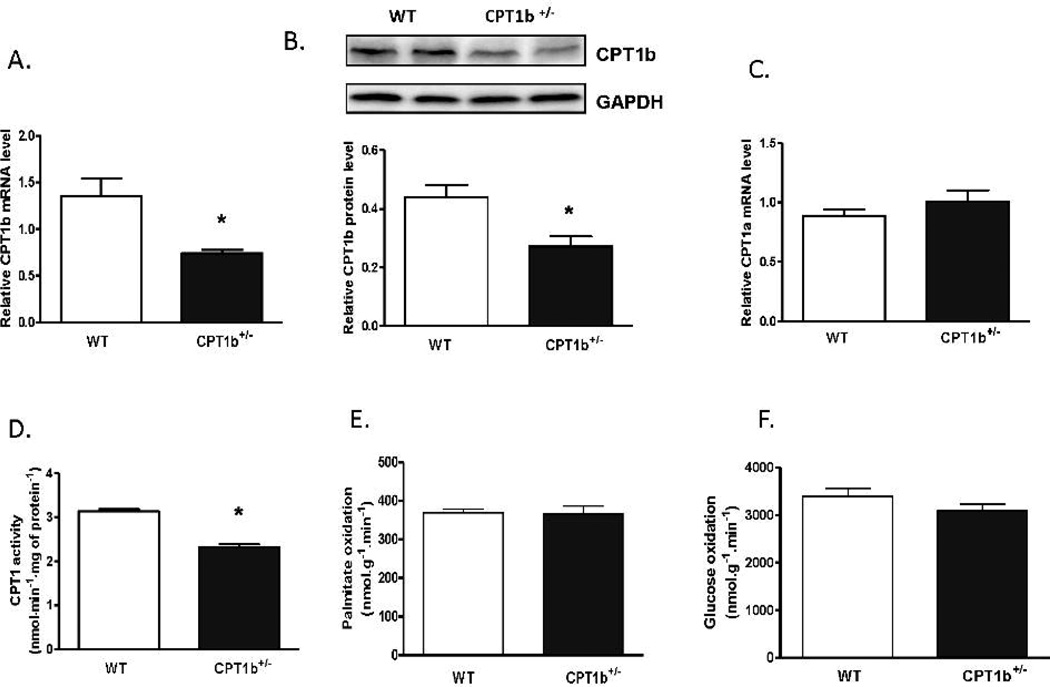
The CPT1b+/− mice were generated as described previously23. While the homozygous CPT1b−/− mice were embryonically lethal, the heterozygous CPT1b+/− mice were overtly normal with the same life span as compared with their wild-type (WT) littermates. The heart weight, body weight and the ratio of heart to body weight were not different between the CPT1b+/− and their WT littermates (supplemental data, Fig.1). CPT1b+/− mice did not exhibit detectable changes in cardiac histology and function. (supplemental data, Fig.2). Real-time PCR revealed that CPT1b transcript expression was attenuated by about 50% in CPT1b+/− relative to WT hearts (Fig.1A). Western blots revealed that CPT1b protein content in the heart was correspondingly decreased by about 50% in CPT1b+/− relative to WT hearts (Fig.1B). CPT1a, a dominant CPT1 isoform in neonatal hearts25, could have been re-upregulated in response to CPT1b deficiency in the heart. However, Q-PCR illustrated no change in CPT1a expression in the CPT1b deficient heart (Fig.1C). Total CPT1 activity in CPT1b+/− hearts was substantially decreased to about 73% of those WT hearts (Fig.1D). Despite the significant CPT1b deficiency in the heart, echocardiographic assessment revealed normal anatomical structure of the heart with comparable cardiac performance in the CPT1b+/− compared with WT hearts (Supplemental data Fig.3 and Supplemental Table 2). Isolated working heart measurements of ex vivo cardiac function revealed no difference between the CPT1b and their WT littermates (Supplemental Table 3). Both cardiac contractility (max dp/dt) and relaxation (min dp/dt) in CPT1b+/− hearts were similar to that of the WT control. Interestingly, the rates of FAO and glucose oxidation were not changed in CPT1b+/− hearts. Therefore, these results indicate that a modest CPT1b deficiency in the heart is not sufficient to cause cardiac dysfunction under a basal physiological condition.
Figure 1.
CPT1b mRNA level and activity in CPT1b+/− mice. Mice were sacrificed at 12~14 weeks of age. RNA samples were extracted from ventricular tissues. A & C) Transcript level of CPT1b and CPT1a were determined by Q-PCR, results from each gene/primer pair were normalized to β-actin (n=4). B) Protein expression was determined by western blot (n=4). D) CPT1 activity was measured using isolated mitochondria according to the method described in the on-line supplement (n=4). E & F) Palmitate oxidation rate and glucose oxidation rate were measured in isolated working heart (n=6). *p<0.05 vs WT.
CPT-1 deficiency aggravates cardiac hypertrophy and dysfunction induced by pressure-overload
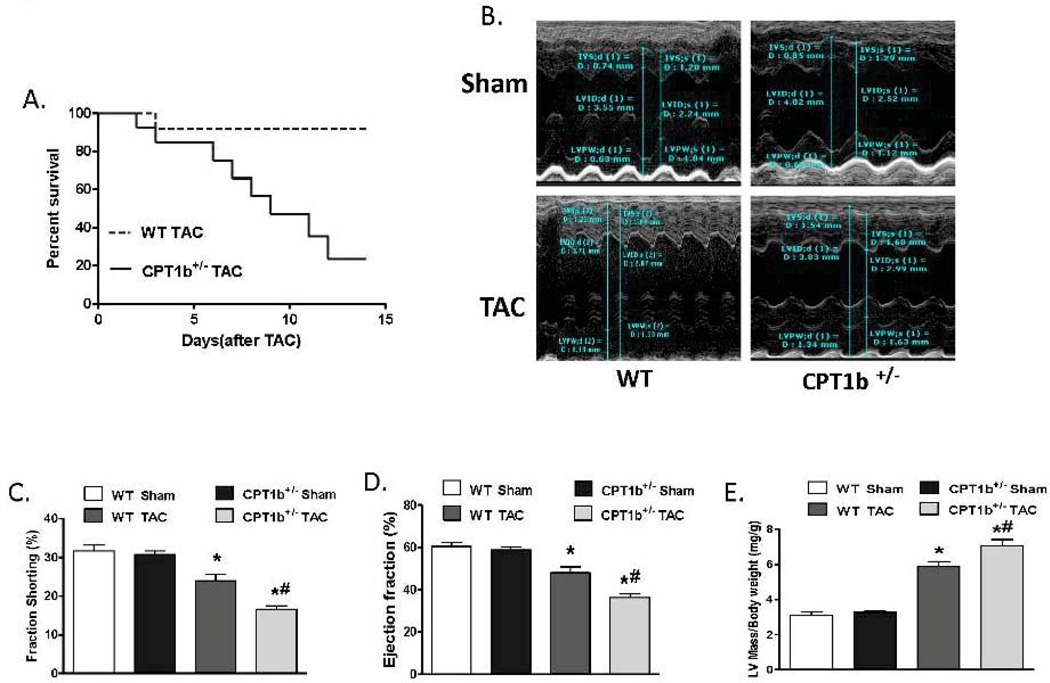
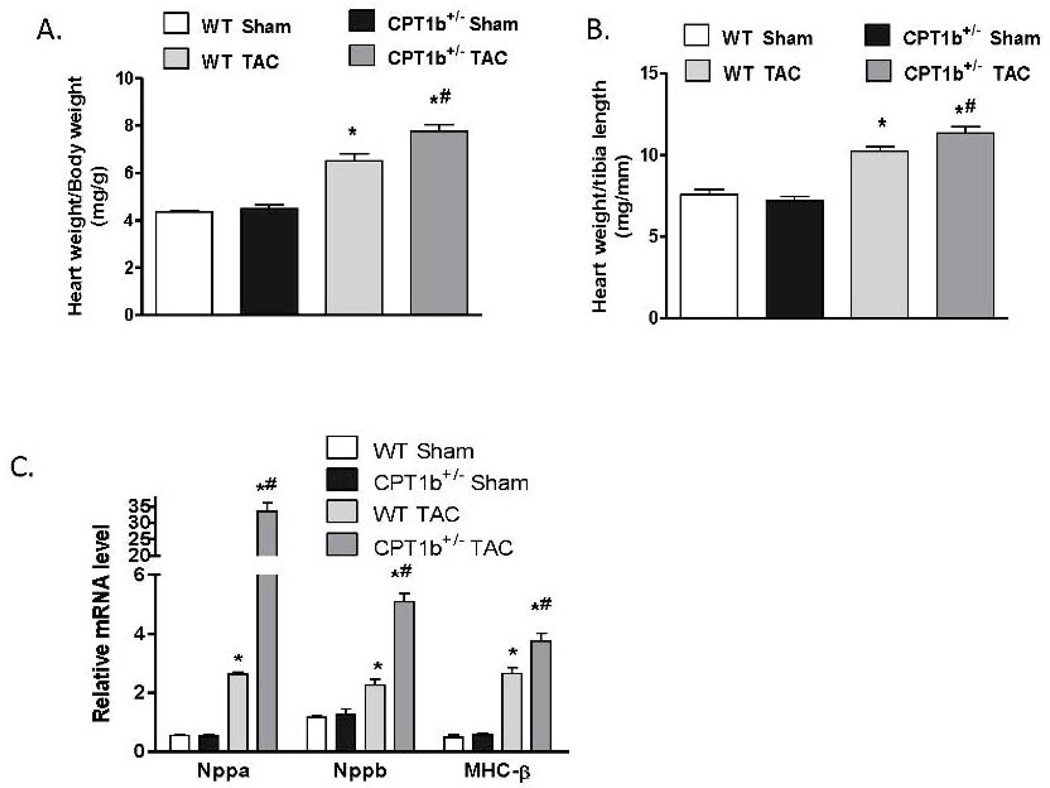
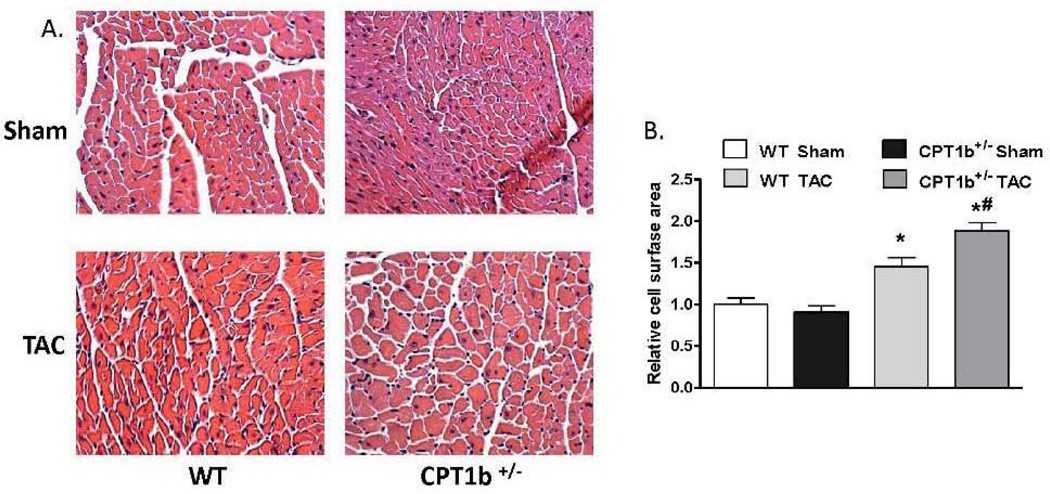
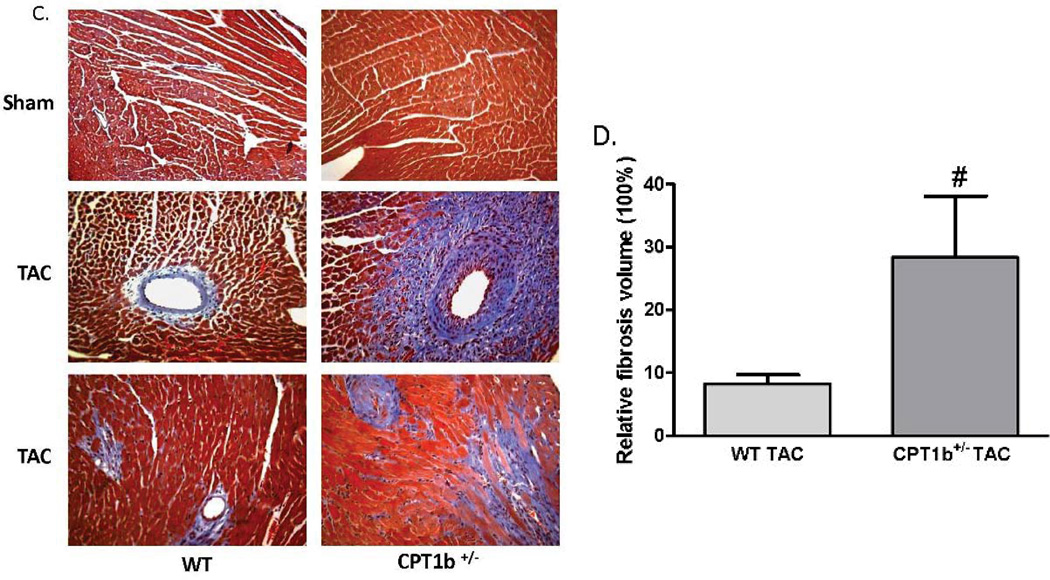
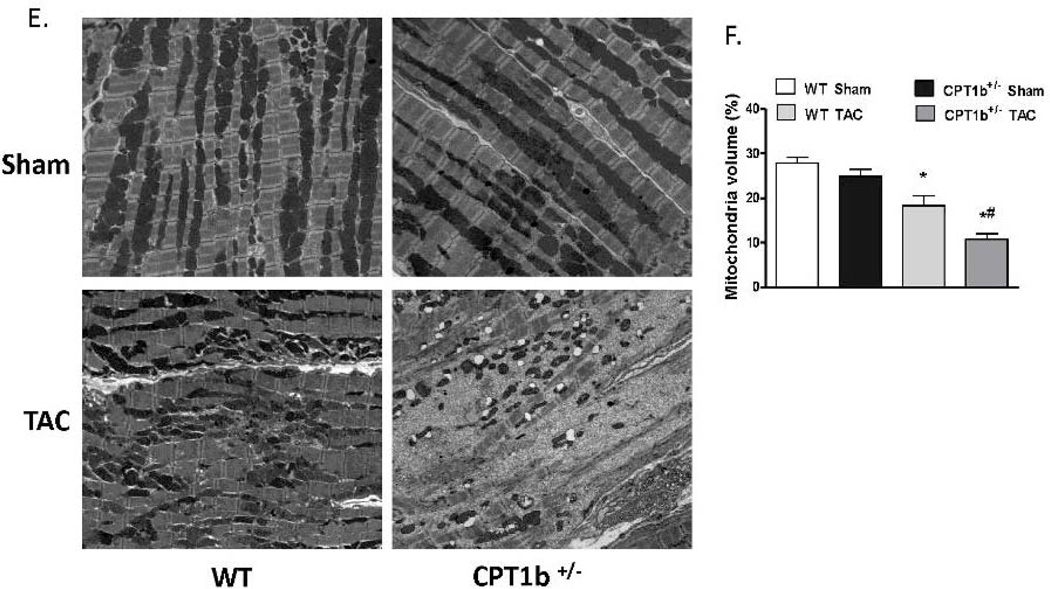
The absence of phenotypic changes in the CPT1b+/− heart provides an ideal animal model to determine whether a partially reduced CPT1 activity can prevent cardiac pathological development under pathological stress conditions. Adult mice were subjected to transverse aorta constriction (TAC)-induced LV pressure-overload. The CPT1b+/− mice were dramatically more susceptible to a severe pressure-overload condition induced by two weeks of TAC than their WT littermates. Majority of the CPT1b+/− mice died before the two-week term of pressure-overload with signs of heart failure (e.g., dilated heart, effluence, shortness of breath, etc.), whereas WT littermates survived (Fig. 2A). Under a milder pressure-overload condition, with a similar level of pressure gradient (Table 1), the CPT1b+/− mice showed more pronounced cardiac hypertrophy than their WT littermates. Echocardiographic assessment showed that left posterior wall thickness (LVPW) at diastole, left ventricular dimension (LVPD) volume at systole, left ventricular mass were further increased in CPT1b+/− mice. Stroke volume, cardiac output, ejection fraction (EF%) and fraction shortening (FS%) were further decreased in CPT1b+/− mice (Table 1 and Fig. 2B, C, D, & E). Heart weight to body weight and heart weight to tibia length ratios were increased in CPT1b+/− mice as compared to WT hearts (Fig. 3A & B). Furthermore, Q-PCR revealed that cardiac expression of molecular markers of cardiac hypertrophy, such as natriuretic peptide precursor A & B (Nppa and Nppb), and myosin heavy chain-β (MHC-β), were increased in CPT1b+/− mice more than their WT littermates (Fig. 3C). In addition, H&E and Trichrome Blue staining on heart sections demonstrated increased cross-sectional area of cardiomyocytes and more pronounced fibrosis in CPT1b+/− mice than in their WT littermates (Fig. 4A, B, C, & D). Transmission electron microscope assessment illustrated a dramatically increased number of swelling mitochondrial with the loss of matrix, and a reduced overall mitochondrial volume in heart sections of CPT1b+/− mice after TAC (Fig. 4E & F). Therefore, these results demonstrate that CPT1b deficiency is detrimental in hearts under mechanical stress-induced cardiac hypertrophy and heart failure.
Figure 2.
Echocardiographic parameters of the mice with pressure-overload. Mice were subjected to TAC procedures at 10~12 weeks of age. A). Kaplan-Meier survival curves for WT and CPT1b+/− mice that were subjected to severe TAC-induced pressure-overload. The survival curves were statistically different (P < 0.05) by log-rank test. B, C, D & E). Mice were subjected to modest TAC at 10~12 weeks of age. Representative echocardiographic images of M-mode measurement and echocardiographic results of EF, FS, and corrected left ventricular mass to body weight (2 weeks after TAC, *p<0.05 vs sham, #P<0.05 vs WT TAC. Sham: n=9; TAC: n=11).
Table 1.
Echocardiography measurement in mice two weeks after TAC.
| Parameters | WT | CPT1b+/− | ||
|---|---|---|---|---|
| Sham (n=9) | TAC (n=11) | Sham (n=9) | TAC (n=11) | |
| Pressure gradient(mm Hg) | NA | 54.91±6.28 | NA | 52.02±7.94 |
| IVS;d (mm) | 0.81±0.17 | 1.21±0.13* | 0.75±0.08 | 1.24±0.16* |
| IVS;s (mm) | 1.13±0.08 | 1.39±0.23* | 1.33±0.05 | 1.42±0.17* |
| LVID;d (mm) | 3.79±0.36 | 4.02±0.24 | 3.82±0.35 | 3.90±0.26 |
| LVID;s (mm) | 2.71±0.40 | 3.04±0.28* | 2.65±0.30 | 3.23±0.27* |
| LVPW;d (mm) | 0.72±0.18 | 1.09±0.19* | 0.75±0.16 | 1.31±0.26*# |
| LVPW;s (mm) | 1.03±0.19 | 1.41±0.11* | 1.04±0.15 | 1.52±0.29* |
| LVID VOL;d(µL) | 65.27±12.03 | 76.15±12.02* | 65.28±11.06 | 70.89±10.92 |
| LVID VOL;s(µL) | 25.18±7.26 | 33.40±5.88* | 23.14±6.56 | 41.61±9.06*# |
| Stroke Volume (μL) | 40.11±7.24 | 41.88±8.54 | 42.14±5.95 | 29.28±7.70*# |
| Cardiac Output (ml/min) | 17.64±2.87 | 17.40±2.97 | 18.62±2.95 | 13.65±4.94*# |
Abbreviations: NA: not available; IVS;s and IVS; d: interventricular septal wall thickness (diastole and systole); LVID;s and LVID;d: left ventricular dimension at systole and diastole; LVPW;s and LVPW;d: left ventricular posterior wall thickness at systole and diastole; LVID VOL;s and LVID VOL;d: left ventricular dimension estimated left ventricular volume at systole and diastole.
p<0.05 vs Sham,
P<0.05 vs WT TAC
Figure 3.
Transcript levels of molecular markers of pathological cardiac hypertrophy in CPT1b+/− mice with pressure-overload. Mice were subjected to a modest pressure-overload condition at 10~12 weeks of age and sacrificed two weeks after TAC. A & B). Heart (mg)/ body weight (g) ratio and heart weight (mg) to tibia length (mm), *p<0.05 vs sham (n=6), # p<0.05 vs WT TAC (n=13). C). Real-time PCR assessment of natriuretic peptide precursor A (Nppa), B (Nppb) and myosin heavy chain-β (MHC-β) transcripts, *p<0.05 vs sham, # p<0.05 vs WT TAC, (n=5).
Figure 4.
Cardiac histology and ultrastructure in CPT1b+/− mice with pressure-overload. Mice were subjected to TAC procedures at 10~12 weeks of age and sacrificed two weeks after TAC. A) Representative histological images (400 ×) with H&E staining on heart section. B) Relative cross-sectional areas. The mean cardiomyocyte cross-sectional area in sham WT mice was set as 1. *p<0.05 vs sham. #p<0.05 vs WT TAC (n=6). C) Representative images of heart sections stained with Trichrome blue. D) Relative fibrosis areas. The whole section area was set as 100%. E) Representative images of left ventricular TEM assessment (×1100). F) Quantification results of mitochondrial volume (%) of heart sections from TEM images. *p<0.05 vs sham. #p<0.05 vs WT TAC (n=8).
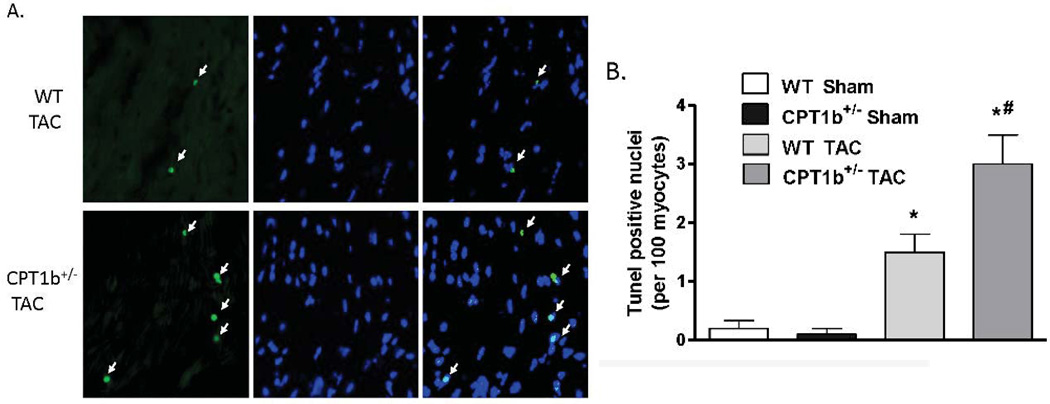
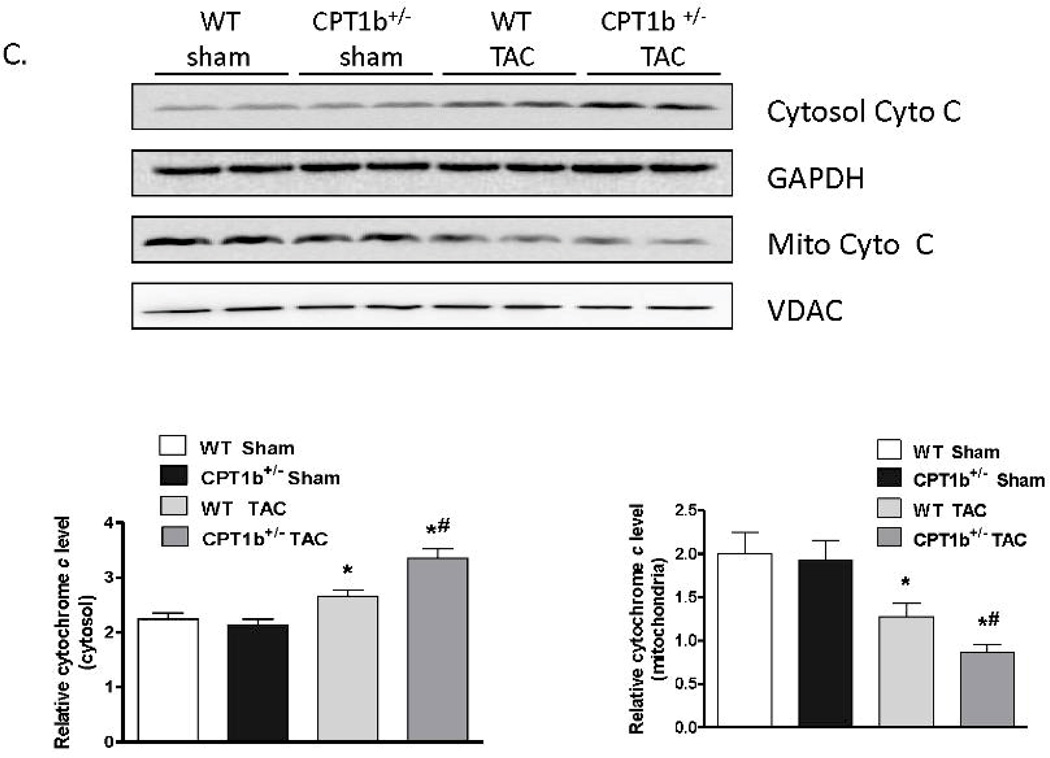
CPT1b deficiency in heart under pressure-overload leads to increased cardiomyocyte apoptosis
Apoptosis has been shown to be one of the major pathological events involved in the development of cardiac hypertrophy and heart failure induced by pressure-overload.26–28 We investigated whether more pronounced cardiomyocyte apoptosis in the CPT1b deficient heart is attributed to the exacerbated pathological development. TUNEL assay on frozen heart sections revealed that an increased number of TUNEL-positive cardiac myocytes on sections from CPT1b+/− hearts (Fig. 5A & B). Western blot analysis on fractionated protein samples revealed that cytochrome c in the cytosol protein samples of CPT1b+/− hearts after TAC was markedly increased compared with that of WT controls (Fig.5C). Therefore, these results indicate that apoptosis is more pronounced in CPT1b+/− relative to WT hearts under the TAC condition.
Figure 5.
Apoptosis assay of CPT1b+/− mice with pressure-overload. Mice were subjected to TAC procedures at 10~12 weeks of age and sacrificed two weeks after TAC. A) Representative image of apoptotic cardiomyocytes in a section of mice hearts. Green staining (see white arrows) indicates apoptotic cells, blue indicates nuclei (TUNEL fluorescence FITC kit, GenScript,USA). B) Quantification results of TUNEL assay (n=5). C) Western blots of cytochrome c in cytosolic and mitochondrial fractions extracted from hearts of mice. *p<0.05 vs sham. #p<0.05 vs WT TAC (n=4).
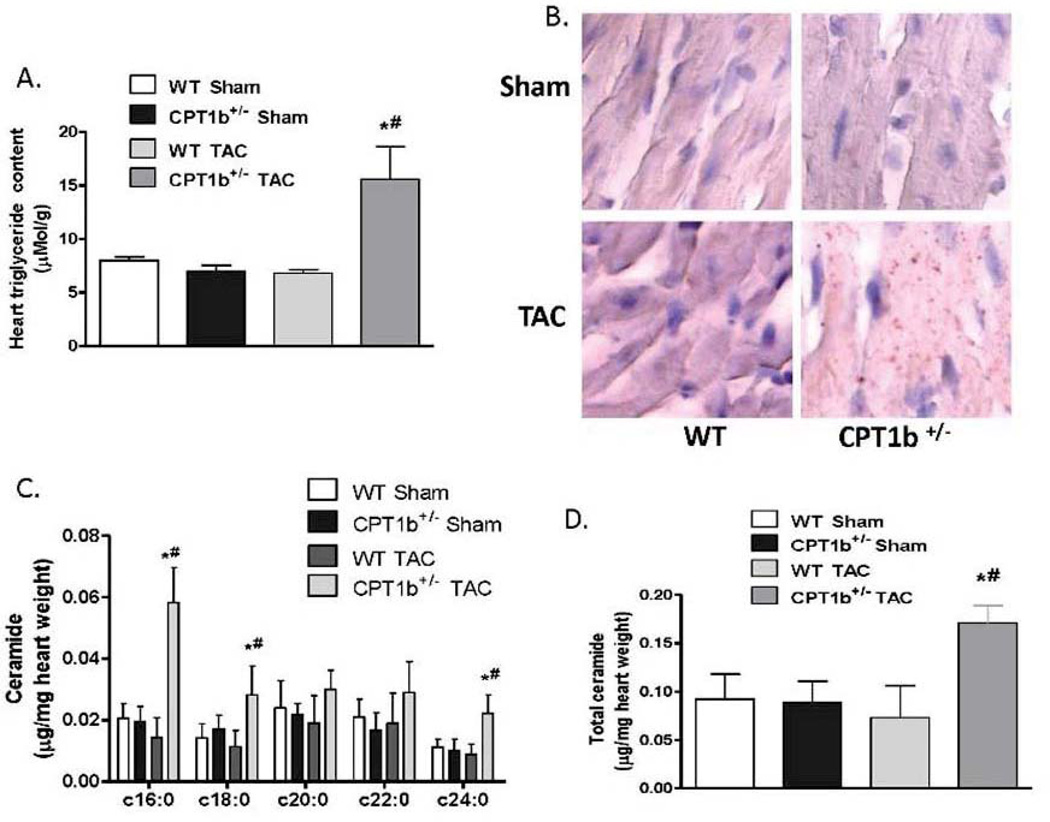
CPT1b+/− hearts under pressure-overload leads to myocardial lipid accumulation
Since CPT1b is a key enzyme for the entry of long-chain fatty acids into the mitochondria, we investigated whether CPT1b deficiency in the heart leads to myocardial triglyceride accumulation. While no change could be detected under basal conditions, myocardial triglyceride concentration was markedly increased in CPT1b+/− relative to WT hearts subjected to TAC (Fig. 6A). Oil-red-O staining on frozen heart sections demonstrated numerous lipid droplets on CPT1b+/−, but not on WT heart sections (Fig. 6B). We further investigated whether myocardial ceramide content is altered with the myocardial accumulation of triglyceride in the CPT1b heart. Mass spectroscopic assessment of the total ceramide and ceramide species composition revealed that total ceramide content in CPT1b+/− hearts was substantially elevated compared with WT controlled hearts (Fig. 6C). C16, C18 and C24 were the ceramide species markedly increased in CPT1b deficient hearts (Fig. 6D).
Figure 6.
Myocardial lipid accumulation of mice with pressure-overload. Mice were subjected to TAC procedures at 10~12 weeks of age and sacrificed two weeks after TAC. A) Myocardial triglyceride content in hearts (n=5). B) Representative photomicrographs depicting the histologic appearance of ventricular tissue sections stained with Oil red O. C) Ceramide species were quantified by ESI-MS/MS as described in the methods (on line supplement). D) Total ceramide was calculated from the sum of C16:0, C18:0, C20:0, C22:0, and C24:0 ceramide subspecies. *p<0.05 vs sham. #p<0.05 vs WT TAC (n=4).
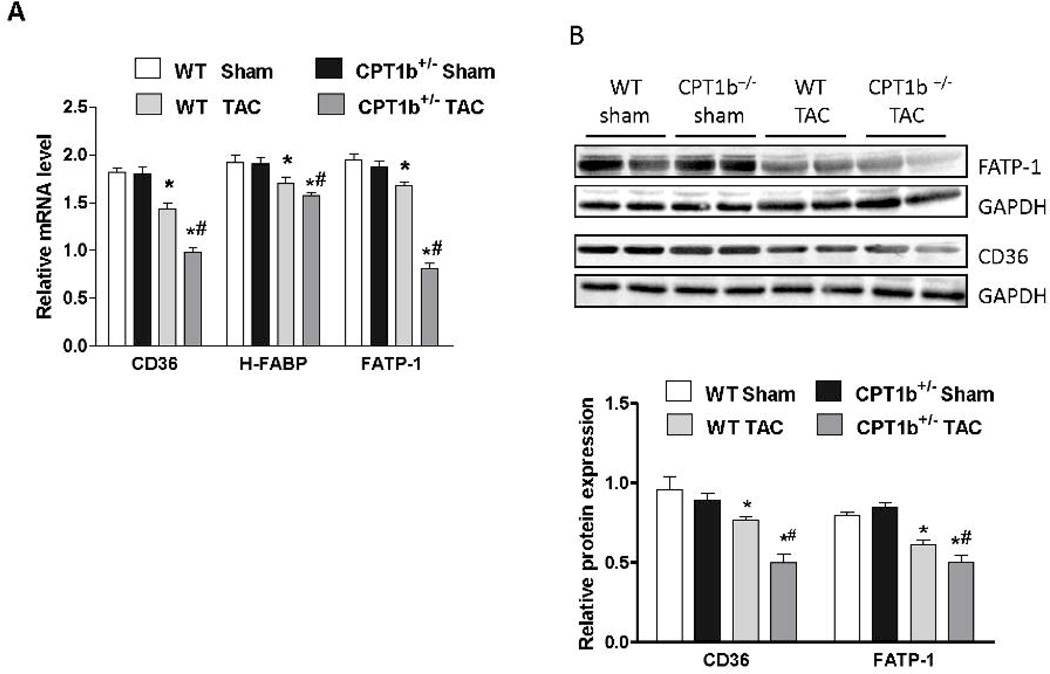
Expression of proteins involved in fatty acid uptake is further downregulated in CPT1b+/− hearts under pressure-overload
To assess whether TAC-induced stress triggers the release of free fatty acids (FFA) from adipose tissues, we measured the serum content of FFA. As expected, serum FFA was modestly increased in both WT and CPT1b+/− mice subjected to TAC at various time points (Supplemental Figure 4). The increased serum FFA under TAC condition may contribute to myocardial lipid accumulation in CPT1b deficient hearts by enhancing fatty acid uptake through peroxisomal proliferator activated-receptor (PPAR) α and PPARγ activation. The expression of PPAR target genes involved in long chain fatty acid (LCFA) uptake or transport were measured. We found that CD36, the heart type fatty acid binding protein (H-FABP) and fatty acid transport protein-1 (FATP-1) were unchanged under basal conditions, but decreased in CPT1b deficient heart subjected to pressure-overload (Fig.7A & B). Therefore, it is not likely that myocardial lipid accumulation in CPT1b+/− hearts is a result of an upregulation of LCFA uptake.
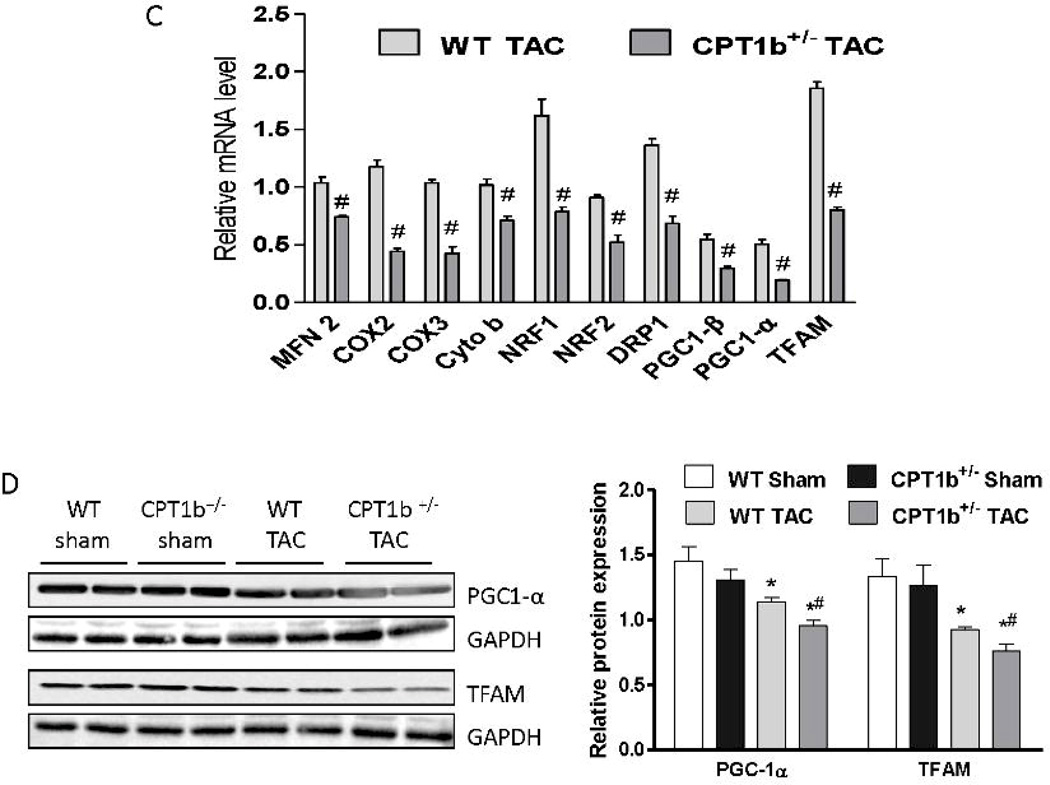
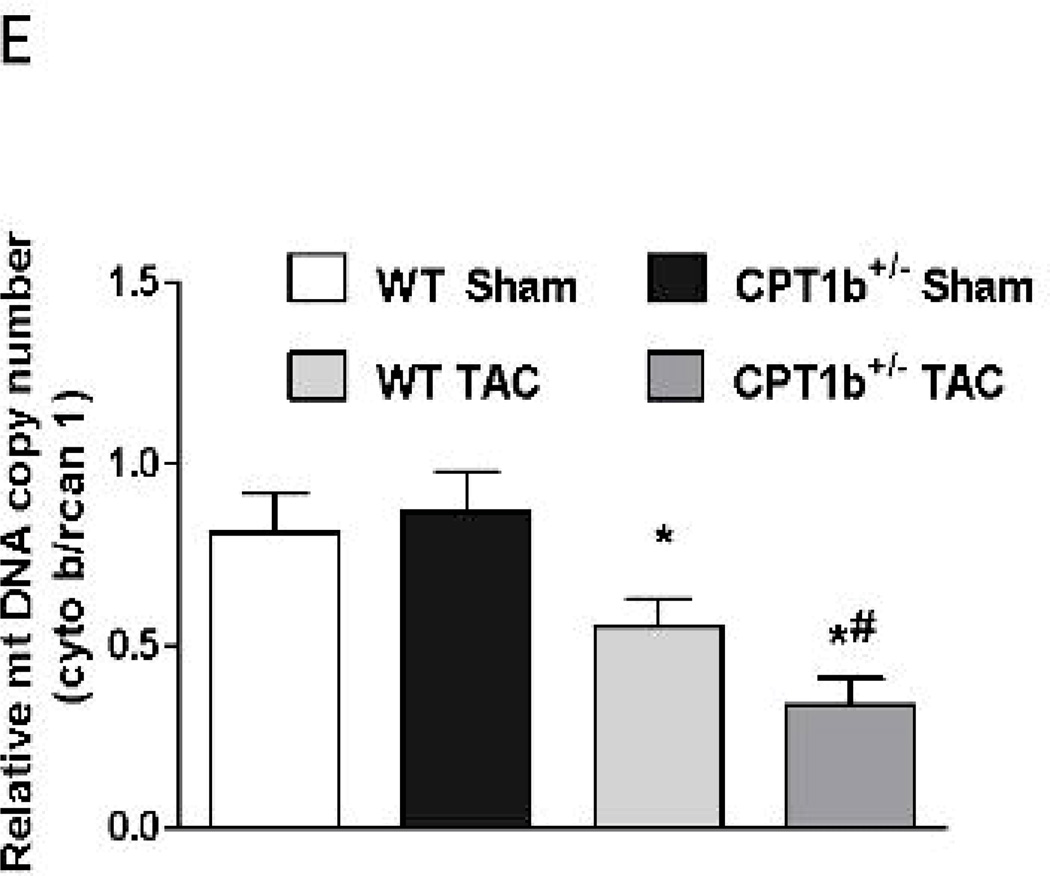
Figure 7.
The expression of fatty acid uptake proteins and mitochondrial biogenesis in mice subjected to pressure-overload. Mice were subjected to TAC procedures at 10~12 weeks of age and sacrificed two weeks after TAC. A) QPCR assessment of CD36, FABP and FATP transcripts (n=4). B) Western blot images and quantification of CD36 and FATP (n=4). C) QPCR assessment of mitochondrial biogenesis genes transcripts (n=4). D) Western blots image and quantification of PGC1-α and TFAM (n=4). E) Mitochondia DNA copy number was assessed by ratio of mitochondrial gene (Cyto b) and nuclear gene (Rcan-1) as described in the methods (on line supplement). *p<0.05 vs sham. #p<0.05 vs WT TAC (n=6).
Mitochondrial biogenesis is decreased in CPT1b+/− hearts under pressure-overload
It has been reported that myocardial lipid accumulation may be associated with impaired mitochondrial biogenesis.29 Q-PCR revealed that the transcript levels of representative mitochondria proteins, such as mitofusin 2 (MFN 2), cytochrome b (Cyto b), cytochrome c oxidase subunit II and III (COX2 and COX3), and dynamin-related protein-1 (DRP1) were substantially attenuated (Fig. 7C). Moreover, transcription factors that are essential for mitochondrial biogenesis, such as transcription factor A, mitochondrial (TFAM), nuclear respiratory factor 1 (NRF1), NRF2 and peroxisome proliferator activated receptor γ coactivator 1α/β (PGC-1α and PGC-1β), were all decreased in CPT1b+/− hearts (Fig. 7C). Protein contents of PGC-1α and TFAM were similarly downregulated in CPT1b+/− relative to WT hearts (Fig. 7D). Mitochondria DNA copy number was also reduced in CPT1b+/− hearts (Fig. 7E). Thus, CPT1b deficiency and subsequent lipid accumulation is associated with a reduction in mitochondrial biogenesis in the heart under pressure-overload conditions.
Fatty acid oxidation is impaired in CPT1b+/− hearts under pressure-overload
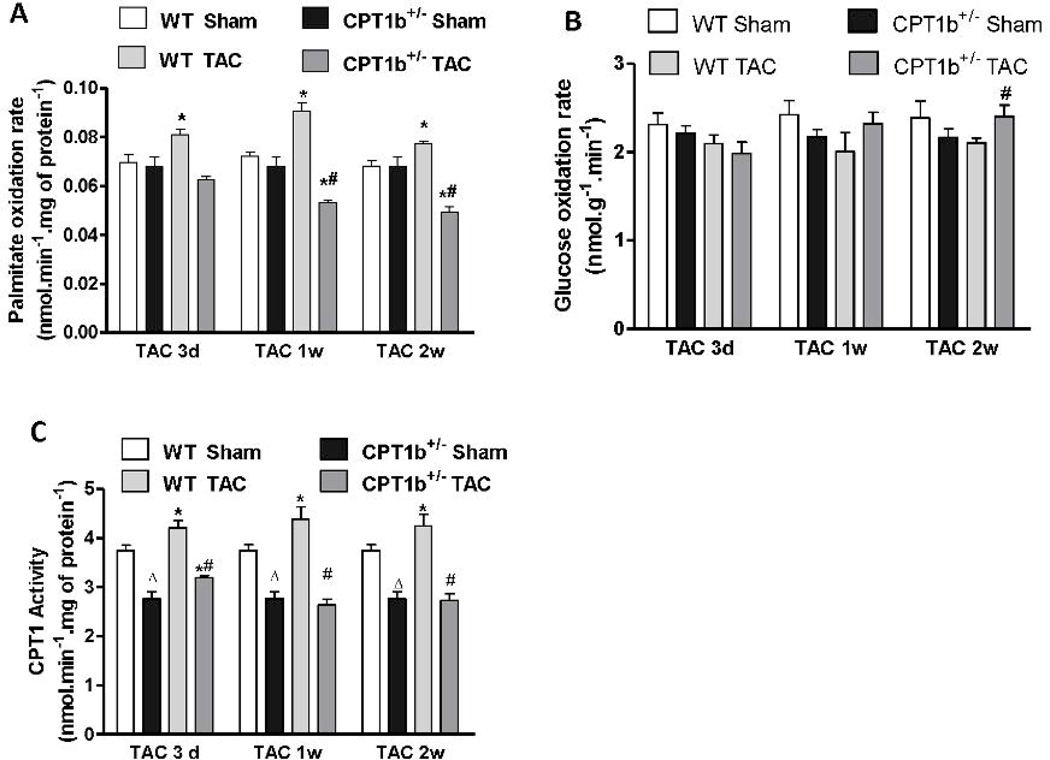
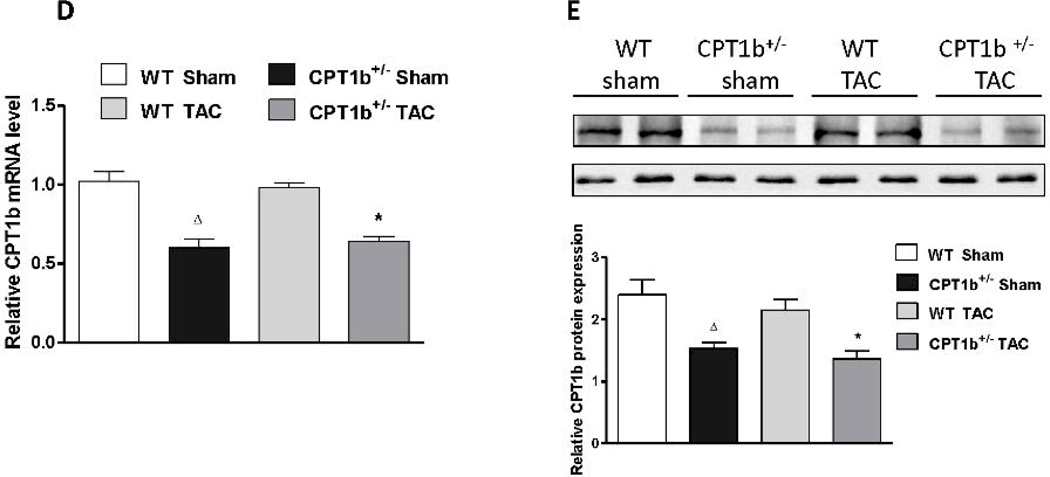
To investigate how CPT-1b deficiency affects myocardial substrate utilization under the TAC-induced pressure-overload condition, we respectively assessed the rates of FAO and glucose oxidation in cardiac samples 3 days, 1 week and 2 weeks after TAC. Interestingly, mitochondrial FAO was increased in WT, but not in CPT1b+/− hearts at all three time points after TAC (Fig. 8A). On the other hand, glucose oxidation rate in cardiac homogenates was not changed at the earlier time points, but increased in CPT1b+/− compared with WT hearts at 2 weeks after TAC (Fig. 8B). CPT1 activity assay showed that mitochondrial CPT1 activity was modestly, but significantly increased at all three time points in WT hearts with TAC. However, CPT1 activity was increased at 3 days, but decreased at 1 week and 2 weeks after TAC in CPT1b+/− hearts compared with hearts with sham for the same time points (Fig. 8C). Similarly, CPT1b expression remained about 50% lower in both transcript and protein levels in CPT1b+/− hearts than in WT hearts (Fig.8 E & F).
Figure 8.
Fatty acid and glucose oxidation rates, and CPT1 activity/expression in pressure-overloaded hearts. Mice were subjected to TAC procedures at 10~12 weeks of age and sacrificed 3 days, 1week and two weeks after TAC, respectively. A) Fatty acid oxidation rate was determined on isolated mitochondria (n=6). B) Glucose oxidation rate was determined on heart homogenate (n=6). C) CPT1 activity was assayed using isolated mitochondria (n=6). D) QPCR assessment of CPT1b, 2 weeks after TAC (n=4). E) Western blots image and quantification of CPT1b, 2 weeks after TAC. *p<0.05 vs sham. #p<0.05 vs WT TAC. Δp<0.05 vs WT Sham (n=6).
Discussion
We investigated the effects of CPT1b deficiency on the pathological development of cardiac hypertrophy and remodeling under the left ventricular pressure-overload condition in mice. We previously demonstrated cardiac hypertrophy in mice with other FAO enzyme deficiencies, which is most predominant in homozygous long-chain acyl-CoA dehydrogenase deficiency.30 The most important finding based on the genetic mouse model of CPT1b deficiency is that CPT1b deficiency causes lipotoxicity in the heart under the pressure-overload condition and leads to exacerbate cardiac pathology.
CPT1b is the predominant CPT1 isoform expressed in the heart and plays an essential role in myocardial FAO. Repressing myocardial FAO has been proposed as a therapeutic target to improve cardiac efficiency in the failing heart. Inhibitors of CPT1 have already been developed and tested in preclinical animal studies and small clinical trials.18, 19 However, it remains controversial due to the mixed results from animal studies. Schwarzer et al. showed that etomoxir failed to reverse pressure-overload induced heart failure in vivo.20 Strikingly, the most studied compound etomoxir has been shown to exert adverse effects that eventually lead to cardiac hypertrophy.31–34 It was proposed that etomoxir treatment may induce cardiac hypertrophy via increased cellular oxidative stress and NF-κB activation.35 Wolkowicz et al. showed that another CPT1 inhibitor, 2-tetradecylglycidic acid induces myocardial hypertrophy via the AT1 receptor.11
It is clear that the potential adverse effects of CPT1 inhibitors may not be just a nonspecific side effect. It may be associated with the irreversible inhibition of CPT1 activity in the heart. The present study provides evidence to support that partial CPT1b deficiency in a mouse model of CPT1 knockout is detrimental to cardiac structure/function due to pressure-overload induced LV systolic dysfunction. CPT1b+/− mice showed a more pronounced systolic dysfunction, but remained in concentric hypertrophy. It is likely that most CPT1b+/− hearts were still at the stage prior to the transition to dilated cardiomyopathy. Therefore, we could not rule out the possibility that CPT1 inhibition might exert beneficial effects only in dilated cardiomyopathy. Additionally, it is possible that inhibitors and the gene knockout will exhibit distinct functions when the corresponding proteins have non-enzymatic functions as a scaffold.
To our knowledge, the present study is the first study based on a gene targeted mouse model with CPT1b deficiency. While a complete knockout of CPT1b causes embryonic lethality, the heterozygous CPT1b knockout mice are overtly normal. It is noted that in the heterozygous CPT-1b knockout heart, CPT1b expression was blunted in both transcript and protein levels. Since CPT1a is also expressed in the adult heart at low level, a compensatory upregulation of CPT1a is possible. In fact, the inverse response of increased Cpt-1b expression in livers of diet challenged CPT-1a+/− mice was reported.36 However, transcript expression of CPT1a was unchanged in the CPT1b deficient hearts. Since the total CPT1 activity was decreased by about 30% compared to WT littermates and CPT1 activity is upregulated during at least the early stage of cardiac hypertrophy, the depression of CPT1 activity in the CPT1b deficient heart appears to be the key determinant for the detrimental response to pressure-overload induced cardiac pathology. The partial CPT1b deficiency in the heart seems insufficient to affect basal cardiac performance and cardiac metabolism. This result does not support previous observations that partial inhibition of CPT1 activity by CPT1 inhibitors, such as etomoxir, leads to cardiac dysfunction and hypertrophy under physiological conditions.35,11 Therefore, it is likely that the detrimental cardiac effects of etomoxir treatment under normal physiological conditions may be associated with effects unrelated to CPT1 inhibition or more severe cardiac CTP1 inhibition (30% in CPT1b+/− vs 40–50% with etomoxir treatment37).
The most important finding in the present study is that the CPT1b+/− mice were much more susceptible to pressure-overload induced pathological cardiac hypertrophy, suggesting that partial CPT1b deficiency is detrimental with pathological development under mechanical stress conditions. This result is in sharp contrast to those using CPT1 inhibitors in human studies.18,19 The reasons for this obvious discrepancy may be derived from different degrees of CPT1 inhibition among studies, non-specific effects of CPT1 inhibitors, or the existence of certain levels of CPT1a activity in CPT1b knockout hearts. Importantly, the current finding on the detrimental effects of CPT1b deficiency is against the basic concept of fatty acid oxidation inhibition as a therapeutic approach in treating heart failure patients. A specific site and specific quantity of the inhibition could be crucial. Moreover, our results demonstrated that mitochondrial FAO and CPT1b activity were upregulated in mitochondria samples from hearts with pressure-overload induced hypertrophy. It is likely that an increased CPT1 activity is crucial to maintain mitochondrial FAO among the remaining mitochondria during the development of pathological cardiac hypertrophy. However, the suppressed mitochondrial biogenesis and function in the heart may potentially impair overall myocardial FAO.
Lipotoxicity in addition to the pressure-overload associated systolic dysfunction appears to be the cause of the detrimental effect of CPT1b deficiency under pathological conditions. Patients with inborn errors of FAO typically manifest cardiomyopathy with diminished systolic function.38 Moreover, intramyocardial lipid accumulation is associated with contractile dysfunction in heart tissues from patients with non-ischemic heart failure.39 The reduction of mitochondrial biogenesis should be the consequence of the progression of cardiac pathology and cardiomyocyte apoptosis. The increased sympathetic activity in response to hemodynamic overload might lead to increased lipolysis. While this appears to be the case, it is insufficient to activate PPAR signaling to increase the expression of fatty acid uptake proteins. Instead, the expression of fatty acid uptake proteins was further decreased, possibly due to the exacerbated cardiac pathological development. Therefore, it is plausible that the increased myocardial triglyceride content in CPT1b+/− hearts is due to the relative reduction of mitochondrial FAO and mitochondrial biogenesis. As a result, myocardial lipid accumulates and feeds the ceramide synthesis. Our observation is in agreement with the previous report that oxfenicine induced myocardial lipid accumulation in rats.40, 41 Myocardial lipid accumulation and the elevation of ceramide content in the heart should be the mechanisms underlying the detrimental effects during the development of pathological cardiac hypertrophy in response to the pressure-overload condition. The cytotoxic effect of ceramide in cardiomyocytes has been well established.42, 43 Ceramide induces apoptosis of cardiomyocyte in vitro and in vivo.44–47 In the PPARγ overexpression heart, myocardial lipid accumulation and increased ceramide content has been observed accompanying cardiomyocyte apoptosis.47 Therefore, the increased ceramide content in the CPT1b deficient heart subjected to TAC is likely to trigger the apoptosis signaling in the heart and aggravates the pathological development.
One limitation of the current study is that the CPT1b deficiency pre-existed, hence we are cautious to avoid over-interpreting this result as CPT-1 inhibitors are used as treatment in heart failure patients. We cannot rule out the possibility that the initial development of the pressure-overload induced cardiac hypertrophy is exceptionally susceptible to CPT1b deficiency. A conditional gene-targeting model of CPT1b could provide more in-depth insights into the effects of CPT1b deficiency in the heart during the various stages of cardiac hypertrophy and heart failure. Despite this limitation, the current finding clearly demonstrates the necessity to further evaluate the use of CPT1 inhibitors as a therapeutic approach to treat patients with cardiac hypertrophy and even heart failure.
The present study demonstrates in a genetic mouse model that partial CPT-1 deficiency is not sufficient to cause cardiac dysfunction under the normal physiological conditions. However, it is detrimental in animals subjected to TAC-induced LV pressure-overload. CPT1b deficiency exacerbates the cardiac pathological development induced by left ventricular pressure-overload due to myocardial lipotoxicity.
Supplementary Material
Acknowledgements
We thank the technical services of the UAB Metabolism Core Laboratory and the Targeted Metabolomics and Proteomics Laboratory. We also thank Kevin Yang for proof-reading and editing the manuscript.
Funding Sources: This work was supported by grants from National Institutes of Health (1R01 HL085499 to QY, 1R01 HL084456 to QY, 1R01 RR02599 to PAW and T32 HL007457 to TK). The technical services of the UAB Metabolism Core Laboratory were supported by grants P30DK56336 and UL 1RR025777. The targeted Metabolomics and Proteomics Laboratory were supported partly by P30DK079337 and P30AR50948.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: Beneficial or detrimental? Cardiovasc Res. 2009;81:420–428. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 2.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 3.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 4.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 5.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 6.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Zarain-Herzberg A, Rupp H. Therapeutic potential of cpt i inhibitors: Cardiac gene transcription as a target. Expert Opin Investig Drugs. 2002;11:345–356. doi: 10.1517/13543784.11.3.345. [DOI] [PubMed] [Google Scholar]
- 8.Panchal AR, Stanley WC, Kerner J, Sabbah HN. Beta-receptor blockade decreases carnitine palmitoyl transferase i activity in dogs with heart failure. J Card Fail. 1998;4:121–126. doi: 10.1016/s1071-9164(98)90252-4. [DOI] [PubMed] [Google Scholar]
- 9.Abozguia K, Clarke K, Lee L, Frenneaux M. Modification of myocardial substrate use as a therapy for heart failure. Nat Clin Pract Cardiovasc Med. 2006;3:490–498. doi: 10.1038/ncpcardio0583. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbert KD, Dyck JR. Malonyl-coa decarboxylase is a major regulator of myocardial fatty acid oxidation. Curr Hypertens Rep. 2005;7:407–411. doi: 10.1007/s11906-005-0034-z. [DOI] [PubMed] [Google Scholar]
- 11.Wolkowicz PE, Urthaler F, Forrest C, Shen H, Durand J, Wei CC, Oparil S, Dell'Italia LJ. 2-tetradecylglycidic acid, an inhibitor of carnitine palmitoyltransferase-1, induces myocardial hypertrophy via the at1 receptor. J Mol Cell Cardiol. 1999;31:1405–1412. doi: 10.1006/jmcc.1999.0977. [DOI] [PubMed] [Google Scholar]
- 12.Cabrero A, Merlos M, Laguna JC, Carrera MV. Down-regulation of acyl-coa oxidase gene expression and increased nf-kappab activity in etomoxir-induced cardiac hypertrophy. J Lipid Res. 2003;44:388–398. doi: 10.1194/jlr.M200294-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Pagliaro P, Chiribiri A, Gattullo D, Penna C, Rastaldo R, Recchia FA. Fatty acids are important for the frank-starling mechanism and gregg effect but not for catecholamine response in isolated rat hearts. Acta Physiol Scand. 2002;176:167–176. doi: 10.1046/j.1365-201X.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- 14.Esser V, Britton CH, Weis BC, Foster DW, McGarry JD. Cloning, sequencing, and expression of a cdna encoding rat liver carnitine palmitoyltransferase i. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J Biol Chem. 1993;268:5817–5822. [PubMed] [Google Scholar]
- 15.Yamazaki N, Shinohara Y, Shima A, Terada H. High expression of a novel carnitine palmitoyltransferase i like protein in rat brown adipose tissue and heart: Isolation and characterization of its cdna clone. FEBS Letters. 1995;363:41–45. doi: 10.1016/0014-5793(95)00277-g. [DOI] [PubMed] [Google Scholar]
- 16.Weis BC, Cowan AT, Brown N, Foster DW, McGarry JD. Use of a selective inhibitor of liver carnitine palmitoyltransferase i (cpt i) allows quantification of its contribution to total cpt i activity in rat heart. Evidence that the dominant cardiac cpt i isoform is identical to the skeletal muscle enzyme. J Biol Chem. 1994;269:26443–26448. [PubMed] [Google Scholar]
- 17.Brown NF, Weis BC, Husti JE, Foster DW, McGarry JD. Mitochondrial carnitine palmitoyltransferase i isoform switching in the developing rat heart. J Biol Chem. 1995;270:8952–8957. doi: 10.1074/jbc.270.15.8952. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 2000;99:27–35. [PubMed] [Google Scholar]
- 19.Valerio A, D'Antona G, Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging (Albany NY) 2011;3:464–478. doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzer M, Faerber G, Rueckauer T, Blum D, Pytel G, Mohr FW, Doenst T. The metabolic modulators, etomoxir and nvp-lab121, fail to reverse pressure overload induced heart failure in vivo. Basic Res Cardiol. 2009;104:547–557. doi: 10.1007/s00395-009-0015-5. [DOI] [PubMed] [Google Scholar]
- 21.Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, d'Agostino C, Hintze TH, Stanley WC, Recchia FA. Carnitine palmitoyl transferase-i inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Turcani M, Rupp H. Modification of left ventricular hypertrophy by chronic etomixir treatment. Br J Pharmacol. 1999;126:501–507. doi: 10.1038/sj.bjp.0702312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji S, You Y, Kerner J, Hoppel CL, Schoeb TR, Chick WS, Hamm DA, Sharer JD, Wood PA. Homozygous carnitine palmitoyltransferase 1b (muscle isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2008;93:314–322. doi: 10.1016/j.ymgme.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 25.Bartelds B, Takens J, Smid GB, Zammit VA, Prip-Buus C, Kuipers JR, van der Leij FR. Myocardial carnitine palmitoyltransferase i expression and long-chain fatty acid oxidation in fetal and newborn lambs. Am J Physiol Heart Circ Physiol. 2004;286:H2243–H2248. doi: 10.1152/ajpheart.00864.2003. [DOI] [PubMed] [Google Scholar]
- 26.Teiger E, Than VD, Richard L, Wisnewsky C, Tea BS, Gaboury L, Tremblay J, Schwartz K, Hamet P. Apoptosis in pressure overload-induced heart hypertrophy in the rat. J Clin Invest. 1996;97:2891–2897. doi: 10.1172/JCI118747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anversa P, Kajstura J, Olivetti G. Myocyte death in heart failure. Curr Opin Cardiol. 1996;11:245–251. doi: 10.1097/00001573-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Anselmi A, Gaudino M, Baldi A, Vetrovec GW, Bussani R, Possati G, Abbate A. Role of apoptosis in pressure-overload cardiomyopathy. J Cardiovasc Med (Hagerstown) 2008;9:227–232. doi: 10.2459/JCM.0b013e328277f1d7. [DOI] [PubMed] [Google Scholar]
- 29.Glenn DJ, Wang F, Nishimoto M, Cruz MC, Uchida Y, Holleran WM, Zhang Y, Yeghiazarians Y, Gardner DG. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension. 2011;57:216–222. doi: 10.1161/HYPERTENSIONAHA.110.160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox KB, Liu J, Tian L, Barnes S, Yang Q, Wood PA. Cardiac hypertrophy in mice with long-chain acyl-coa dehydrogenase or very long-chain acyl-coa dehydrogenase deficiency. Lab Invest. 2009;89:1348–1354. doi: 10.1038/labinvest.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rupp H, Elimban V, Dhalla NS. Modification of subcellular organelles in pressure-overloaded heart by etomoxir, a carnitine palmitoyltransferase 1 inhibitor. FASEB J. 1992;6:2349–2353. doi: 10.1096/fasebj.6.6.1531968. [DOI] [PubMed] [Google Scholar]
- 32.Vetter R, Kott M, Rupp H. Differential influences of carnitine palmitoyltransferase-1 inhibition and hyperthyroidism on cardiac growth and sarcoplasmic reticulum phosphorylation. Eur Heart J. 1995;16(Suppl C):15–19. doi: 10.1093/eurheartj/16.suppl_c.15. [DOI] [PubMed] [Google Scholar]
- 33.Hulsmann WC, Peschechera A, Schneijdenberg CT, Verkleij AJ. Comparison of the effects of carnitine palmitoyltransferase-1 and -2 inhibitors on rat heart hypertrophy. Cardioscience. 1994;5:193–197. [PubMed] [Google Scholar]
- 34.Yotsumoto T, Naitoh T, Kitahara M, Tsuruzoe N. Effects of carnitine palmitoyltransferase 1 inhibitors on hepatic hypertrophy. Eur J Pharmacol. 2000;398:297–302. doi: 10.1016/s0014-2999(00)00288-0. [DOI] [PubMed] [Google Scholar]
- 35.Cabrero A, Merlos M, Laguna JC, Carrera MV. Down-regulation of acyl-coa oxidase gene expression and increased nf-kappab activity in etomoxir-induced cardiac hypertrophy. J Lipid Res. 2003;44:388–398. doi: 10.1194/jlr.M200294-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Nyman LR, Tian L, Hamm DA, Schoeb TR, Gower BA, Nagy TR, Wood PA. Long term effects of high fat or high carbohydrate diets on glucose tolerance in mice with heterozygous carnitine palmitoyltransferase-1a (CPT-1a) deficiency: Diet influences on cpt1a deficient mice. Nutr Diabetes. 2011;1:e14. doi: 10.1038/nutd.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopaschuk GD, Kelly DP. Signalling in cardiac metabolism. Cardiovasc Res. 2008;79:205–207. doi: 10.1093/cvr/cvn134. [DOI] [PubMed] [Google Scholar]
- 38.Kelly DP, Strauss AW. Inherited cardiomyopathies. N Engl J Med. 1994;330:913–919. doi: 10.1056/NEJM199403313301308. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 40.Jodalen H, Ytrehus K, Moen P, Hokland B, Mjos OD. Oxfenicine-induced accumulation of lipid in the rat myocardium. J Mol Cell Cardiol. 1988;20:277–282. doi: 10.1016/s0022-2828(88)80060-9. [DOI] [PubMed] [Google Scholar]
- 41.Bachmann E, Weber E. Biochemical mechanisms of oxfenicine cardiotoxicity. Pharmacology. 1988;36:238–248. doi: 10.1159/000138390. [DOI] [PubMed] [Google Scholar]
- 42.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brindley DN, Kok BP, Kienesberger PC, Lehner R, Dyck JR. Shedding light on the enigma of myocardial lipotoxicity: The involvement of known and putative regulators of fatty acid storage and mobilization. Am J Physiol Endocrinol Metab. 2010;298:E897–E908. doi: 10.1152/ajpendo.00509.2009. [DOI] [PubMed] [Google Scholar]
- 44.Dyntar D, Eppenberger-Eberhardt M, Maedler K, Pruschy M, Eppenberger HM, Spinas GA, Donath MY. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 2001;50:2105–2113. doi: 10.2337/diabetes.50.9.2105. [DOI] [PubMed] [Google Scholar]
- 45.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Usta E, Mustafi M, Artunc F, Walker T, Voth V, Aebert H, Ziemer G. The challenge to verify ceramide's role of apoptosis induction in human cardiomyocytes--a pilot study. J Cardiothorac Surg. 2011;6:38. doi: 10.1186/1749-8090-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, Goldberg IJ. PPAR gamma-induced cardiolipotoxicity in mice is ameliorated by PPAR alpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.