Abstract
Toxoplasma gondii is an opportunistic, zoonotic pathogen with a worldwide distribution. There are large variations in the seroprevalence of T. gondii infection in different regions of the world. Although toxoplasmosis became a notifiable communicable disease in Taiwan in 2007, little is known about its epidemiology among the general population. This cross-sectional study aimed to survey the seroprevalence of T. gondii infection and its risk factors among healthy blood donors in Taiwan. Through collaborating with the Taiwan Blood Services Foundation, a total of 1,783 healthy blood donors from all six-branch blood service centers participated in this study. The blood samples were tested for the presence of T. gondii antibodies and DNA using enzyme immunoassays and real-time PCR, respectively. Structured questionnaires were used to gather information on risk factors for T. gondii infection. Of the 1,783 participants, 166 (9.3%) tested positive for anti-Toxoplasma IgG, while 5 (0.28%) tested positive for anti-Toxoplasma IgM. The five IgM positive donors had high avidity antibodies suggestive of past infection. No active parasitemia was detected by real-time PCR assays. Multivariate logistic regression showed that undercooked pork meat consumption (adjusted odds ratio [OR] = 2.9; 95% confidence interval [CI]: 1.3–6.5), raw mussels consumption (adjusted OR = 5.3; 95% CI: 1.5–19.1), having a cat in the household (adjusted OR = 2.0; 95% CI: 1.2–3.2), a lower education level (adjusted OR = 1.6; 95% CI: 1.1–2.3), and donation place in eastern Taiwan (adjusted OR = 2.5; 95% CI: 1.6–3.9) were independent risk factors for Toxoplasma seropositivity. These findings provide information on the seroprevalence and epidemiology of T. gondii infection among healthy blood donors in Taiwan.
Introduction
Toxoplasma gondii is a parasitic protozoan found throughout the world that can be carried by most species of warm blooded animals and infects nearly one-third of the world’s human population [1]–[3]. Although toxoplasmosis occurs worldwide, the seroprevalence of T. gondii infection can vary greatly between countries (10–80%) and even within a given country [4]–[5]. T. gondii infections in immunocompetent people are usually mild and self-limiting; however, severe disease and complication can occur in immunocompromised individuals and newborns [2], [5]. People typically become infected by three principal routes of transmission: foodborne transmission (consuming undercooked, contaminated meat), animal-to-human transmission (ingesting oocysts shed in the feces of infected cats), and vertical transmission from mother to fetus through the placenta during pregnancy. Additionally, T. gondii can be transmitted via blood transfusion or organ transplantation from infected donors [6]–[8].
Toxoplasmosis became a notifiable communicable disease in Taiwan in 2007. Previous studies on the seroprevalence of T. gondii antibodies have focused on special groups, such as human immunodeficiency virus (HIV)-infected adults (10.2%) [9], pregnant women (9.1–11.6%) [10]–[11], and mountain aborigines (19.4%–26.7%) [12]–[13]; little is known about the epidemiology of T. gondii among the general population. This cross-sectional study aimed to survey the seroprevalence of T. gondii infection and associated risk factors among healthy blood donors in Taiwan. We collaborated with the Taiwan Blood Services Foundation, and a total of 1,783 healthy blood donors from all six branch blood service centers participated in this study.
Materials and Methods
Study Design
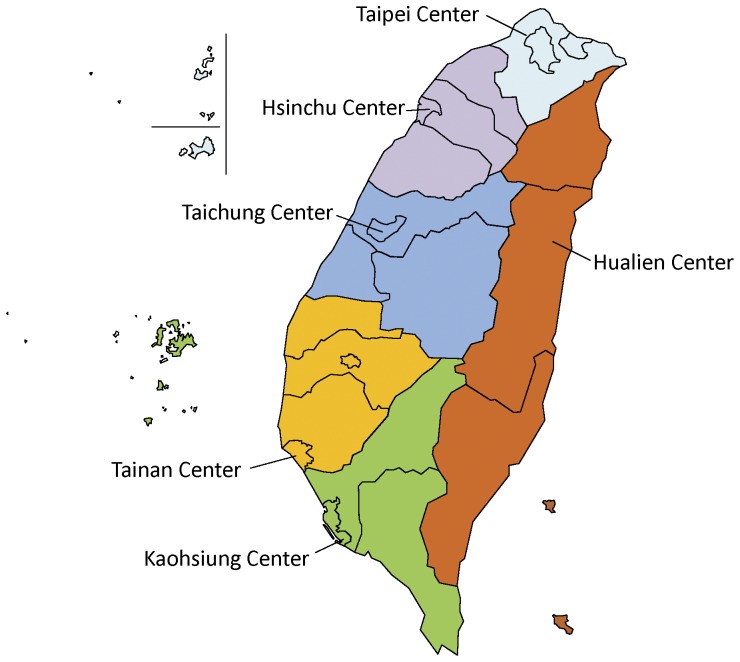
We performed a cross-sectional study in all of the six district blood centers of the Taiwan Blood Services Foundation. The six blood centers are located in Taipei (Northern Taiwan), Hsinchu (Northwestern Taiwan), Taichung (Central Taiwan), Tainan (Southwestern Taiwan), Kaohsiung (Southern Taiwan), and Hualien (Eastern Taiwan), respectively (Figure 1).
Figure 1. Geographical Distribution of District Blood Service Centers in Taiwan.
Participants
All samples from blood donors were routinely tested for HIV, hepatitis B virus surface antigen (HBsAg), hepatitis C virus (HCV), Human T-Lymphotropic virus (HTLV), and Treponema pallidum. Study participants were voluntary blood donors who met the following inclusion criteria: 1) individuals ≥18 years of age; 2) individuals who agreed to participate in the study and gave written informed consent; 3) individuals who tested negative for HIV, HCV, HTLV, T. pallidum, and HBsAg.
A total of 1,783 blood samples from healthy blood donors were collected from June 2010 to October 2010. Sample size estimation for each center was based on the proportion of each center’s blood donation from 2008 to 2009 (see Table 1 footnote for details).
Table 1. Demographic characteristics of the 1,783 blood donors.
| Characteristics | Blood donors No. | Donors with anti-T. gondii IgG | |
| No. | % | ||
| Donation center† | |||
| Taipei | 483 | 44 | 9.1 |
| Hsinchu | 250 | 21 | 8.4 |
| Taichung | 350 | 29 | 8.3 |
| Tainan | 250 | 22 | 8.8 |
| Kaohsiung | 280 | 18 | 6.4 |
| Hualien | 170 | 32 | 18.8 |
| Gender | |||
| Male | 1,127 | 114 | 10.1 |
| Female | 654 | 52 | 8.0 |
| Age | |||
| 18–25 | 330 | 33 | 10.0 |
| 26–35 | 473 | 42 | 8.9 |
| 36–45 | 433 | 37 | 8.5 |
| 46–55 | 370 | 38 | 10.3 |
| >55 | 158 | 16 | 10.1 |
| Childbearing-aged (18–45 y/o)woman | 454 | 36 | 7.9 |
| Blood group type | |||
| O | 814 | 74 | 9.1 |
| A | 492 | 45 | 9.1 |
| B | 331 | 28 | 8.5 |
| AB | 146 | 19 | 13.0 |
| Educational level | |||
| College and above | 1,138 | 88 | 7.7 |
| High school and below | 638 | 78 | 12.2 |
| Occupation | |||
| Laborer | 332 | 25 | 7.5 |
| Businessman/employee | 1,061 | 109 | 10.3 |
| Student/unemployed | 382 | 32 | 8.4 |
To ensure good representativeness for each region (see Figure 1), sample size estimation for each center was based on the proportion of each center’s blood donation from 2008 to 2009, as listed below:
Taipei (northern Taiwan, regional population: 6,963,205) had 518,696 blood donations in 2008 and 533,399 blood donations in 2009.
Hsinchu (northwestern Taiwan, regional population: 3,462,995) had 253,678 blood donations in 2008 and 243,416 blood donations in 2009.
Taichung (central Taiwan, regional population: 4,479,052) had 366,167 blood donations in 2008 and 365,843 blood donations in 2009.
Tainan (southwestern Taiwan, regional population: 3,429,697) had 276,218 blood donations in 2008 and 290,406 blood donations in 2009.
Kaohsiung (southern Taiwan regional population: 3,749,737) had 311,525 blood donations in 2008 and 313,686 blood donations in 2009.
Hualien (eastern Taiwan, regional population: 1,035,086) had 81,473 blood donations in 2008 and 82,434 blood donations in 2009.
Ethics
This work was approved by the Institutional Review Board of Taiwan Centers for Disease Control (CDC) (Taipei, Taiwan) and the Institutional Review Board of Taiwan Blood Donor Services Foundation. Written informed consent was obtained from all participants before blood sampling.
Serologic Testing
Whole blood and serum samples were transported from the six district blood centers to the Parasitic Disease Laboratory of Taiwan CDC (Taipei, Taiwan) and stored at −20°C until analyzed. Serum samples of blood donors were tested for anti-T. gondii IgG and IgM antibodies by commercially available enzyme immunoassays (bioMérieux, Marcy l’Etoile, France) with an automated Vitek Immuno Diagnostic Assay System (VIDAS). Analyses were performed as instructed by the manufacturers. For the IgG and IgM assays, positive results were defined as values of ≥8 international units (IU)/ml and index values of ≥0.65. Equivocal results range from 4 to 8 IU/ml and index values of 0.55 to 0.65. Negative results were defined as <4 IU/ml and index values of <0.55. In addition, all IgG-positive/IgM-positive sera were tested using the IgG avidity assay (bioMérieux, Marcy l’Etoile, France). Using the toxoplasmosis IgG avidity assay, low IgG avidity was defined as an index value <0.200, equivocal IgG avidity was defined as 0.200≤ index <0.300, and high IgG avidity was defined as an index value ≥0.3. A high avidity test result using the IgG avidity assay excludes a recently acquired infection within 4 months of serum sampling. The blood group of each donor was confirmed using reverse blood grouping.
Real-time Polymerase Chain Reaction (PCR)
DNA from the whole blood samples was extracted using the QIAamp DNA Mini Kit (QIAGEN, Valencia, California, USA), according to the manufacturer’s protocol. A real-time PCR assay targeting the 529-bp repeat element (RE) of T. gondii was performed as published previously [14], with some modification. The cycling parameters were as follows: preheat at 95°C for 10 min, followed by 45 cycles at 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 15 seconds. Amplification was carried out with the CFX96 real-time PCR detection system (Bio-Rad Laboratories, Hercules, California, USA). The final reaction mix contained 12.5 µl 2× KAPA PROBE FAST qPCR Master Mix (KAPA Biosystems, Boston, USA), 400 nM of each primer, 800 nM of TaqMan probe, and 5 µl of template DNA in a 25 µl reaction volume. A sample was considered to be positive if the cycle threshold (Ct) value was <40.
Questionnaire
The questionnaire surveyed basic demographic data, including age, gender, education, residence, and occupation. Possible risk factors, including the sources of drinking water (running water, valley water, ground water, and bottled water), raw/undercooked meat (fish, lamb, beef, pork, oyster, clams, and mussels) consumption, raw vegetables consumption, animal contacts (cats, dogs), gardening or agriculture, prior blood transfusion, and living abroad, were also surveyed.
Statistical Analysis
The statistical analysis was performed using SPSS 18.0 software package (IBM, Armonk, New York, USA). P values less than 0.05 were considered statistically significant. Logistic regression was used to analyze the association between T. gondii seropositivity and potential risk factors. Multivariate logistic analysis was performed with the full model, including all potential risk factors in the analyses.
Results
Participants
A total of 1,783 healthy blood donors met the inclusion criteria and were enrolled in the study. Demographic characteristics of the 1,783 healthy blood donors are shown in Table 1 and Table S1. The mean age of the participants in this study was 38.1 years (median age 37 years; range 18 to 65 years). Most participants were male, aged 31–45 years, non-laborers (businessmen or employees), and had a college education or above.
Seroprevalence of Anti-T. gondii Antibodies
Of the 1,783 blood donors, 166 (9.3%) tested seropositive for anti-T. gondii IgG antibody, 161 (9.0%) donors tested seropositive for only IgG, and 5 (0.28%) tested seropositive for both IgM and IgG. None were positive for IgM antibody alone. The titers of anti-T. gondii IgG positive samples ranged from 9 to 685 IU/ml (median titers: 55.5 IU/ml). Five of the anti-T. gondii IgG and IgM positive donors had high avidity antibodies suggestive of prior infection.
By geographic region, anti-T. gondii IgG-seropositive rates in Taipei, Hsinchu, Taichung, Tainan, Kaohsiung, and Hualien were 9.1%, 8.4%, 8.3%, 8.8%, 6.4%, and 18.8% (Table 1). By gender, the anti-T. gondii IgG-seropositive rates were 10.1% and 8.0% in men and women (p = 0.131), respectively. The anti-T gondii IgG-seropositive rate was 7.9% among women of childbearing age (18–45 years). There was no significant difference in the prevalence among different age groups (p = 0.788).
Real Time PCR
A total of 1,783 blood samples were tested using the real-time PCR assays, and all of the blood samples revealed negative results. For comparison, we performed PCR-restriction fragment length polymorphism (PCR-RFLP) analyses of T. gondii DNA from the five clinical specimens (from symptomatic toxoplasmosis cases) stored in our laboratory. GRA6 marker showed that the T. gondii strains in two of the cases (P1 and P3) harbor the type I allele (Figure S1).
Risk Factors of being Anti-T gondii IgG-seropositive
In the univariate analysis, six variables were found to be associated with anti-T gondii IgG seropositivity, including donation place at Hualien, lower education level, consumption of valley water, consumption of undercooked pork meat, consumption of raw mussels, and having a cat in the household (Table 2). Other demographic and behavioral characteristics of the blood donors did not show an association with T. gondii infection. Further analysis using multivariate logistic regression revealed that the consumption of undercooked pork meat, the consumption of raw mussels, having a cat in the household, a lower education level, and donation place in eastern Taiwan were independent risk factors for Toxoplasma seropositivity (Table 3).
Table 2. Univariate analysis of the variables associated with T. gondii seroprevalence.
| Variable | Blood donor No. | Seropositivity(%) | Odds Ratio(95% Confidence interval) | p-value |
| Donation place | ||||
| Hualien | 170 | 18.8 | 2.6 (1.7–3.9) | <0.001* |
| Non-Hualien | 1,613 | 8.3 | 1.0 | |
| Gender | ||||
| Male | 1,127 | 10.1 | 1.3 (0.9–1.8) | 0.131 |
| Female | 654 | 8.0 | 1 | |
| Educational level | ||||
| High school and below | 638 | 12.2 | 1.7 (1.2–2.3) | 0.002* |
| College and above | 1,138 | 7.7 | 1.0 | |
| Blood group type | ||||
| O | 814 | 9.1 | 1.0 | |
| A | 492 | 9.1 | 1.0 (0.7–1.5) | 0.973 |
| B | 331 | 8.5 | 0.9 (0.6–1.5) | 0.734 |
| AB | 146 | 13.0 | 1.5 (0.9–2.6) | 0.142 |
| Valley water consumption | ||||
| Yes | 229 | 13.1 | 1.6 (1.03–2.4) | 0.036* |
| No | 1,553 | 8.8 | 1.0 | |
| Undercooked beef meat consumption | ||||
| Yes | 463 | 9.3 | 0.99 (0.7–1.4) | 0.974 |
| No | 1,317 | 9.3 | 1.0 | |
| Undercooked pork meat consumption | ||||
| Yes | 51 | 21.6 | 2.8 (1.4–5.6) | 0.003* |
| No | 1,728 | 8.9 | 1.0 | |
| Raw fish consumption | ||||
| Yes | 1,258 | 9.5 | 1.1 (0.7–1.5) | 0.763 |
| No | 522 | 9.0 | 1.0 | |
| Raw mussels consumption | ||||
| Yes | 14 | 28.6 | 4.0 (1.2–12.8) | 0.034* |
| No | 1,762 | 9.1 | 1.0 | |
| Uncooked vegetables consumption | ||||
| Yes | 1,287 | 9.7 | 1.2 (0.8–1.7) | 0.365 |
| No | 493 | 8.3 | 1.0 | |
| Cats in the neighborhood | ||||
| Yes | 451 | 10.0 | 1.1 (0. 8–1.6) | 0.564 |
| No | 1,313 | 9.1 | 1.0 | |
| Cat in the household | ||||
| Yes | 187 | 14.4 | 1.8 (1.1–2.8) | 0.012* |
| No | 1,593 | 8.7 | 1.0 | |
| Gardening or agriculture | ||||
| Yes | 649 | 10.9 | 1.4 (0.98–1.9) | 0.069 |
| No | 1,128 | 8.3 | 1.0 | |
| Blood transfusion | ||||
| Yes | 181 | 9.9 | 1.1 (0.7–1.8) | 0.765 |
| No | 1,597 | 9.3 | 1.0 | |
| Living abroad (>3 month) | ||||
| Yes | 78 | 2.6 | 1.0 | |
| No | 1,698 | 9.5 | 4.0 (0.98–16.5) | 0.054 |
Statistically significant.
Table 3. Multivariate logistic regression with full model for risk factors of T. gondii infection.
| Variable | Adjusted odds ratio(95% Confidence interval) | p-value |
| Gender | 1.3 (0.9–1.8) | 0.235 |
| Donation place (Hualien vs. Non-Hualien) | 2.5 (1.6–3.9) | <0.001* |
| Education: high school and below | 1.6 (1.1–2.3) | 0.007* |
| Blood group type (A vs. O) | 1.1 (0.7–1.6) | 0.802 |
| Blood group type (B vs. O) | 0.9 (0.6–1.5) | 0.688 |
| Blood group type (AB vs. O) | 1.6 (0.9–2.7) | 0.119 |
| Running water consumption | 1.2 (0.7–1.8) | 0.554 |
| Valley water consumption | 1.6 (1.0–2.7) | 0.052 |
| Ground water consumption | 1.0 (0.4–2.5) | 0.984 |
| Bottled water consumption | 0.9 (0.6–1.3) | 0.465 |
| Undercooked beef meat consumption | 0.8 (0.5–1.2) | 0.270 |
| Undercooked lambmeat consumption | 0.8 (0.3–2.6) | 0.759 |
| Undercooked pork meat consumption | 2.9 (1.3–6.5) | 0.011* |
| Raw fish consumption | 0.9 (0.6–1.3) | 0.510 |
| Raw oysters consumption | 1.3 (0.8–2.2) | 0.290 |
| Raw clams consumption | 1.0 (0.5–1.9) | 0.971 |
| Raw mussels consumption | 5.3 (1.5–19.1) | 0.010* |
| Uncooked vegetables consumption | 1.2 (0.8–1.8) | 0.404 |
| Cat in the household | 2.0 (1.2–3.2) | 0.004* |
| Cats in the neighborhood | 1.0 (0.7–1.4) | 0.814 |
| Gardening or agriculture | 1.2 (0.8–1.7) | 0.297 |
| Blood transfusion | 1.0 (0.6–1.8) | 0.873 |
| Living abroad (>3 month) | 0.2 (0.1–1.0) | 0.052 |
Statistically significant.
Discussion
This is the first systematic countrywide seroepidemiologic study on T. gondii infection among Taiwanese healthy blood donors, with a large sample size of 1,783 subjects to allow for good representativeness. We found an overall prevalence of T. gondii antibodies among the 1,783 blood donors of 9.3%. The seroprevalences in donors from northern, northwestern, central, southwestern, southern, and eastern region of Taiwan were 9.1%, 8.4%, 8.3%, 8.8%, 6.4%, and 18.8%, respectively. These results indicate that T. gondii infection exists in all major geographical regions in Taiwan, and that the seroprevalence is significantly higher in eastern Taiwan than in other regions.
Among the blood donors in this study, both the presence of a cat in the household and the consumption of undercooked pork meat are significant risk factors for T. gondii seropositivity. This indicates that both infection routes, namely the ingestion of oocysts (animal-to-human transmission) and the ingestion of tissue cysts in meat (foodborne transmission), existed in our study group, similar to the infection routes reported in other countries [15]–[18].
Compared to other countries, our overall seroprevalence was similar to the seroprevalence reported in Mexico [19] and Thailand [20]–[21] but lower than that reported in blood donors from countries including Brazil [22], Chile [23], Malaysia [24], India [25], Egypt [26], Czech Republic [27], and New Zealand [28], where seroprevalences varied from 20.3% to 75.0%. The relatively low overall seroprevalence in Taiwan is probably because the majority of the population in Taiwan eats well-cooked food.
In Taiwan, the general population of ethnic Han Chinese seldom consumes raw pork. Nevertheless, most aborigines have the traditional customs of eating the raw meat of wild pigs, rats, and goats. Previous studies indicated that seroprevalences of T. gondii ranged from 19.4% to 26.7% among mountain aboriginal people, and that eating raw meat seems to render individuals more susceptible to T. gondii infection [12]–[13]. In 2010, a case of neonate with congenital toxoplasmosis was notified to Taiwan CDC. Field investigation found that the aboriginal mother had eaten raw marinated pork at a gestational age of 24 weeks; the raw pork was highly suspected as the transmission source [29]. In the present study, we found that the prevalence of T. gondii seropositivity was significantly higher in the donors from eastern Taiwan (adjusted OR = 2.5; 95% CI: 1.6–3.9), a region with a high proportion of aboriginals among population. The donors from eastern Taiwan were also significantly more likely to report undercooked pork meat consumption (10/169 [5.9%] in eastern Taiwan versus 41/1610 [2.5%] in rest of other regions, p-value = 0.012), suggesting a higher proportion of aboriginal people. However, under the regulations of blood donating services, ethnic information could not be obtained. Therefore, we are unable to verify the association of aboriginal ethnicity and Toxoplasma seropositivity. The donation place in eastern Taiwan remains an independent significant risk after adjusting for the effect of pork consumption in multivariate analysis. Therefore, genetic composition of the population might also affect the risk of acquiring toxoplasmosis.
In addition to the association between consumption of raw meat and T. gondii infection, our study found that the consumption of raw mussels was also associated with seropositivity of T. gondii. Previous experimental studies indicated that T. gondii oocysts in seawater could survive long-term and remain infective for several months [30]. In the natural environment, viable oocysts can become concentrated by filter feeders and have been found in various species of shellfish [31]–[33]. Environmentally resistant oocysts that are shed in the feces of infected cats and passed through freshwater flow into the marine environment are considered a contamination source of mussels [34]. Studies in the United States also found that the consumption of raw oysters, clams, and mussels was a risk factor of Toxoplasma infection [17], [18].
Through analyzing the demographic characteristics of blood donors, our study found that the seropositivity to T. gondii decreased with increasing education level, similar to other reports [19]. We did not find a significant correlation between age or gender with T. gondii seroprevalence. The seropositive rate (7.9%) among female donors of childbearing age (aged 18–45) was similar to a previous hospital-based survey on pregnant women (9.1–10.2%) [10]–[11]. Thus, up to 92.1% of childbearing women in Taiwan have not yet been infected and could be at risk to acquire T. gondii infection. Furthermore, we found that women of childbearing age are more likely to raise cats in the household than older women (61/454 [13%] women of childbearing age versus 14/200 [7%] older women, p-value = 0.017). Our results suggest that, to prevent congenital toxoplasmosis, health education targeted toward women of childbearing age may be required to minimize the risk of animal-to-human transmission during prenatal and gestation periods.
Moreover, blood transfusion is another potential transmission route for Toxoplasma infection. Some studies suggest that toxoplasmosis transmitted through blood transfusion could lead to serious clinical consequences in immunocompromised, immunosuppressed patients and multiple blood transfusion recipients [2], [7]. However, routine screening for T. gondii in blood and blood products is not mandatory in Taiwan. In our study, five of our blood donors had IgM antibodies against T. gondii and a high avidity index. Therefore, cases with an acute phase of infection could be excluded. Furthermore, the absence of parasitemia by real-time PCR within our blood donors might also exclude the possibility of transmission through blood transfusion.
In Caucasian population, the carriers of AB group are the most susceptible to T. gondii infection [35]. In our study, Taiwanese people of blood group AB also had a higher T. gondii seroprevalence than people of blood group O. However, the difference was not statistically significant (Table 2 and Table 3).
Conclusion
We found that T. gondii infection among tested healthy blood donors, as evidence by positive anti-Toxoplasmosis IgG antibodies, exists in all major regions in Taiwan, with an overall seroprevalence of 9.3%. The consumption of raw pork, which is more common in eastern Taiwan, the consumption of raw mussels, and exposure to domestic cats are most likely the major transmission routes of T. gondii among the participants in our study.
As compared to previous studies that focused on specific groups and small-scale samples, this study used countrywide survey data to explore the epidemiology of T. gondii in Taiwan and could provide important information on the seroprevalence and potential risk factors for T. gondii transmission. However, ethnic information and subjects aged less than 18 years old could not be included in the present study due to ethical regulations. Further studies are needed to focus on these under-surveyed groups, and more surveillance efforts are required for this neglected disease.
Supporting Information
PCR-restriction fragment length polymorphism (PCR-RFLP) analyses with GRA6 marker. M: DNA molecular marker; P1–P5: patient1 to patient 5; RH88: reference strain from ATCC.
(TIF)
Characteristics of male vs. female blood donors.
(DOC)
Acknowledgments
The authors gratefully acknowledge the supporting six district blood centers of Taiwan Blood Services Foundation for specimen collection and Dr. Edward Guy for helpful suggestions.
Funding Statement
This work was supported by a grant (DOH100-DC-2029) from the Centers for Disease Control, Department of Health, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hill DE, Chirukandoth S, Dubey JP (2005) Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev 6: 41–61. [DOI] [PubMed] [Google Scholar]
- 2. Montoya JG, Liesenfeld O (2004) Toxoplasmosis. Lancet 363: 1965–1976. [DOI] [PubMed] [Google Scholar]
- 3. Pappas G, Roussos N, Falagas ME (2009) Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 39: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 4. Sukthana Y (2006) Toxoplasmosis: beyond animals to humans. Trends Parasitol 22: 137–142. [DOI] [PubMed] [Google Scholar]
- 5. Robert-Gangneux F, Darde ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25: 264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remington JS, Klein JO (2001) Infectious diseases of the fetus and newborn infant. Philadelphia: Saunders. Xiv.
- 7. Siegel SE, Lunde MN, Gelderman AH, Halterman RH, Brown JA, et al. (1971) Transmission of toxoplasmosis by leukocyte transfusion. Blood 37: 388–394. [PubMed] [Google Scholar]
- 8. Derouin F, Pelloux H (2008) Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect 14: 1089–1101. [DOI] [PubMed] [Google Scholar]
- 9. Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, et al. (2005) Prevalence of Toxoplasma gondii infection and incidence of toxoplasma encephalitis in non-haemophiliac HIV-1-infected adults in Taiwan. Int J STD AIDS 16: 302–306. [DOI] [PubMed] [Google Scholar]
- 10. Yu JC (1985) [A seroepidemiological study on Toxoplasma gondii infection among pregnant women and neonates in Taiwan]. Taiwan Yi Xue Hui Za Zhi 84: 286–295. [PubMed] [Google Scholar]
- 11. Hu IJ, Chen PC, Su FC, Hsieh CJ, Jeng SF, et al. (2006) Perinatal toxoplasmosis, northern Taiwan. Emerg Infect Dis 12: 1460–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan CK, Su KE, Chung WC, Tsai YJ, Chiou HY, et al. (1998) Seroprevalence of Toxoplasma gondii antibodies among Atayal aboriginal people and their hunting dogs in northeastern Taiwan. Jpn J Med Sci Biol 51: 35–42. [DOI] [PubMed] [Google Scholar]
- 13. Fan CK, Su KE, Wu GH, Chiou HY (2002) Seroepidemiology of Toxoplasma gondii infection among two mountain aboriginal populations and Southeast Asian laborers in Taiwan. J Parasitol 88: 411–414. [DOI] [PubMed] [Google Scholar]
- 14. Edvinsson B, Lappalainen M, Evengard B (2006) Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Clin Microbiol Infect 12: 131–136. [DOI] [PubMed] [Google Scholar]
- 15. Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, et al. (2000) Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis. BMJ 321: 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baril L, Ancelle T, Goulet V, Thulliez P, Tirard-Fleury V, et al. (1999) Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scand J Infect Dis 31: 305–309. [DOI] [PubMed] [Google Scholar]
- 17. Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, et al. (2001) Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol 154: 357–365. [DOI] [PubMed] [Google Scholar]
- 18. Jones JL, Dargelas V, Roberts J, Press C, Remington JS, et al. (2009) Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 49: 878–884. [DOI] [PubMed] [Google Scholar]
- 19. Alvarado-Esquivel C, Mercado-Suarez MF, Rodriguez-Briones A, Fallad-Torres L, Ayala-Ayala JO, et al. (2007) Seroepidemiology of infection with Toxoplasma gondii in healthy blood donors of Durango, Mexico. BMC Infect Dis 7: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinlaor S, Ieamviteevanich K, Pinlaor P, Maleewong W, Pipitgool V (2000) Seroprevalence of specific total immunoglobulin (Ig), IgG and IgM antibodies to Toxoplasma gondii in blood donors from Loei Province, Northeast Thailand. Southeast Asian J Trop Med Public Health 31: 123–127. [PubMed] [Google Scholar]
- 21. Sukthana Y, Chintana T, Supathanapong W, Siripanth C, Lekkla A, et al. (2000) Prevalence of toxoplasmosis in selected populations in Thailand. J Trop Med Parasitol 23: 53–58. [Google Scholar]
- 22. Coelho RA, Kobayashi M, Carvalho LB Jr (2003) Prevalence of IgG antibodies specific to Toxoplasma gondii among blood donors in Recife, Northeast Brazil. Rev Inst Med Trop Sao Paulo 45: 229–231. [DOI] [PubMed] [Google Scholar]
- 23. Zamorano CG, Contreras MC, Villalobos S, Sandoval L, Salinas P (1999) [Seroepidemiological survey of human toxoplasmosis in Osorno, Region X, Chile, 1998]. Bol Chil Parasitol 54: 33–36. [PubMed] [Google Scholar]
- 24. Nissapatorn V, Kamarulzaman A, Init I, Tan LH, Rohela M, et al. (2002) Seroepidemiology of toxoplasmosis among HIV-infected patients and healthy blood donors. Med J Malaysia 57: 304–310. [PubMed] [Google Scholar]
- 25. Elhence P, Agarwal P, Prasad KN, Chaudhary RK (2010) Seroprevalence of Toxoplasma gondii antibodies in North Indian blood donors: implications for transfusion transmissible toxoplasmosis. Transfus Apher Sci 43: 37–40. [DOI] [PubMed] [Google Scholar]
- 26. Elsheikha HM, Azab MS, Abousamra NK, Rahbar MH, Elghannam DM, et al. (2009) Seroprevalence of and risk factors for Toxoplasma gondii antibodies among asymptomatic blood donors in Egypt. Parasitol Res 104: 1471–1476. [DOI] [PubMed] [Google Scholar]
- 27. Svobodova V, Literak I (1998) Prevalence of IgM and IgG antibodies to Toxoplasma gondii in blood donors in the Czech Republic. Eur J Epidemiol 14: 803–805. [DOI] [PubMed] [Google Scholar]
- 28. Zarkovic A, McMurray C, Deva N, Ghosh S, Whitley D, et al. (2007) Seropositivity rates for Bartonella henselae, Toxocara canis and Toxoplasma gondii in New Zealand blood donors. Clin Experiment Ophthalmol 35: 131–134. [DOI] [PubMed] [Google Scholar]
- 29. Chiang TY, Ji DD (2011) Epidemics of toxoplasmosis in Taiwan. Taiwan Epidemiol Bull 27: 353–360. [Google Scholar]
- 30. Lindsay DS, Dubey JP (2009) Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J Parasitol 95: 1019–1020. [DOI] [PubMed] [Google Scholar]
- 31. Esmerini PO, Gennari SM, Pena HF (2010) Analysis of marine bivalve shellfish from the fish market in Santos city, Sao Paulo state, Brazil, for Toxoplasma gondii . Vet Parasitol 170: 8–13. [DOI] [PubMed] [Google Scholar]
- 32. Miller MA, Miller WA, Conrad PA, James ER, Melli AC, et al. (2008) Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int J Parasitol 38: 1319–1328. [DOI] [PubMed] [Google Scholar]
- 33. Putignani L, Mancinelli L, Del Chierico F, Menichella D, Adlerstein D, et al. (2011) Investigation of Toxoplasma gondii presence in farmed shellfish by nested-PCR and real-time PCR fluorescent amplicon generation assay (FLAG). Exp Parasitol 127: 409–417. [DOI] [PubMed] [Google Scholar]
- 34. Dubey JP (2004) Toxoplasmosis - a waterborne zoonosis. Vet Parasitol 126: 57–72. [DOI] [PubMed] [Google Scholar]
- 35. Kolbekova P, Kourbatova E, Novotna M, Kodym P, Flegr J (2007) New and old risk-factors for Toxoplasma gondii infection: prospective cross-sectional study among military personnel in the Czech Republic. Clin Microbiol Infect 13: 1012–1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR-restriction fragment length polymorphism (PCR-RFLP) analyses with GRA6 marker. M: DNA molecular marker; P1–P5: patient1 to patient 5; RH88: reference strain from ATCC.
(TIF)
Characteristics of male vs. female blood donors.
(DOC)