Abstract
Background
The prevalence of isolated hearing loss (HL) associated with the m.3243A>G mutation is unknown. The aim of this study was to assess the frequency and heteroplasmy level of the m.3243A>G mutation in a large group of Polish patients with postlingual bilateral sensorineural HL of unidentified cause.
Methodology/Principal Findings
A molecular search was undertaken in the archival blood DNA of 1482 unrelated patients with isolated HL that had begun at ages between 5 and 40 years. Maternal relatives of the probands were subsequently investigated and all carriers underwent audiological tests. The m.3243A>G mutation was found in 16 of 1482 probands (an incidence of 1.08%) and 18 family members. Of these 34 individuals, hearing impairment was detected in 29 patients and the mean onset of HL was at 26 years. Some 42% of the identified m.3243A>G carriers did not develop multisystem symptomatology over the following 10 years. Mean heteroplasmy level of m.3243A>G was lowest in blood at a level of 14% and highest in urine at 58%. These values were independent of the manifested clinical severity of the disease.
Conclusions
A single m.3243A>G carrier can usually be found among each 100 individuals who have postlingual hearing loss of unknown cause. Urine samples are best for detecting the m.3243A>G mutation and diagnosing mitochondrially inherited hearing loss.
Introduction
Postlingual hearing loss (HL) occurs in 85–95% of patients with multisystem syndromes associated with an m.3243A>G mutation in the tRNAleu (UUR) gene [1]–[4]. In the MELAS phenotype (mitochondrial encephalomyopathy lactic acidosis stroke-like episodes) [5], [6], HL is the fourth most frequent of 17 symptoms [7]; in the MIDD phenotype (mitochondrially inherited diabetes-deafness syndrome), hearing impairment is usually the second most-frequent symptom [8], [9]. The origin of the mitochondrial sensorineural defect may be cochlear [3], [10] or retro-cochlear if there is central neurological involvement [11]. Major findings in the cochleas of subjects with the m.3243A>G mutation are degeneration and decreased cochlear neurons [12], fewer outer hair cells and endolymphatic sac cells [13], and atrophy of stria vascularis [12]–[14].
Although the prevalence of the most frequent m.3243A>G tRNA leucine (UUR) transition (MTTL1) in the general population is known [13], [15]–[17], and the occurrence of mitochondrial disease associated with various mtDNA mutations is estimated as more than 1 in 5,000 [18], knowledge of the frequency of m.3243A>G mutation associated with isolated hearing impairment is limited [19]–[23] since usually, only m.1555A>G mitochondrial 12S rRNA mutation, and a few other specific mtDNA mutations associated with aminoglycoside susceptibility, are tested in patients with nonsyndromic HL [24]–[26].
Methods
Objectives
The aim of the study was to assess the frequency and heteroplasmy level of the m.3243A>G mutation in a large group of Polish patients with postlingual bilateral sensorineural HL of unidentified cause.
Patients
The study group was recruited from a cohort of 7000 individuals who consulted the Genetics Department of the Institute of Physiology and Pathology of Hearing (the Polish national referral centre) between the years 2000 and 2010. Only cases with bilateral HL, starting at an age between 5 and 40 years, and with available archived DNA sample, were included in the study. Patients were excluded if they had an established cause of hearing impairment, had syndromic HL of known genetic origin, or had two mutations in the connexin genes GJB2 and GJB6 (homozygosity or compound heterozygosity status) [27], [28]. In total, 1482 archived blood DNA samples were made available for the m.3243A>G mutation search.
The data retrieved from medical reports of the recruited patients included: (1) the age of the patient, (2) gender, (3) age of HL onset (defined as the age at which the patient or his/her parents first became aware of hearing impairment), (4) the result of the first audiological examination based on pure tone audiometry (PTA), (5) the degree of HL (defined as the arithmetic mean of the frequencies 0.5, 1, 2, and 4 kHz for the better-hearing ear), (6) whether the HL was progressive or stable (determined by comparing the mean hearing threshold of each patient's initial and last audiogram), (7) the presence of additional symptoms of dysfunction of the hearing pathway like tinnitus and/or vertigo, (8) other health problems of the patient, and (9) the family history of hearing impairment, the basic health status of maternal relatives with hearing problems, and sudden deaths in the family. Characteristics of the study group are shown in Table 1.
Table 1. Characteristics of the study group: all examined patients with hearing loss (HL), and the 34 patients identified as having the m.3243A>G mutation.
| Studied patients | m.3243A>G mutation carriers | |
| Number | 1482 | 34* |
| Males | 649 (44%) | 13 (38%) |
| Females | 833 (56%) | 21 (62%) |
| Mean age at the study (years) | 5–62, mean 27.0 | 6–61, mean 31.0 |
| Mean age of onset of HL (years) | 5–40, mean 15; SD = 10.4 | 8–63, mean 261 SD = 13.7; N = 29*** |
| Profound degree of hearing loss** | 3.8% | 3.4% |
| Severe degree of hearing loss** | 6.6% | 13.8% |
| Moderate degree of hearing loss** | 43.6% | 51.8% |
| Mild degree of hearing loss** | 46% | 31% |
| Normal hearing | 0 | 5 |
| Other dysfunction of the inner ear (tinnitus/vertigo) | 399/103; (27%/7%) | 12/5; (35%/15%) |
| Progression of hearing loss during observation | ND | 14 |
| Positive family history of hearing loss | 528 (35.6%) | 33 (97.0%)2 |
| Development of additional multi-organ 3243A>G pathology | ND | 17 |
| Number of 35delG GJB2 heterozygotes | 80 (5.5%) | 1/16 (6.3%) |
16 probands and 18 relatives.
Arithmetic mean of 0.5, 1, 2 and 4 kHz for the better-hearing ear.
N = number of patients; 29 of 34 had HL; 5 subjects (aged 6–20) had normal hearing.
p<0.000001 vs patients without m.3243A>G (t-test).
p<0.00001.
ND = no data.
Clinical and audiological status of the carriers of the m.3243A>G mutation were re-assessed, and they underwent genetic counselling. Maternal relatives of the m.3243A>G probands were also invited for audiological and genetic consultation. Severity of mitochondrial disease was assessed according to the Newcastle Mitochondrial Diseases Adult Scale (NMDAS) [29].
Clinical and laboratory investigations
Hearing levels were determined by pure tone audiometry. Evaluation of the degree of hearing loss was based on ANSI (American National Standards Institute) and ISO (International Standards Organization) standards. On these scales, mild hearing loss is that up to 40 dB HL, moderate is 41–70 dB HL, severe is 71–90 dB HL, and profound is more than 90 dB HL. Additional hearing tests – otoacoustic emissions, speech audiometry, tympanometry with stapedial reflexes, and brainstem evoked auditory potentials (BEAP) – were conducted to identify the character of the hearing loss (sensorineural hearing loss SNHL of cochlear origin, retro-cochlear, or conductive).
Cerebral alterations and brain lactates were assessed by magnetic resonance imaging with magnetic resonance spectroscopy (Siemens 3T). Organic acids excreted in urine were analysed using gas chromatography-mass spectroscopy (GC-MS).
Additional samples for the heteroplasmy study (urine sediment, nails, hair follicles, and buccal mucosa smear) were collected from individuals with confirmed or suspected m.3243A>G mutation and who gave signed consent.
A search for the m.3243A>G mutation was performed using TaqMan Assay on Demand from Applied Biosystems, Foster City, CA, according to the manufacturer's instructions. All positive samples were directly sequenced to confirm the presence of the mutation using primers M_F and M_R without 5′JOE modification (Table 2). The heteroplasmy level was assessed by PCR-RFLP using the primers and ApaI (Fermentas, Lithuania) enzyme (the m.3243A>G mutation creates a restriction site for this enzyme). Digested products were analyzed on an ABI 3130 capillary sequencer. Finally, by analysing the area under the peak the mutated/non-mutated DNA ratio was assessed.
Table 2. The sequences of the primers M_F and M_R.
| Primer | Sequence | modification |
| M_F | 5′CCTCCCTGTACGAAAGGACA3′ | none |
| M_R | 5′AGGAGTAGGAGGTTGGCCATGG 3′ | 5′ JOE |
Ethics statement
The bioethical commission of the Institute of Physiology and Pathology of Hearing approved the study and all subjects or their guardians gave signed consent.
Statistical analysis
Comparisons of mean onset of HL among m.3243A>G carriers versus other patients were done using a student t-test for independent samples. Comparison of heteroplasmy levels in different tissues was performed by a student t-test for dependent samples. Correlation between total disease score and heteroplasmy levels was performed by linear regression. All analyses were done using the Statistica software package.
Results
Prevalence of m.3243A>G
Our study of 1482 archival DNA samples of HL patients revealed m.3243A>G mutation in 16 cases (1.08%) indicating that on average the m.3243A>G mutation was found in one of 92 examined patients with isolated HL that had begun between the ages of 5 and 40 years.
In comparison with the whole studied HL group, patients with the m.3243A>G mutation had similar age, gender, degree of HL, and frequency of symptoms or other dysfunctions of the inner ear. The major features of the subgroup positive for m.3243A>G were maternal inheritance in all families (except one) and relatively later onset of HL in the m.3243A>G subgroup (Table 1).
The total of 34 identified individuals carrying the m.3243A>G mutation included 16 probands and 18 of their relatives detected at the second step by molecular investigation. Presence of the m.3243A>G mutation was not previously tested nor suspected in any of these cases.
The study group consisted of 29 patients with m.3243A>G HL (16 probands and 13 relatives). Five m.3243A>G relatives (children and young females) without hearing problems, but testing positively for the m.3243A>G mutation, were analysed separately.
Over the course of 10 years of observations, 17 of 29 HL patients developed multi-system mitochondriopathy while 12 remained oligosymptomatic. Clinical characteristics of the studied patients are shown in Table 3.
Table 3. Characteristics of the patients carrying the m.3243A>G mutation patients 1–17 with multi-organ presentation (subgroup I), patients 18–29 with isolated hearing loss (subgroup II), and asymptomatic carriers 30–34 (subgroup III).
| Patient number | Gen der | Age (yrs) | Age of onset of HL (yrs) | HL severity | HL progression | Disease severity (NMDAS scale) | Organic acids profile in urine (GC-MS) | Increased lactates in brain (MRS) | Brain MRI | Symptoms and onset | Remarks |
| 1 | F | 20 | 19 | mild | Yes | 7 | NP | NP | NP | RP (20), SS | Aminogly* |
| 2 | F | 43 | 32 | mild | No | 15 | MGCA | 2 | IMGP | M (40), RP (43) | Episode of blindness lasting 2 days |
| 3 | M | 12 | 8 | mild | No | 55 | NP | NP (died) | NP (died) | H (8), M (8), N (8), S (11) | Died at age 12 |
| 4 | F | 38 | 35 | moderate | Yes | 19 | MGCA LA | 2 | MCA IMGP | H (35), N (37), SS | Pancreatitis* |
| 5 | F | 41 | 10 | moderate | ND | 20 (part 2 only) | NP | NP | NP | DM (15), H (40) | |
| 6 | M | 13 | 9 | moderate | No | 81 | NP | NP | NP | H (9), M (9), N (9), S (9), RP (10) | |
| 7 | M | 29 | 24 | moderate | Yes | 26 | NA | NP (pacemaker) | NP | C (24), M (24), SS | Stress * |
| 8 | M | 27 | 20 | moderate | ND | 33 (part 2 only) | NP | NP (died) | NP | M (21), N (21), S (27), SS | Died at age 27 |
| 9 | M | 42 | 32 | moderate | No | 33 | NA | 1 | MCA | DM (38), K (40), RP (42), I | Impaired spermatogenesis at biopsy, end-stage renal failure |
| 10 | M | 52 | 40 | moderate | No | 77 | MGCA | 3 | MCA Focal lesion after S in occ-temp-parietal area. IMGP | M (40), N (40), RP (45), S (45), C (45), DM (46), H (50), I, SS | Impaired spermatogenesis at biopsy |
| 11 | F | 16 | 8 | severe | Yes | 38 | NA | 2 | Diffuse bilateral lesions after S in frontal, occ-temp-parietal areas, insular cortex. IMGP MCA | N (8), S (8) | Stress * |
| 12 | F | 47 | 37 | severe | Yes | 14 | MGCA | 2 | MCA IMGP | C (40), M (45), I | Aminogly* |
| 13 | F | 72 | 47 | severe | No | 74 | NP | NP | NP | H (47), M (47), N (47), C (50), DM (50) | |
| 14 | M | 37 | 19 | severe | Yes | 4 | NA | 1 | MCA IMGP | SS | Noise* |
| 15 | F | 28 | 19 | profound | Yes | 31 | LA | NP (CI) | NP | N (25), M (25) | Aminogly * |
| 16 | F | 38 | 22 | profound | Yes | 33 | NA | 2 | MCA IMGP | H (30), DM (35), K (36), M (38), SS | End-stage renal failure. Stress * |
| 17 | F | 51 | 17 | profound | Yes | 99 | NP | NP (CI) | NP | H (20), C (40), DM (40), N (40), RP (40), S (40), M (49), I, SS | Aminogly * |
| 18 | F | 18 | 17 | mild | No | 6 | NP | 1 | NA | - | |
| 19 | F | 24 | 10 | mild | No | 1 | NP | NP | NP | - | |
| 20 | F | 24 | 23 | mild | No | 1 | NA | 1 | NA | - | |
| 21 | F | 35 | 34 | mild | Yes | 4 | NA | 1 | IMGP | - | Stress* |
| 22 | F | 49 | 48 | mild | Yes | 12 | MGCA | 0 | IMGP | - | Stress* |
| 23 | M | 29 | 9 | mild | No | 1 | NA | 0 | NA | - | |
| 24 | F | 78 | 63 | moderate | No | 34 | NA | 1 | Focal spread vascuar lesions of white matter (non-specific). | - | NMDAS score probably false positive, connected with advanced age |
| 25 | M | 32 | 29 | moderate | Yes | 8 | NA | 1 | - | Noise * | |
| 26 | M | 33 | 33 | moderate | Yes | 11 | NP | NP | NP | - | Stress* |
| 27 | M | 51 | 20 | moderate | ND | NP | NP | NP | NP | - | |
| 28 | F | 49 | 42 | severe | No | 25 (13 of part I - QoL) | NA | 1 | MCA | - | |
| 29 | F | 61 | 25 | profound | Yes | 7 | NA | NP (CI) | NP | - | Pregnancy * |
| 30 | F | 5 | No HL | - | No HL | 11 | NA | NP | NP | - | Hair heteroplasmy 20.4% |
| 31 | F | 10 | No HL | - | No HL | 24 (15 of part I - QoL) | NA | NP | NP | - | Buccal heteroplasmy 100% |
| 32 | F | 10 | No HL | - | No HL | 0 | MGCA | 0 | NA | - | |
| 33 | F | 19 | No HL | - | No HL | 5 | LA | 1 | IMGP | - | |
| 34 | M | 8 | No HL | - | No HL | 0 | NA | 1 | NA | - | Hair heteroplasmy 63.7% |
Probands are shown in bold.
GC-MS = gas chromatography–mass spectroscopy; NMDAS = Newcastle Mitochondrial Disease Adult Scale; MGCA = 3-methylglutaconic aciduria; LA = lactic aciduria; HL = hearing loss; NP = not performed; NA = normal value; QoL = quality of life; CI = cochlear implant user; IMGP = increased mineralisation of globus pallidus; MCA = minimal cerebellar atrophy; MRS = magnetic resonance spectroscopy; MRS score: 0 = negative LA, 1 = uncertain LA, 2 = positive LA, 3 = strong LA.
C = cardiomyopathy; DM = diabetes mellitus; H = migraine; I = infertility; K = renal insufficiency; M = myopathy; N = peripheral neuropathy; RP = pigmentary degeneration of retina; S = stroke-like episodes; SS = short stature.
No HL = normal hearing.
Stress = hearing deterioration following stressful event; Aminogly = hearing deterioration following aminoglycoside administration; Noise = hearing deterioration following noise exposure; Pancreatitis = hearing deterioration following acute pancreatitis; Pregnancy = hearing deterioration after pregnancy.
According to the clinical status, the m.3243A>G carriers were divided into three subgroups:
Multi-symptomatic subgroup (17 patients)
Isolated hearing loss subgroup (12 patients)
Asymptomatic subgroup (5 individuals).
Audiological assessment
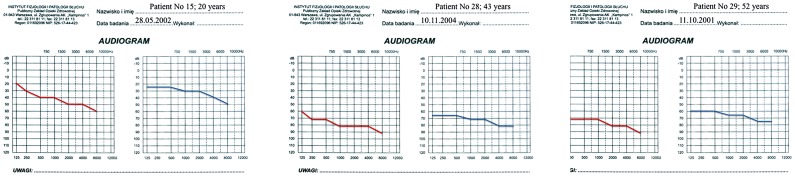
Bilateral sensorineural hearing loss was the first presentation of the disease in all m.3243A>G patients (Figure 1). The mean age of onset of hearing impairment was 26 years: 18 years for men and 28 years for women (Table 4). HL started earlier in the multi-symptomatic subgroup (21 years) than in the isolated HL subgroup (32 years).
Figure 1. Audiograms of patients 15, 28, and 29 when first diagnosed with isolated hearing impairment.
Table 4. Heteroplasmy levels (% of total tissue DNA) of m.3243A>G mutation in mtDNA samples isolated from: urinary sediment, hair follicles, buccal mucosa, nails and blood (put in order of heteroplasmy intensity).
| No of patients in group; mean age | Females/Males | Gender-age of onset (mean age of onset in group) | No of studied samples of urinary sediment; Mean %; (Range %) | No of studied samples of hair follicles; Mean %; (Range %) | No of studied samples of buccal mucosa smear; Mean %; (Range %) | No of studied samples of nails; Mean %; (Range %) | No of studied samples of blood; Mean %; (Range %) |
| I group (17) 36 yrs | 10/7 | F–24 yrs; M–16 yrs (21 yrs) | 13; 67%; (31.4%–96.5%) | 13; 21.2%; (0%–77.2%) | 11; 26.1%; (5.6%–60.2%) | 12; 26.4%; (6.7%–65.2%) | 14; 14,4%; (5,4%–27,8%) |
| II group (12) 40 yrs | 8/4 | F–37 yrs; M–23 yrs (32 yrs) | 11; 51.5%; (7.6%–85.8%) | 8; 25.8%; (1.6%–63.7%) | 10; 21.0%; (1.2%–33.2%) | 9; 14.2%; (1.8%–24.6%) | 12; 9,3%; (0%–32,1%) |
| III group (5) 8 yrs | 4/1 | NA | 3; 39.5%; (16.2%–81.3%) | 4; 44.8%; (20.4%–66.5%) | 5; 35.9%; (3.8%–100%) | 4; 34.1%; (16.7%–49.6%) | 5; 21,1%; (12,8%–32,5%) |
| Total 34 patients 33 yrs | 22/12 | F–28 yrs; M–18 yrs (26 yrs) | 27; 57.6%; (7.6%–96.5%) | 26; 26.5% (0%–77.2%) | 26; 26.0%; (1.2%–100%) | 25; 23.2%; (1.8%–65.2%) | 31; 13,5%; (0%–32,5%) |
F = female, M = male, yrs = years, NA = not applicable.
The subgroups of patients: I – multi-organ presentation, II - isolated hearing loss and III - asymptomatic are presented separately.
Progression of the HL was observed in 14 out of 29 (48%) patients: in 10 women and 4 men (Table 3). The proportion of patients with a progressive course of HL was similar in both multi-symptomatic and isolated HL subgroups (Table 5).
Table 5. Comparison of clinical status among all groups of patients (I, II and III) carrying m.3423A>G mutation: I = Multi-symptomatic subgroup, II = Isolated HL subgroup, III = Asymptomatic subgroup.
| No of patients in groups | Mild degree of HL | Moderate degree of HL | Severe degree of HL | Profound degree of HL | Progression of HL | Mean value of NMDAS score (parts 1–3) | Normal value/Examined (GC-MS) | LA/MGCA (GC-MS) | 0 = no brain LA (MRS) | 1 = uncertain brain LA (MRS) | 2 = positive brain LA (MRS) | 3 = strong brain LA (MRS) | Brain MRI alterations/Examined | Heteroplasmy level in urine (%) | Heteroplasmy level in blood (%) |
| I (17) | 3 | 7 | 4 | 3 | 53% 9/17 | 39* | 5/10 | 2/4 | 0 | 2 | 5 | 1 | 8/8 | 67 | 14 |
| II (12) | 5 | 4 | 2 | 1 | 42% 5/12 | 10** | 7/8 | 0/1 | 2 | 6 | 0 | 0 | 4/8 | 51 | 9 |
| III (5) | NA | NA | NA | NA | NA | 8*** | 3/5 | 1/1 | 1 | 2 | 0 | 0 | 1/3 | 40 | 31 |
| AL (34) | 8 | 11 | 6 | 4 | 14/29 | 15/23 | 3/6 | 3 | 10 | 5 | 1 | 13/19 | 53 | 18 |
2 patients had only part 2 assessed.
11/12 patients were assessed.
probably false positive related to high score for quality of life (part 1) of two patients.
HL = hearing loss; NMDAS = Newcastle Mitochondrial Disease Adult Scale; MRS = magnetic resonance spectroscopy,
LA = lactic acidosis, MGCA = 3-methylglutaconic aciduria, MRI = magnetic resonance imaging; NA = not applicable.
GC-MS = Gas Chromatography–Mass Spectroscopy.
Degree of HL: mild HL<40 dB; moderate 41–70 dB; severe 71–90 dB; profound >91 dB.
Hearing deterioration was triggered by stressful situations (6 times), medications (aminoglycosides, 4 times), noise (2 times), or other major events such as acute pancreatitis or pregnancy (Table 3) with similar frequency in both the multi-symptomatic and isolated HL subgroups.
The majority of audiograms showed a pantonal shape with high frequencies more severely affected (Figures 1, 2, and 3). BEAP did not show retro-cochlear pathology in any case and always confirmed audiometric hearing thresholds. Otoacoustic emissions were absent in all HL patients. Type A tympanograms and stapedial reflexes were registered in all ears (excluding severe and profound HL).
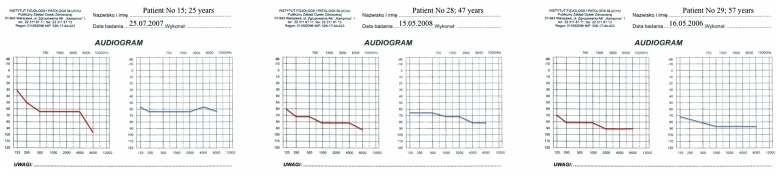
Figure 2. Subsequent audiograms of the patients presented in Fig. 1 .
Patient 15 has developed multisystem disorder; patients 28 and 29 have remained oligosymptomatic. Hearing loss has not progressed in patient 28.
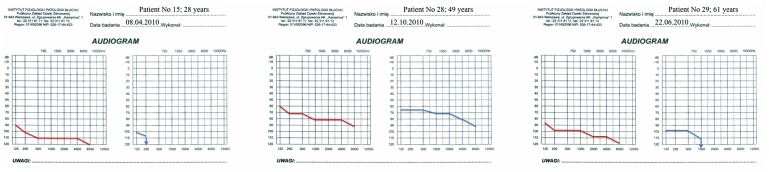
Figure 3. Latest audiograms of the three patients shown in Figs. 1 and 2 .
Patients 15 and 29 have become cochlear implant users. The HL threshold of patient 28 has still not changed.
Heteroplasmy study
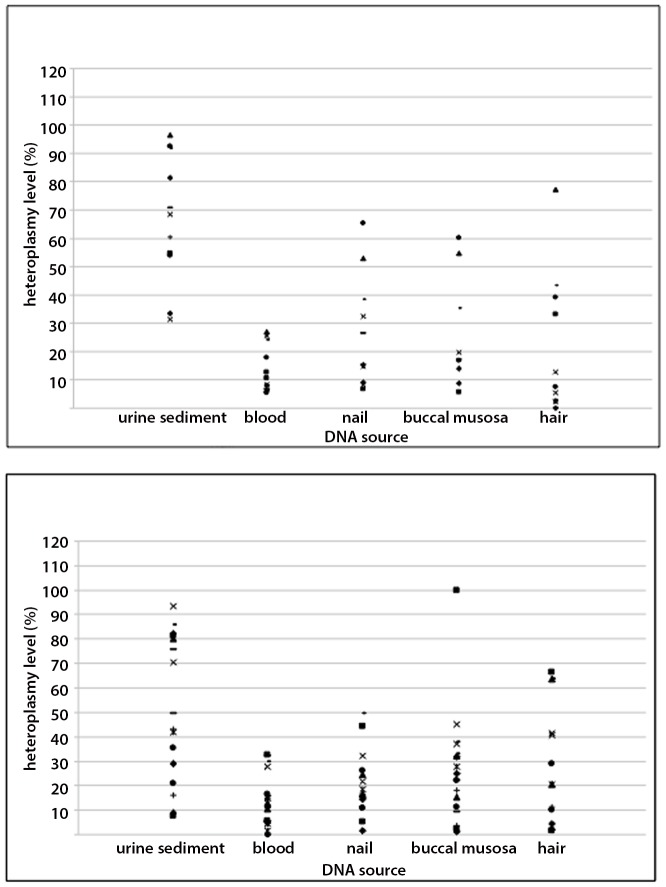
The levels of heteroplasmy in the whole m.3243A>G group decreased in the following order: urine, hair, buccal mucosa, nails, and blood (Table 4). The mean level of heteroplasmy detected in urine (57.6%) was significantly higher (p<10−6) than in any other tested tissue, whereas blood heteroplasmy levels were significantly lower compared to other tissues (p<0.002, Table 4). Notably, the most pronounced mean difference (44.1%) was found in a comparison between urine and blood (p<10−7). Analysis of heteroplasmy levels among other tissues did not reveal significant differences (data not shown). Mean heteroplasmy load was relatively higher in the multi-systemic subgroup than in the isolated HL and asymptomatic subgroups (Table 4), but the differences were relatively small. The patients from the isolated HL and the asymptomatic subgroups showed low as well as high mtDNA load in all tissues studied (Figure 4).
Figure 4. Heteroplasmy level of the m.3243A>G mutation in examined tissues.
Above: the scores of patients with multi-symptomatic presentation (subgroup I). Below: the scores of patients with isolated hearing loss and asymptomatic carriers (subgroups II and III together).
After adjustment for age we detected a relatively weak correlation between total disease score and heteroplasmy level in urine (p = 0.034) or nails (p = 0.015), but the distribution of m.3243A>G mutation load among tissues and individuals did not allow clinically useful prediction of disease severity. There was no relation between kidney disease in the patients 9 and 16 (Table 3) and level of urine heteroplasmy [30].
Brain imaging and lactates
Magnetic resonance imaging (MRI) was abnormal in 13 of 19 examined patients (Table 5). The most frequent changes were cerebellar atrophy and increased mineralisation of the globus pallidus. Chronic and/or acute stroke-like changes were seen in two cases. Cerebral abnormalities were found in MRI, mainly in the multi-symptomatic subgroup (in all examined patients), but also in the four patients from the isolated HL and one patient from the asymptomatic subgroups. Details of the MRI findings are given in Table 3.
Magnetic resonance spectroscopy (MRS) of the brain showed increased lactate signal in 6 out of 19 examined patients (Table 5). The pathology was seen only in the multi-symptomatic subgroup. The lactate signal was absent in three MRS scans, and was uncertain in 10 patients (Table 3). Profiling of urinary organic acids revealed an increased excretion of lactic acid in three patients and/or 3-methylglutaconic acid (3-MGCA) in six patients (Table 3).
Discussion
In this hearing loss-oriented study, we found that about 1% of Polish patients presenting with isolated HL that had begun between the ages of 5 and 40 years harboured the m.3243A>G mutation in their blood DNA. This figure is lower than ∼2.4% reported for Japanese HI patients [20], [31], [32], but similar to that reported in a diabetic Caucasian cohort [33] and 3 times less than the prevalence of m.1555A>G mutation (3.4%) reported for HL patients [34]. A similar hearing loss study was carried out by Majamaa et al. who found m.3243A>G mutation in 2.1% of 250 HL patients by applying pre-selection based on maternal inheritance [16].
The association between isolated HL and m.3243A>G is consistent with the data of Manwaring et al. who studied hair follicle mtDNA in 900 elderly individuals and found six m.3243A>G carriers, all having mild to moderate hearing impairment [35]. Uimonen et al. showed that isolated HL associated with m.3243A>G mutation resembled presbyacusis, but its progression rate was faster [22].
The m.3243A>G HL in our study was not always progressive and remained the only complaint in 42% of patients (Table 3). Mitochondrial etiology of long-standing hearing loss in a 78 year-old woman was established after development of m.3243A>G associated MELAS phenotype in her son. Molecular testing for m.3243A>G mutation was positive in at least four young adults with stable HL which had lasted more than 10 years. Data on the non-progressive course of m.3243A>G induced long-lasting HL is limited. In the key paper of Uimonen et al., the progressive pattern was the most common, with high frequencies affected first [22]. A relationship between HL progression and severity of m.3243A>G phenotype has been found [23]. This suggests that a low progression rate of hearing impairment is a good prognostic marker.
The m.3243A>G positive subgroup differed from the whole study group by having relatively later onset of HL, as well as presenting maternal inheritance of HL in all except one case. However, at the first presentation of HL in m.3243A>G carriers, the risk of developing multisystem disease cannot be predicted.
Analysis of circumstances of hearing deterioration in m.3243A>G mutation revealed various triggering factors, some of which have been suggested previously, such as exposure to aminoglycosides, valproates, noise exposure, or stress [36], [37]. Other triggers, such as pregnancy or pancreatitis, have not been convincingly reported in the literature.
In this study the onset of m.3243A>G associated HL was earlier in men than in women (18 and 28 years, respectively). Uimonen et al. found that being male, as well as a high heteroplasmy level of the mutation, may aggravate the severity of hearing impairment in m.3243A>G carriers [22]. More epidemiological data are needed to properly assess a sex influence on the m.3243A>G phenotype.
In our patients the m.3243A>G mutation load was lowest in the DNA derived from archived blood samples compared to other analysed tissues. It has previously been found that the mtDNA mutation load in blood decreases in older patients due to the negative selection of leukocytes carrying impaired mitochondria [38], [39]; because of this, significantly better detection of m.3243A>G can be achieved by analysing urine [40], buccal mucosa, or hair [35], [36], [41], [42].
In this study, the heteroplasmy level of m.3243A>G mutation was markedly (2–4 times) higher in DNA derived from urine compared to other tissues. Interestingly, the urinary heteroplasmy of over 30% was found not only in the classical MELAS patients, but also in the oligosymptomatic and even asymptomatic m.3243A>G persons.
Our results are consistent with those of Whittaker et al. who showed that urine is the most suitable material for detection of the m.3243A>G mutation. The authors concluded that the screening of urine for m.3243A>G mutation load is a better predictor of outcome than the gold standard of skeletal muscle [40].
Short stature [43], abnormal excretion of 3-MGCA in urine [44], [45], and elevation of lactates in the brain MRS, which were seen in our oligo- and asymptomatic m.3243A>G carriers, may be helpful in identifying affected individuals, but these are not sensitive tests either.
Summarizing
People with postlingual isolated HL require testing for m.3243A>G mutation. Early detection of patients with mitochondrial isolated HL and identification of mutation-carrying healthy relatives would improve proper prophylactic management – i.e. avoidance of harmful environmental factors (noise and stress) and certain medications (aminoglicosides and valproate). New therapies may become available in the near future. Urinary sediment – not blood – is the material of choice for assessing the mtDNA mutation load in people with isolated hearing loss. The possible prognostic value of high and low levels of mtDNA heteroplasmy in urine sediment needs further study.
Funding Statement
The study was supported by grant from the Polish Ministry of Science and High Level Education NN 403 130 636, NN 407 118 939 and Polish mitochondrial network MITONET.pl. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No current external funding sources for this study.
References
- 1. Michaelides M, Jenkins SA, Bamiou DE, Sweeney MG, Davis MB, et al. (2008) Macular dystrophy associated with the A3243G, mitochondrial DNA mutation: distinct retinal and associated features, disease variability, and characterization of asymptomatic family members. Arch Ophthalmol 126: 320–28. [DOI] [PubMed] [Google Scholar]
- 2. Deschauer M, Muller T, Wieser T, Schulte-Mattler W, Kornhuber M, et al. (2001) Hearing impairment is common in various phenotypes of mitochondrial DNA A3243G mutation. Arch Neurol 58: 1885–8. [DOI] [PubMed] [Google Scholar]
- 3. Damian MS, Siebel P, Reichmann H (1995) Clinical spectrum of the MELAS mutation. Acta Neurol Scand 92: 409–15. [DOI] [PubMed] [Google Scholar]
- 4. Mosewich RK, Donat R, DiMauro S (1993) The syndrome of mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes presenting without stroke. Arch Neurol 50: 275–8. [DOI] [PubMed] [Google Scholar]
- 5. Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP (1984) Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke like episodes: a distinctive clinical syndrome. Ann Neurol 16: 481–8. [DOI] [PubMed] [Google Scholar]
- 6. Goto Y, Nonaka I, Horai S (1990) A mutation in the tRNA leu (UUR) gene associated with the MELAS subgroup of mitochondrial encephalopaties. Nature 348: 651–3. [DOI] [PubMed] [Google Scholar]
- 7. Chinnery PF, Howell N, Lightowlers RN, Turnbull DM (1997) Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain 120: 1713–21. [DOI] [PubMed] [Google Scholar]
- 8. Narbonne H, Paquis-Fluckinger V, Valero R, Heyries L, Pellissier JF, et al. (2004) Gastrointestinal tract symptoms in maternally inherited diabetes and deafness (MIDD). Diabetes Metab 30: 61–66. [DOI] [PubMed] [Google Scholar]
- 9. van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, et al. (1992) Mutation in mitochondrial tRNA(Leu) (UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet 1: 368–71. [DOI] [PubMed] [Google Scholar]
- 10.Chinnery PF, Griffiths TS (2006) Mitochondrial otology. In: DiMauro S., Hirano M., Schon EA. Mitochondrial Medicine Informa UK Limited, pp.161–77.
- 11. Sawada S, Takeda T, Kakigi A, Saito H, Suehiro T, et al. (1997) Audiological findings of sensorineural deafness associated with a mutation in the mitochondrial DNA. Am J Otol 18: 332–5. [PubMed] [Google Scholar]
- 12. Nadol JB, Merchant SN (2001) Histopathology and molecular genetics of hearing loss in the human. Int J Pediatr Otorhinolaryngol 61: 1–15. [DOI] [PubMed] [Google Scholar]
- 13. Uusimaa J, Moilanen JS, Vainionpaa L, Tapanainen P, Lindholm P, et al. (2007) Prevalence, segregation, and phenotype of the mitochondrial DNA 3243A>G mutation in children. Ann Neurol 62: 278–87. [DOI] [PubMed] [Google Scholar]
- 14.Merchant SN (2010) Genetically determined and other developmental defects. In: Schuknecht's Pathology of the Ear, 3th Edition PMPH-USA, 209 pp.
- 15. Schaefer A, Taylor R, Turnbull DM, Chinnery PF (2004) The epidemiology of mitochondrial disorders - past, present and future. Bioch Biophys Acta 1659: 115–20. [DOI] [PubMed] [Google Scholar]
- 16. Majamaa K, Moilanen J, Uimonen S, Remes A, Salmela P, et al. (1998) Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and stroke like episodes: prevalence of the mutation in an adult population. Am J Hum Genet 63: 447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elliot HR, Samuels DC, Eden JA, Relton CL, Chinnery PF (2008) Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet 8: 254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chinnery PF, Wardell TM, Singh-Kler R, Hayes C, Brown DT, et al. (2000) The epidemiology of pathogenic mitochondrial DNA mutation. Ann Neurol 48: 188–93. [PubMed] [Google Scholar]
- 19. Leveque M, Marlin S, Jonard L, Procaccio V, Reynier P, et al. (2007) Whole mitochondrial genome screening in maternally inherited non-syndromic hearing impairment using a microarray resequencing mitochondrial DNA chip. Eur J Hum Genet 15: 1145–55. [DOI] [PubMed] [Google Scholar]
- 20. Kato T, Nishigaki Y, Noguchi Y, Ueno H, Hosoya H, et al. (2010) Extensive and rapid screening for major mitochondrial DNA point mutations in patients with hereditary hearing loss. J Hum Genet 55: 147–54. [DOI] [PubMed] [Google Scholar]
- 21. Sue CM, Quigley A, Katsabanis S, Kapsa R, Crimmins DS, et al. (1998) Detection of MELAS A3243G point mutation in muscle, blood and hair follicle. J Neurol Sci 161: 36–39. [DOI] [PubMed] [Google Scholar]
- 22. Uimonen S, Moilanen JS, Sorri M, Hassinen IE, Majamaa KS (2001) Hearing impairment in patients with 3243A>G mtDNA mutation: phenotype and rate of progression. Hum Genet 108: 284–9. [DOI] [PubMed] [Google Scholar]
- 23. Majamaa-Voltti KA, Winqvist S, Remes AM, Tolonen U, Pyhtinen, et al (2006) A 3-year clinical follow-up of adult patients with 3243 A>G in mitochondrial. DNA Neurology 66: 1470–75. [DOI] [PubMed] [Google Scholar]
- 24. Kokotas H, Petersem MB, Wilems PJ (2007) Mitochondrial deafness. Clin Genet 71: 379–91. [DOI] [PubMed] [Google Scholar]
- 25. Guan M-X (2011) Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion 11: 237–45. [DOI] [PubMed] [Google Scholar]
- 26. Rydzanicz M, Cywińska K, Wróbel M, Pollak A, Gawęcki W, et al. (2011) The contribution of the mitochondrial COI/tRNA(Ser(UCN)) gene mutations to non-syndromic and aminoglycoside-induced hearing loss in Polish patients. Mol Genet Metab Sep–Oct;104 1–2:153–9. [DOI] [PubMed] [Google Scholar]
- 27. Pollak A, Mueller-Malesinska M, Skórka A, Kostrzewa G, Ołdak M, et al. (2008) GJB2 and hearing impairment: promoter defects do not explain the excess of monoallelic mutations. J Med Genet 45 9:607–8. [DOI] [PubMed] [Google Scholar]
- 28. Pollak A, Skórka A, Mueller-Malesińska M, Kostrzewa G, Kisiel B, et al. (2007) M34T and V37I mutations in GJB2 associated hearing impairment: evidence for pathogenicity and reduced penetrance. Am J Med Genet A.1;143A 21:2534–43. [DOI] [PubMed] [Google Scholar]
- 29. Schaefer AM, Phoenix C, Elson JL, McFarland R, Chinnery PF, et al. (2006) Mitochondrial disease in adults: A scale to monitor progression and treatment. Neurology 66: 1932–4. [DOI] [PubMed] [Google Scholar]
- 30. Dinour D, Mini S, Polak-Charcon S, Lotan D, Holtzman J (2004) Progressive nephropathy associated with mitochondrial tRNA gene mutation. Clin Nephrol 62: 149–54. [DOI] [PubMed] [Google Scholar]
- 31. Oshima T, Ueda N, Ikeda K, Abe K, Takasaka T (1999) Hearing loss with a mitochondrial gene mutation is highly prevalent in Japan. Laryngoscope 109: 334–8. [DOI] [PubMed] [Google Scholar]
- 32. Usami Si, Abe S, Akita J, Namba A, Shinkawa H, et al. (2000) Prevalence of mitochondrial gene mutations among hearing impaired patients. J Med Genet 37: 38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malecki M, Klupa T, Wanic K, Frey J, Cyganek K, et al. (2001) Searching for mitochondrial A3243G tRNALeu mutation in Polish patients with type 2 diabetes mellitus. Med Sci Monit 7: 249–50. [PubMed] [Google Scholar]
- 34. Rydzanicz M, Wróbel M, Pollak A, Gawecki W, Brauze D, et al. (2010) Mutation analysis of mitochondrial 12S rRNA gene in Polish patients with non-syndromic and aminoglycoside-induced hearing loss. Biochem Biophys Res Commun 395: 116–21. [DOI] [PubMed] [Google Scholar]
- 35. Manwaring N, Jones MM, Wang JJ, Rochtchina E, Howard C, et al. (2007) Population prevalence of the MELAS A3243G mutation. Mitochondrion 7: 230–33. [DOI] [PubMed] [Google Scholar]
- 36. Finsterer J (2007) Genetic, pathogenetic, and phenotypic implications of the mitochondrial A3243G tRNALeu (UUR) mutation. Acta Neurol Scand 116: 1–14. [DOI] [PubMed] [Google Scholar]
- 37. Chinnery PF, Elliott C, Green GR, Rees A, Coulthard A, et al. (2000) The spectrum of hearing loss due to mitochondrial DNA defects. Brain 123: 82–92. [DOI] [PubMed] [Google Scholar]
- 38. Sue CM, Lipsett LJ, Crimmins DS, Tsang C, Boyages SC, et al. (1998) Cochlear origin of sensorineural deafness in MELAS syndrome. Ann Neurol 43: 350–59. [DOI] [PubMed] [Google Scholar]
- 39. Shanske S, Pancrudo J, Kaufmann P, Engelstad K, Jhung S, et al. (2004) Varying loads of the mitochondrial DNA A3243G mutation in different tissues: implication for diagnosis. Am J Med Genet 130: 134–7. [DOI] [PubMed] [Google Scholar]
- 40. Whittaker RG, Blackwood JK, Alston CL, Blakely EL, Elson JL, et al. (2009) Urine heteroplasmy is the best predictor of clinical outcome in the m.3243A>G mtDNA mutation. Neurology 72: 568–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manwaring N, Jones MM, Wang JJ, Rochtchina E, Howard C, et al. (2007) Mitochondrial DNA haplogroups and age-related hearing loss. Arch. Otolaryngol. Head Neck Surg 133: 929–33. [DOI] [PubMed] [Google Scholar]
- 42. Narbonne H, Perucca-Lostanlen D, Desnuelle C, Vialettes B, Saunieres A, et al. (2001) Searching for A3243G mitochondrial DNA mutation in buccal mucosa in order to improve the screening of patients with mitochondrial diabetes. Eur J Endocrinol 145: 541–2. [DOI] [PubMed] [Google Scholar]
- 43. Pronicki M, Sykut-Cegielska J, Mierzewska H, Tońska K, Iwanicka K, et al. (2002) Diversity of clinical symptoms in A3243G mitochondrial DNA mutation (MELAS syndrome mutation). Med Sci Monit 8: 767–73. [PubMed] [Google Scholar]
- 44. Wortmann SB, Kluijtmans LA, Engelke UF, Wevers RA, Morava E (2010) The 3-methylglutaconic acidurias: what's new? J Inherit Metab Dis on line DOI 10.1007/s10545-010-9210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karkucinska-Wieckowska A, Lebiedzinska M, Jurkiewicz E, Pajdowska M, Trubicka J, et al. (2011) Increased reactive oxygen species (ROS) production and low catalase level in fibroblasts of a girl with MEGDEL association (Leigh syndrome, deafness, 3-methylglutaconic aciduria). Folia Neuropathol 49: 56–63. [PubMed] [Google Scholar]