Abstract
Chicken eggs are the main source of human Salmonella enterica serovar Enteritidis infection. S. Enteritidis infects the oviduct and ovary of the chicken leading to infection of developing eggs. Therefore, control in poultry production is a major public health priority. Vaccination of hens has proved successful in control strategies in United Kingdom leading to a 70% drop in human cases since introduced. However, as hens reach sexual maturity they become immunosuppressed and it has been postulated this leads to increased susceptibility to Salmonella infection. In this study we define the changes to the systemic and reproductive tract-associated immune system of hens throughout sexual development by flow cytometry and histology and determine changes in susceptibility to experimental S. Enteritidis challenge in naive and vaccinated hens. Changes to both systemic and local immune systems occur in chickens at sexual development around 140 days of age. The population of several leukocyte classes drop, with the greatest fall in CD4+ lymphocyte numbers. Within the developing reproductive tract there an organised structure of lymphocytic aggregates with γδ-T lymphocytes associated with the mucosa. At point-of-lay, this organised structure disappears and only scattered lymphocytes remain. Protection against Salmonella challenge is significantly reduced in vaccinated birds at point-of-lay, coinciding with the drop in CD4+ lymphocytes. Susceptibility to reproductive tract infection by Salmonella increased in vaccinated and naïve animals at 140 and 148 days of age. We hypothesise that the drop in γδ-T lymphocytes in the tract leads to decreased innate protection of the mucosa to infection. These findings indicate that systemic and local changes to the immune system increase the susceptibility of hens to S. Enteritidis infection. The loss of protective immunity in vaccinated birds demonstrates that Salmonella control should not rely on vaccination alone, but as part of an integrated control strategy including biosecurity and improved animal welfare.
Introduction
Salmonella enterica serovar Enteritidis is the most common cause of human non-typhoidal salmonellosis in Europe and North America and is frequently associated with consumption of infected eggs. The public health and economic impact of egg infection is illustrated by the recall of over 500 million potentially contaminated eggs during an outbreak in the USA in August 2010 [1]. Egg contamination with S. Enteritidis is a consequence of its ability to colonise the chicken reproductive tract leading to transovarian vertical transmission to both eggs and chicks [2]–[5]. It is considered that infection of the ovary by Salmonella leads to yolk contamination, infection of the upper parts of the oviduct prior to the shell-gland (infundibulum and magnum) lead to contamination of the albumen or egg white and faecal contamination of the egg surface may occur after laying [6]. Tropism for the reproductive tract in S. enterica is largely confined to S. Enteritidis and the genetically similar avian-adapted serovar S. Pullorum and as such transovarian transmission is largely confined to these serovars [4], [5], [7]. The mechanisms that allow S. Enteritidis to colonise the avian reproductive tract are complex and multi factorial but include adhesins such as fimbriae that facilitate attachment to the reproductive mucosa [8]–[10]. Immunological changes in the host may also underlie infection of the reproductive tract and previously we have shown that suppression of cell-mediated immunity at point-of-lay in hens is a critical factor in reproductive tract infection by S. Pullorum [11]. Infection with S. Pullorum is characterised by a persistent systemic carrier state with reproductive tract infection occurring as part of a recrudescent infection at point-of-lay [7]. It has been postulated that immunosuppression in production hens may lead to reproductive tract infection with Salmonella carried systemically or in the gastrointestinal tract, or that Salmonella bacteria within the environment of the poultry house may utilise this immunosuppressive event as a ‘window of opportunity’ to infect hens.
Cost-effective control of S. Enteritidis is a major concern both to maintaining public health and for the poultry industry that operates on marginal costs. Within Europe, most notably the United Kingdom, changes in legislation and voluntary control schemes have led to the successful reduction in S. Enteritidis in eggs and as a consequence in reducing human cases from over 22000 confirmed cases in 1997 in England and Wales to fewer than 3000 by 2010 [12]. The fall in cases of S. Enteritidis Phage Type 4, responsible for the majority of egg-associated infection in the UK in the 1980s and 1990s, was even more marked, with a drop from over 15000 cases in 1992 to 459 in 2010. Central to the control strategy was the introduction of vaccination for laying hens in 1998. Initially vaccination was through intramuscular delivery of inactivated killed vaccines, although this has been largely superseded by the use of live attenuated vaccines that are both more effective and can be delivered through drinking water thereby reducing labor costs [13]. Vaccination has been successful to the extent that cases of salmonellosis linked to consumption of eggs produced commercially in the UK are rare. However, not all countries in Europe permit the use of live Salmonella vaccines, and there is considerable pressure to extend vaccine withdrawal periods prior to the point-of-lay. It is also unclear to what extent immunosuppression at point-of-lay effects protection afforded by vaccination. In the UK, the introduction of a voluntary code of practice of which vaccination was a component (Lion Mark Scheme) and subsequently legislation through the National Control Plans for Salmonella has also led to considerable improvements in biosecurity, hygiene and husbandry practice in the poultry industry that complement vaccination and as such it is impossible to determine whether vaccination alone would have had such an impact on the control of Salmonella in eggs.
In this study we aimed to determine the systemic and local changes in the reproductive tract immune system that underlie immunosuppression at point-of-lay and whether these have an effect on susceptibility to S. Enteritidis infection in naive or vaccinated chickens. Relatively little is known about the immunological structure and particularly adaptive responses in the healthy or infected avian reproductive tract other than basic descriptive histology. We set out to investigate the changes in cell populations and structure and cytokine repertoire expressed in the hen ovary and oviduct, along with changes in the circulating T lymphocyte populations. We then determined whether these changes correspond to increased susceptibility to Salmonella infection. Understanding susceptibility to Salmonella and any deficiency in vaccine protection at point-of-lay will allow development of more effective vaccination and other control strategies, reducing the risk of S. Enteritidis infection and subsequent transmission to eggs.
Materials and Methods
Ethics Statement
All work was conducted in accordance with UK legislation governing experimental animals under project licence PPL 40/3063 and was approved by the University of Liverpool ethical review process prior to the award of the licence. All animals were checked a minimum of twice daily to ensure their health and welfare.
Experimental animals
1-day old female Lohmann Brown Egg laying Chicks were obtained from a commercial hatchery. Birds were vaccinated against Marek's Disease Virus in the hatchery, but not against any other pathogen. Chicks were maintained in floor pens and given ad libitum access to water and a vegetable protein based diet (SDS, Witham, Essex UK). Chicks were maintained at a temperature of 30°C until 21 days of age then lowered to 20°C.
Determining Immunological changes at point-of-lay
45 chicks were housed as described above. At 32, 60, 77, 102, 124, 132, 140, 148 and 165 days of age, five birds were killed by neck dislocation and immediately dissected. Spleens were removed and processed individually for flow cytometry and histology as described below. At 102 days of age onwards, ovarian tissue and tissue from three regions of the oviduct: isthmus, magnum and uterus were taken for histology or frozen in RNAlater (Life Technologies, Paisley, UK) for analysis of cytokine expression. Breast muscle was taken as a control tissue for comparison.
Analysis of changes to systemic immune system by flow cytometry
Splenic tissue taken from individual birds at post mortem examination was placed into 5 ml Dulbecco's Modified Essential Media containing 5% foetal calf serum (Life Technologies, Paisley, UK). Cell suspensions were prepared by passing tissue through a 40 μM cell strainer (Beckton Dickinson, Oxford, UK,). The suspension was overlaid onto 10 ml room temperature Histopaque 1083 and spun at 220×g for 10 minutes. The buffy coat layer was then removed and resupended into FACS Buffer (PBS with 1% BSA and 0.005% sodium azide). Cells were counted in a haemocytometer and adjusted to 106 cells/ml prior to staining.
For antibody staining, 106 cells in a volume of 1 ml were pelleted in a microcentruge for 3 ml. Supernatant was removed and 2 μl of primary antibody added and the pellet resuspended in residual buffer. The following antibodies were used: mouse anti-chicken CD4, CD8α, CD8β, MHC Class II, TCRγδ, the B lymphocyte marker Bu-1 and the monocyte-macrophage marker KUL01 (Southern Biotech, Birmingham, Alabama, USA) along with an IgG1 isotype control, clone MOPC 21 (Sigma, Poole, UK) as an isotype control. Cells were then incubated at room temperature for 10 minutes. 1 ml of FACS buffer was added, the tube inverted to mix and cells pelleted in a microcentrifuge and supernatant removed. This step was repeated to wash the primary antibody. After washing, 2 μl of FITC- conjugated rabbit anti-mouse IgG (AbD Serotec Cambridge, UK) was added and incubated in the dark at room temperature for 10 minutes. The cells were then washed as described above and resuspended in 600 μl of FACS buffer containing 1% paraformaldehyde and then stored at 4°C until analysed. Cells were analysed on a Becton Dickinson FACScalibur flow cytometer. Analysis of cells populations was performed using CellQuestPro (Beckton Dickinson) and WinMDI 2.8 (www.http://facs.scrips.edu/software.html) software.
Immunhistochemistry
Tissue samples were embedded in OCT, snap frozen, cut into 8 μm sections on a cryostat and stained with mouse anti-chicken monoclonal antibodies (Southern Biotech,) to CD4, CD8α, CD8β, γδ T cell receptor, MHC Class II and the macrophage marker KUL01. Antibody concentrations and incubation conditions were based on those described by WIthanage et al [14], [15]. Visualisation was determined by use of the Vectastain Elite ABC kit containing biotinylated anti-mouse IgG, avidin and biotinylated horseradish peroxidase. The complex was then visualised with the NovaRED substrate kit (Vector Labs, Peterborough, Cambs, UK). Sections were examined and photographed using a NIKON Eclipse 80i microscope. Analysis of cell populations was determined by the methods described by WIthanage et al [15], [16].
Cytokine expression in the reproductive tract
Expression of the key cytokines IL-2, IL-4, IL-6, IL-12, IFN-γ and the chemokines CXCLi2 and CXCLi4 were determined in oviduct and ovarian tissue by qRT-PCR as described previously [7], [15]. Breast muscle tissue was taken as a negative control as it considered to have no cytokine expression. RNA was isolated from tissue samples using RNeasy mini kits (Qiagen, Crawley, Sussex. UK). Samples stored in RNAlater were removed and placed into the lysis buffer provided and homogenised using a TissueLyser (Qiagen). RNA was then isolated according to manufacturer's instructions. RNA levels between samples was normalised based on expression on 28S rRNA and expression data presented as a 40-Ct value minus the mean Ct value for breast muscle [7].
S. Enteritidis challenge in vaccinated and naive animals
52 one-day old commercial female egg-laying chicks were housed in two equal sized groups under the conditions described above. One group were vaccinated at 8, 41 and 95 days of age with a commercial live vaccine (Avipro SE, Lohmann Animal Health, Cuxhaven, Germany) according to manufacturer's instructions. At 77, 102, 132, 140 and 148 days of age 5 chickens from each group were housed separately and infected intravenously via the wing vein with 106 cfu of S. Enteritidis 125109 PT4 [4], [17] in a volume of 0.2 ml Luria Bertani Broth. At four days post challenge, birds were killed by neck dislocation. At post mortem examination spleen and liver samples were aseptically collected for quantitative bacteriology with counts determined on Brilliant Green Agar (Oxoid) using previously described methods [17]. The reproductive tract was removed and ovary and oviduct tissue samples from upper (infundibulum) and mid (magnum) of the oviduct were collected and pooled from each individual bird, incubated overnight with Selenite Broth and then plated onto Brilliant Green Agar to detect presence or absence of Salmonella [17]. Developing eggs in the oviduct or ones laid by birds after challenge were collected into jars containing Selenite Broth for detection of Salmonella as previously described [18].
Statistical analysis
Statistical analysis was performed using SPSS v.20 (IBM). Comparison between cell populations was made by ANOVA. For comparison of bacterial load between infected groups through the Kruskal-Wallis test, an equivalent non-parametric test to ANOVA, was used as the data was not distributed normally.
Results
Changes in leucocyte populations at point-of-lay
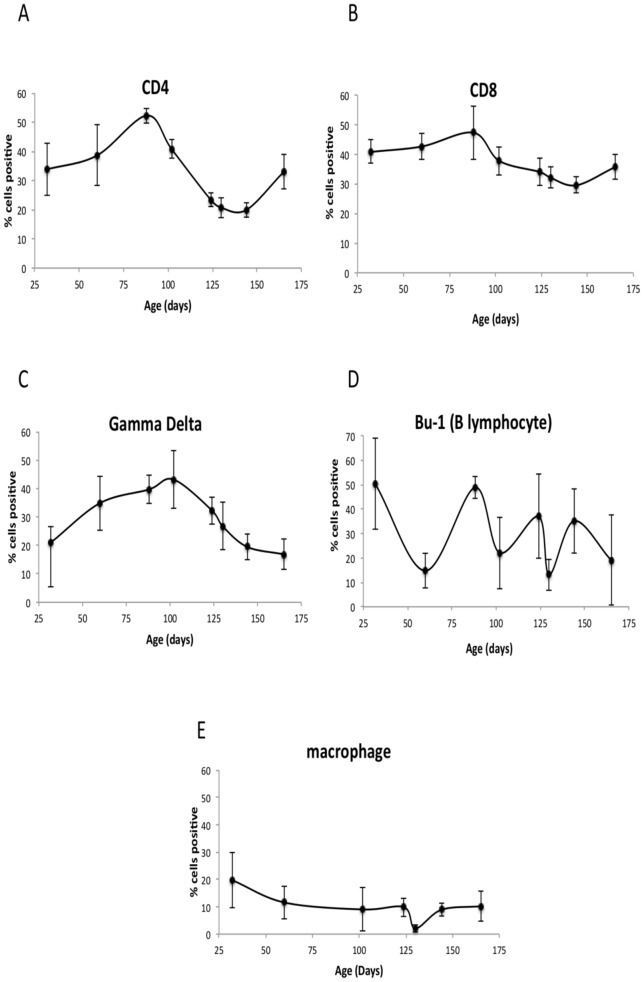
As birds move towards sexual maturity at 130 to 140 days of age there is a decline in T lymphocyte populations in the spleen (Fig. 1a, 1b, 1c, whereas there is no consistent change in macrophage or B lymphocyte populations (Figure 1d, 1e). Comparison of the population at 88 or 102 days of age to that at 130 days of age shows a significant falls in both CD4+ (Fig. 1a) and γδ T cell (Fig. 1b) populations (P = <0.001). CD8+ populations (CD8α and CD8β combined) (Fig. 1c) also show a decline between 102 and 130 days of age, though this is not statistically significant (P = 0.062). The decline in these lymphocyte populations matches the start of the egg-laying period (typically 125–140 days of age in commercial layers). At 165 days of age when hens are fully productive there is a significant recovery in CD4+ populations from the levels at 130 days (P = 0.03). However, γδ levels remain low as birds mature. The B lymphocyte population was considerably variable from bird-to-bird and between time points though there was no discernable change in population.
Figure 1. Changes in splenic mononuclear leucocyte populations through development of and onset of sexual maturity in commercial laying hens determined by flow cytometry following specific single color staining with monoclonal antibodies to major chicken cell markers.
Fig. 1A shows the CD4+ population which falls significantly between 102 and 130 days of age as does the γδ population (Figure 1C). Figure 1B shows the CD8+ population (data for CD8α and CD8β combined), Figure 1D shows B lymphocyte (Bu-1) and Figure 1E macrophage (KUL01) stained populations. Data are shown as means of the percentage of positive cells (± SD). Data shown are based on five birds per time point.
Cellular populations and changes in the chicken reproductive tract
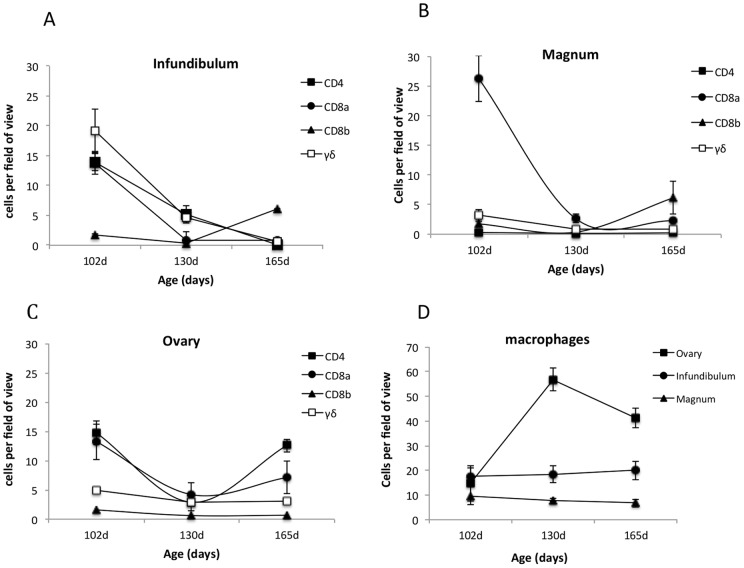
Leucocyte populations in the reproductive tract show considerable changes in numbers and organisation through the onset of sexual maturity (Figure 2). In the developing oviduct of the immature hen at 105 days of age, T lymphocytes are organised into aggregates and scattered in small numbers throughout the infundibulum (upper) (Figure 2a) and magnum (central) oviduct (Figure 2b), although there are more CD4+ cells in the infundibulum and CD8a+ cells in the magnum. Both are numerous in ovarian tissue (Fig. 2c). As birds approach point-of-lay at 130 days of age, lymphoid aggregates disappear with lower overall numbers of lymphocytes scattered through the reproductive tract (Figure 3). Significantly, there is a large population of γδ T lymphocytes in the infundibulum of the developing oviduct that declines at sexual maturity. Although numbers are fewer in the magnum and ovary, a similar decline is seen. By 165 days of age there is an increase in lymphocyte numbers from the levels at 130 days of age throughout the reproductive tract with the ovary showing the greatest increase in lymphocyte numbers. The exception being γδ T lymphocytes which remain at low levels. In contrast, there is little change in macrophage populations in the oviduct throughout sexual development, although there is an increase in macrophages in ovary towards at point-of-lay (Fig. 2d). Staining for MHC Class II that are neither stained by macrophage or B lymphocyte markers also reveals an organised structure of cells below the epithelium that may represent an organised population of antigen presenting cells in the reproductive that have not previously described.
Figure 2. Changes in cell populations in the chicken reproductive tract as determined by immunohistological examination.
2A shows T lymphocyte populations in the infundibulum (upper oviduct), 2B in the magnum (mid oviduct) and 2Cthe ovary. Figure 2D shows the changes in macrophage populations in the ovary and oviduct. Data are based on 10 fields of view for two sections of each tissue from each animal. Between five to seven birds were examined at each time point. Data are given as a mean (±SD).
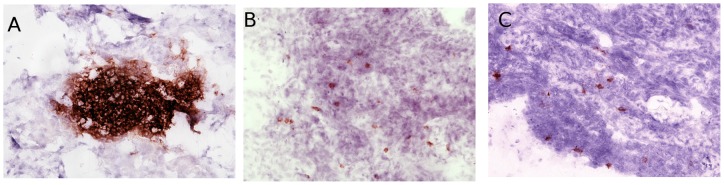
Figure 3. Changes to CD4+ lymphocyte populations in the reproductive tract.
At 102 days of age CD4+ populations are primarily organised into aggregates in the ovary (Figure 3A) and oviduct. Birds lack lymph nodes and form more loose lymphoid structures. At 130 days (Figure 3B) these structures are no longer found, with smaller populations of cells found throughout the ovary. At 165 days (Figure 3C) CD4+ numbers increase slightly, but remain scattered throughout the tissue. Photomicrographs taken at a magnification of ×400.
Expression of cytokines in the reproductive tract
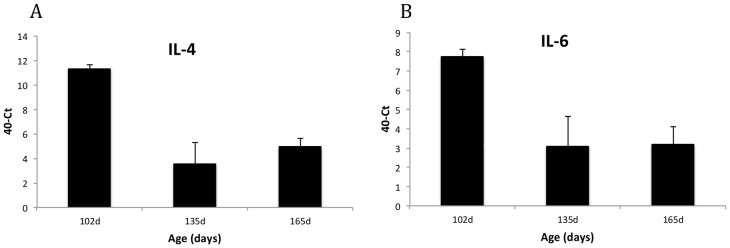
Expression of IL-4 and some expression of IL-6 were detected in the ovaries at all time points examined (Fig. 4). Similar levels expression of IL-6 was found in both the infundibulum and magnum within the oviduct, though expression of IL-4 was detectable throughout the oviduct, levels were lower than the ovaries (data nor shown). Given that secretion of IgA has been shown in the ovary and oviduct, IL-4 may be playing a role in regulation of antibody secretion. No expression of other cytokines or chemokines could be detected.
Figure 4. Expression of Interleukin-4 (A) and Interleukin-6 (B) in the ovary.
Data is expressed as 40-Ct values in ovarian tissue in comparison to expression in breast muscle tissue as a control for no expression (40-Ct = 0). RNA levels are normalised between tissues using 28S RNA expression. Data are shown as means based on expression levels of 5 birds at each time point (±SD).
Susceptibility to S. Enteritidis infection
Salmonella levels in the spleen, liver and reproductive tract following challenge in vaccinated and naive chickens are shown in Table 1. Prior to sexual maturity vaccinated birds showed both lower levels of Salmonella and fewer positive animals compared to naive animals. Significantly lower bacterial numbers were found in the spleen of vaccinated chickens challenged at 77 (P = 0.048) and 102 days of age (P = 0.009) than the naïve group indicating a significant level of protection. However protection decreased as birds reached point-of-lay. Salmonella levels in the spleen were not lowered in the vaccinated group in birds challenged at 132 (P = 0.75), 140 (P = 0.33) 0r 148 days of age (P = 0.61). Reproductive tract infection also coincides with the reaching of sexual maturity in vaccinated birds with the ovaries or oviduct only being infected following challenge at 140 and 148 days of age. There also appeared to be an increased susceptibility to reproductive tract infection in naïve animals at point-of-lay with three out of five birds positive at 148 days of age. Developing and laid eggs from both groups post-challenge were found to contain Salmonella with 3 of 21 eggs laid by naïve hens and 1 of 14 eggs laid by vaccinated hens cultured positive for the presence of Salmonella.
Table 1. Salmonella Enteritidis infection levels in spleen, liver and reproductive tract following challenge of vaccinated and naïve hens four days following intravenous challenge at varying ages through sexual development.
| Group | Tissue | Age at challenge (days) | |||||||||
| 77d | 102d | 132d | 140d | 148d | |||||||
| No positive/total number | Median log10 cfu/g (range) | No positive/total number | Median log10 cfu/g (range) | No positive/total number | Median log10 cfu/g (range) | No positive/total number | Median log10 cfu/g (range) | No positive/total number | Median log10 cfu/g (range) | ||
| Vaccinated | Spleen | 2/5 | 0.85 (0–3.0) | 2/5 | 1.09 (0–2.18) | 5/5 | 1.85 (1.2–2.48) | 4/5 | 1.0 (0–2.18) | 1/5* | <1 |
| Liver | 0/5 | <1 | 1/5 | 0.58 (0–1.70) | 2/5* | <1 | 3/5 | 1.08 (0–2.90) | 2/5* | <1 | |
| Ovary & Oviduct | 0/5 | ND | 0/5 | ND | 0/5 | ND | 2/5 | ND | 2/5 | ND | |
| Naive | Spleen | 3/5 | 2.6 (0–2.18) | 5/5 | 2.49 (1.7–3.48) | 3/5 | 2.57 (0–4.18) | 3/5 | 1.33 (0–2.70) | 3/5 | 0.68 (0–2.74) |
| Liver | 1/5 | 0.58 (0–1.70) | 2/5 | 1.15 (0–2.80) | 3/5 | 0.66 (0–3.30) | 3/5 | 0.56 (0–2.8) | 3/5 | 1.15 (0–2.90) | |
| Ovary & Oviduct | 0/5 | ND | 1/5 | ND | 1/5 | ND | 2/5 | ND | 3/5 | ND | |
Data are presented as median values with ranges based on five birds per group at each age and number positive for Salmonella out of total number challenged per group. Quantitation was not performed on pooled reproductive tract samples and are shown as Salmonella culture positive or negative following enrichment culture.
Positive only after enrichment.
ND = Not done.
Discussion
The data presented here show that susceptibility to S. Enteritidis infection in the chickens is increased around the point-of-lay and that the efficacy of vaccination is significantly decreased at this time, although vaccination offers protection prior to sexual maturity. Moreover, challenge at point-of-lay can lead to infection of the reproductive tract and eggs even in vaccinated birds. Previously we have shown that suppression of T lymphocyte activity was associated with infection of the reproductive tract by Salmonella [19]. Here we show that a sharp decrease in T lymphocytes and particular CD4+ cells underlies this loss of activity. Furthermore there are substantial changes to the local organisation of lymphocytes within the reproductive tract and, in particular, a sharp drop in γδ T lymphocytes. These changes are likely to increase the susceptibility of the ovarian and oviductal epithelium to infection.
Hens at point-of-lay have increased susceptibility for infection with Salmonella even following vaccination and this has potential consequences for its control. Whilst the relatively high challenge inoculum delivered by the intravenous route used in these experiments is more likely to lead to reproductive tract infection or egg infections than a low-level challenge in the field, the model used clearly shows that vaccinated hens are more susceptible to Salmonella infection at point-of-lay. There is good epidemiological evidence from the UK and other countries that vaccination is effective in reducing egg infection [20], however the data presented here would indicate that protection afforded by vaccination is not complete. It is perhaps significant to consider that vaccination in the UK has also been accompanied by improvements in hygiene, biosecurity and animal welfare within the poultry industry [21]–[23]. This has reduced levels of contamination in poultry houses and considerably reduced the likelihood of exposure to Salmonella infection. It does, however, suggest that both vaccination and improved biosecurity and husbandry should be employed in concert to control salmonellosis in egg production and that vaccination alone may not offer complete protection.
The decrease in T lymphocyte proliferation activity at point-of-lay previously described appears to be related to or coincide with a reduction in cell numbers. There is a reduction in several T cell subsets at point-of-lay indicating that immunosuppression is not liked to changes in a single cell type. However the most marked reductions are in CD4+ and γδ cells that is likely to lead to reduced production of Th1 cytokines such as interferon-γ that drive the cellular responses associated strongly with clearance and protection against salmonellosis in the chicken [7], [15], [24]. It is probable that these changes are mediated by changes in steroid hormones that drive development of the reproductive tract and the onset of oviposition and could be considered analogous to immunosuppression during pregnancy or post-partuition in mammalian species, although the increase in nutritional requirements that underlies rapid development of the reproductive tract and oviposition may also have effects upon the immune system [25]. The role of local immunological changes in the chicken reproductive tract on infection is less clear. In the developing oviduct and ovary there is an organised structure of lymphocytes in aggregates that disperse at point-of-lay. Birds, unlike mammals, lack encapsulated lymph nodes instead developing less structured lymphoid aggregates particularly upon antigenic stimulation of the immune system [26]. Previously, lymphoid structures such as those containing CD4+ cells described here had been found in the oviduct of immature and older hens, although their cellular composition was not described [27], [28]. The dispersion of these lymphocyte aggregates at point-of-lay has not been described previously, nor has the relatively high numbers of γδ T lymphocytes within the reproductive tract. Although there is some drop in T cell numbers overall in the reproductive tract at point-of-lay, CD4, CD8α, CD8β and γδ T cells remain scattered throughout the oviduct and ovary throughout the onset of lay. The drop in γδ T lymphocytes may be significant in the increased susceptibility to reproductive tract infection, particularly as both vaccinated and non-vaccinated birds show increased infection levels. Whilst it is considered that γδ T lymphocytes play an important role in innate defences in mucosal tissues including the mammalian reproductive system, such function in the avian reproductive tract has not yet been described. Furthermore it is known that circulating γδ T lymphocytes play an important role in the initiation of immune responses to S. Enteritidis in the chicken [29], so a decrease in these cells in the ovary and oviduct may have consequences in reproductive tract infection. In contrast, there is little change in macrophage numbers in the oviduct whereas there is an influx of macrophages or macrophage-like cells into the ovaries, as previously described [30]. However neither the function nor the phenotype of these cells is known.
The relatively scattered nature and small numbers of leucocytes in the reproductive tract are perhaps reflected in the lack of detectable expression of cytokines in the reproductive tract, with the obvious exception of IL-4. Previously the presence of IgA, IgM and IgG secreting B cells and secreted antibodies have been shown in the oviduct and ovary and, as such, the expression of IL-4 in these tissues is not surprising and fits well with the known humoral immune responses of the ovaries and oviduct [28].
Although susceptibility to infection of the reproductive tract increases in both naïve and vaccinated birds increases at point-of-lay, susceptibility to splenic infection is only increased in vaccinated birds. The drop in T lymphocytes in the spleen is a potential mechanism for this. Cellular immunity is key to both clearance of primary infection and to protection to systemic re-challenge with Salmonella [24]. The significant reduction in T lymphocyte numbers in the spleen could potentially lead to a failure to mount an effective rapid anamnestic response in vaccinates leading to increased susceptibility to systemic infection leading to increased the increased bacterial numbers in the spleen found. The changes in T lymphocytes populations appear to have no impact on susceptibility in naïve animals. However, in the Salmonella challenge model used it is likely that there would be a limited adaptive response within four days of primary challenge and so little difference in susceptibility if mediated by cellular immunity. It is possible that subsequent clearance of infection during the period of immunosuppression is slower. Although the data presented here and previously show that there is both a decrease in protection in vaccinated hens and increased susceptibility to recrudescent Salmonella infection in carrier hens [31], the effect of the drop of T lymphocyte numbers on a primary systemic Salmonella infection requires further investigation, though susceptibility to reproductive tract infection does appear to increase.
Data from the field and experimental observations clearly indicate that vaccination is an invaluable tool in the control of infection of table eggs with Salmonella Enteritidis [20], [32]. However, the immunological changes that accompany sexual maturity and the onset of egg-laying in hens mean that at this point there is increased susceptibility to infection in both naive and vaccinated animals. It may be possible to improve vaccines and a number of promising live attenuated and sub-unit vaccine candidates have been trialled experimentally [33]–[38] as have attempts to stimulate immunity to S. Enteritidis through cytokines and Toll-like receptor agonists [39]–[42]. However, it is not clear if these approaches would overcome any gap in immunity and issues of cost along with regulatory issues on the use of genetically modified bacterial vaccines in many countries may limit their use. As such it would be prudent for the poultry industry worldwide to adopt a holistic approach to Salmonella control that combines vaccination with other measures such as good husbandry, biosecurity and welfare.
Acknowledgments
The authors wish to thanks Professor Pete Kaiser and Lisa Rothwell, Roslin Institute, University of Edinburgh for helpful advice and discussions on staining and cytokine gene expression and Professor Tom Humphrey, University of Liverpool for reviewing the manuscript.
Funding Statement
This work was funded by the UK Biotechnology and Biological Sciences Research Council, www.bbsrc.ac.uk, Grant Number BB/D007542/1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.CDC (2010) Investigation Update: Multistate Outbreak of Human Salmonella Enteritidis Infections Associated with Shell Eggs. Centers for Disease Control.
- 2. Gast RK, Guraya R, Guard-Bouldin J, Holt PS, Moore RW (2007) Colonization of specific regions of the reproductive tract and deposition at different locations inside eggs laid by hens infected with Salmonella Enteritidis or Salmonella Heidelberg. Avian diseases 51: 40–44. [DOI] [PubMed] [Google Scholar]
- 3. Guard-Petter J (2001) The chicken, the egg and Salmonella enteritidis. Environmental microbiology 3: 421–430. [DOI] [PubMed] [Google Scholar]
- 4. Thomson NR, Clayton DJ, Windhorst D, Vernikos G, Davidson S, et al. (2008) Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome research 18: 1624–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Gast R, et al. (2009) Mechanisms of egg contamination by Salmonella Enteritidis. FEMS microbiology reviews 33: 718–738. [DOI] [PubMed] [Google Scholar]
- 6. Van Immerseel F (2010) Stress-induced survival strategies enable Salmonella Enteritidis to persistently colonize the chicken oviduct tissue and cope with antimicrobial factors in egg white: A hypothesis to explain a pandemic. Gut pathogens 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, et al. (2009) The immunobiology of avian systemic salmonellosis. Veterinary immunology and immunopathology 128: 53–59. [DOI] [PubMed] [Google Scholar]
- 8. Clayton DJ, Bowen AJ, Hulme SD, Buckley AM, Deacon VL, et al. (2008) Analysis of the role of 13 major fimbrial subunits in colonisation of the chicken intestines by Salmonella enterica serovar Enteritidis reveals a role for a novel locus. BMC microbiology 8: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies R, Breslin M (2003) Effects of vaccination and other preventive methods for Salmonella enteritidis on commercial laying chicken farms. The Veterinary record 153: 673–677. [PubMed] [Google Scholar]
- 10. Woodward MJ, Sojka M, Sprigings KA, Humphrey TJ (2000) The role of SEF14 and SEF17 fimbriae in the adherence of Salmonella enterica serotype Enteritidis to inanimate surfaces. Journal of medical microbiology 49: 481–487. [DOI] [PubMed] [Google Scholar]
- 11. Iqbal M, Philbin VJ, Withanage GS, Wigley P, Beal RK, et al. (2005) Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar typhimurium. Infection and immunity 73: 2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DEFRA (2011) Zoonoses Report UK 2010. London: Department for Environment, Food and Rural Affairs.
- 13. Gantois I, Ducatelle R, Timbermont L, Boyen F, Bohez L, et al. (2006) Oral immunisation of laying hens with the live vaccine strains of TAD Salmonella vac E and TAD Salmonella vac T reduces internal egg contamination with Salmonella Enteritidis. Vaccine 24: 6250–6255. [DOI] [PubMed] [Google Scholar]
- 14. Withanage GS, Kaiser P, Wigley P, Powers C, Mastroeni P, et al. (2004) Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar typhimurium. Infection & Immunity 72: 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Withanage GS, Wigley P, Kaiser P, Mastroeni P, Brooks H, et al. (2005) Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infection and immunity 73: 5173–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Withanage GS, Kaiser P, Wigley P, Powers C, Mastroeni P, et al. (2004) Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar typhimurium. Infection and immunity 72: 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berchieri A Jr, Wigley P, Page K, Murphy CK, Barrow PA (2001) Further studies on vertical transmission and persistence of Salmonella enterica serovar Enteritidis phage type 4 in chickens. Avian pathology: journal of the WVPA 30: 297–310. [DOI] [PubMed] [Google Scholar]
- 18. Wigley P, Berchieri A Jr, Page KL, Smith AL, Barrow PA (2001) Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infection and immunity 69: 7873–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wigley P, Hulme SD, Powers C, Beal RK, Berchieri A Jr, et al. (2005) Infection of the reproductive tract and eggs with Salmonella enterica serovar pullorum in the chicken is associated with suppression of cellular immunity at sexual maturity. Infection and immunity 73: 2986–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barrow PA (2007) Salmonella infections: immune and non-immune protection with vaccines. Avian pathology: journal of the WVPA 36: 1–13. [DOI] [PubMed] [Google Scholar]
- 21. Cogan TA, Humphrey TJ (2003) The rise and fall of Salmonella Enteritidis in the UK. Journal of applied microbiology 94 Suppl: 114S–119S [DOI] [PubMed] [Google Scholar]
- 22. Davies R, Liebana E, Breslin M (2003) Investigation of the distribution and control of Salmonella enterica serovar Enteritidis PT6 in layer breeding and egg production. Avian pathology: journal of the WVPA 32: 225–237. [DOI] [PubMed] [Google Scholar]
- 23. Gillespie IA, O'Brien SJ, Adak GK, Ward LR, Smith HR (2005) Foodborne general outbreaks of Salmonella Enteritidis phage type 4 infection, England and Wales, 1992–2002: where are the risks? Epidemiology and infection 133: 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beal RK, Powers C, Davison TF, Barrow PA, Smith AL (2006) Clearance of enteric Salmonella enterica serovar Typhimurium in chickens is independent of B-cell function. Infection and immunity 74: 1442–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wigley P, Barrow P, Schat KA (2008) The Avian reproductive Immune System. In: Davison S, Kaspers B, Schat KA, editors. Avian Immunology. London: Academic Press. 289–298.
- 26.Olah I, Vervelde L (2008) Structure of the avian lymphoid system. In: Davison F, Kaspers B, Schat KA, editors. Avian Immunology. London: Academic Press. pp. 13–49.
- 27. Biswal G (1954) Additional Histological Findings in the Chicken Reproductive Tract. Poultry science 33: 843–851. [Google Scholar]
- 28. Withanage GSK, Baba E, Sasai K, Fukata T, Kuwamura M, et al. (1997) Localization and enumeration of T and B lymphocytes in the reproductive tract of laying hens. Poultry science 76: 671–676. [DOI] [PubMed] [Google Scholar]
- 29. Berndt A, Pieper J, Methner U (2006) Circulating gamma delta T cells in response to Salmonella enterica serovar enteritidis exposure in chickens. Infection and immunity 74: 3967–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng WM, Yoshimura Y (1999) Localization of macrophages in the chicken oviduct: Effects of age and gonadal steroids. Poultry science 78: 1014–1018. [DOI] [PubMed] [Google Scholar]
- 31. Wigley P, Hulme SD, Powers C, Beal RK, Berchieri A Jr, et al. (2005) Infection of the reproductive tract and eggs with Salmonella enterica serovar pullorum in the chicken is associated with suppression of cellular immunity at sexual maturity. Infect Immun 73: 2986–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillespie I, Elson R (2005) Successful reduction of human Salmonella Enteritidis infection in England and Wales. Euro surveillance: bulletin europeen sur les maladies transmissibles = European communicable disease bulletin 10: E051117 051112. [DOI] [PubMed]
- 33. Methner U, Barrow PA, Berndt A, Rychlik I (2011) Salmonella Enteritidis with double deletion in phoPfliC-A potential live Salmonella vaccine candidate with novel characteristics for use in chickens. Vaccine 29: 3248–3253. [DOI] [PubMed] [Google Scholar]
- 34. Toyota-Hanatani Y, Kyoumoto Y, Baba E, Ekawa T, Ohta H, et al. (2009) Importance of subunit vaccine antigen of major Fli C antigenic site of Salmonella enteritidis II: a challenge trial. Vaccine 27: 1680–1684. [DOI] [PubMed] [Google Scholar]
- 35. Desin TS, Wisner AL, Lam PK, Berberov E, Mickael CS, et al. (2011) Evaluation of Salmonella enterica serovar Enteritidis pathogenicity island-1 proteins as vaccine candidates against S. Enteritidis challenge in chickens. Veterinary microbiology 148: 298–307. [DOI] [PubMed] [Google Scholar]
- 36. Jawale CV, Chaudhari AA, Jeon BW, Nandre RM, Lee JH (2012) Characterization of a novel inactivated Salmonella enterica serovar Enteritidis vaccine candidate generated using a modified cI857/lambda PR/gene E expression system. Infection and immunity 80: 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng W, Si W, Yin L, Liu H, Yu S, et al. (2011) Salmonella enteritidis ghost vaccine induces effective protection against lethal challenge in specific-pathogen-free chicks. Immunobiology 216: 558–565. [DOI] [PubMed] [Google Scholar]
- 38. Nandre RM, Chaudhari AA, Matsuda K, Lee JH (2011) Immunogenicity of a Salmonella Enteritidis mutant as vaccine candidate and its protective efficacy against salmonellosis in chickens. Veterinary immunology and immunopathology 144: 299–311. [DOI] [PubMed] [Google Scholar]
- 39. He H, Lowry VK, Swaggerty CL, Ferro PJ, Kogut MH (2005) In vitro activation of chicken leukocytes and in vivo protection against Salmonella enteritidis organ invasion and peritoneal S. enteritidis infection-induced mortality in neonatal chickens by immunostimulatory CpG oligodeoxynucleotide. FEMS immunology and medical microbiology 43: 81–89. [DOI] [PubMed] [Google Scholar]
- 40. Kogut MH, Rothwell L, Kaiser P (2005) IFN-gamma priming of chicken heterophils upregulates the expression of proinflammatory and Th1 cytokine mRNA following receptor-mediated phagocytosis of Salmonella enterica serovar enteritidis. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research 25: 73–81. [DOI] [PubMed] [Google Scholar]
- 41. Mackinnon KM, He H, Swaggerty CL, McReynolds JL, Genovese KJ, et al. (2009) In ovo treatment with CpG oligodeoxynucleotides decreases colonization of Salmonella enteriditis in broiler chickens. Veterinary immunology and immunopathology 127: 371–375. [DOI] [PubMed] [Google Scholar]
- 42. Hartley C, Salisbury AM, Wigley P (2012) CpG oligonucleotides and recombinant interferon-gamma in combination improve protection in chickens to Salmonella enterica serovar Enteritidis challenge as an adjuvant component, but have no effect in reducing Salmonella carriage in infected chickens. Avian Pathology 41: 77–82. [DOI] [PubMed] [Google Scholar]