Abstract
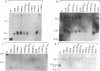
We have used Southern's blotting technique to determine the extent to which the genes encoding the constant (C) regions of μ, α, γ1, and γ2b immunoglobulin heavy (H) chains are altered in number and context from their germline (embryo) state in a series of 14 plasmacytomas expressing various H chain classes. In the three μ chain-producing plasmacytomas studied there was no evidence of rearrangement of CH genes other than Cμ. In contrast, rearrangement and deletion of nonexpressed CH genes was frequent in plasmacytomas that produce γ or α chains. The observed pattern of deletions is consistent with the idea that the ontogenetic switch in H chain class requires CH gene deletion. Frequently, though not always, such deletions as well as other types of rearrangement occur in both allelic loci. Particularly noteworthy are three γ2a-expressing tumors in which Cα gene rearrangement is evident in both alleles. We incorporate these observations into a probabilistic model of B cell development: in the first phase, deletions may occur between the Cμ gene and the variable (VH) gene array, which result in the formation of a productive fused VH-Cμ gene. The cell may then enter a second phase, which allows deletions within the CH gene arrays of both homologous chromosomes. Some deletions juxtapose the expressed VH gene with a second CH gene and result in a H chain class switch; others delete or alter the context of CH genes without changing the phenotype of the cell. We predict that switching can be both a single-step and a multi-step process, and that in the latter case those rearrangements that do not result in a switch may be physiologically significant in that they may limit the options of further switching.
Keywords: heavy chain constant region genes, gene rearrangement, heavy chain class switch
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. O., Freeman K. B. DNA sequences neighboring the duck hemoglobin genes. Cold Spring Harb Symp Quant Biol. 1974;38:707–716. doi: 10.1101/sqb.1974.038.01.076. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Kataoka T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Obata M., Yamawaki-Katoaka Y., Kataoka T., Kawakami T., Takahashi N., Mano Y. Cloning and complete nucleotide sequence of mouse immunoglobulin gamma 1 chain gene. Cell. 1979 Oct;18(2):559–568. doi: 10.1016/0092-8674(79)90072-2. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Yamawaki-Kataoka Y., Yamagishi H., Honjo T. Cloning immunoglobulin gamma 2b chain gene of mouse: characterization and partial sequence determination. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4240–4244. doi: 10.1073/pnas.76.9.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. R., Kincade P. W., Cooper M. D. Sequential expression of germ line genes in development of immunoglobulin class diversity. Fed Proc. 1975 Jan;34(1):33–39. [PubMed] [Google Scholar]
- Lenhard-Schuller R., Hohn B., Brack C., Hirama M., Tonegawa S. DNA clones containing mouse immunoglobulin kappa chain genes isolated by in vitro packaging into phage lambda coats. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4709–4713. doi: 10.1073/pnas.75.10.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Schibler U., Perry R. P. Nuclear transcripts of mouse heavy chain immunoglobulin genes contain only the expressed class of C-region sequences. Science. 1979 Jun 8;204(4397):1087–1088. doi: 10.1126/science.109919. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Cooper M. D. A model for the differentiation of B lymphocytes with implications for the biological role of IgD. Immunol Rev. 1977;37:105–126. doi: 10.1111/j.1600-065x.1977.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Schibler U., Huebner K., Croce C. M. Selective suppression of the transcription of ribosomal genes in mouse-human hybrid cells. J Cell Physiol. 1979 Mar;98(3):553–559. doi: 10.1002/jcp.1040980313. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Birshtein B. K., Scharff M. D. Variants of a mouse myeloma cell line that synthesize immunoglobulin heavy chains having an altered serotype. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1427–1430. doi: 10.1073/pnas.72.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers J., Clarke P., Salser W. Sequence analysis of cloned cDNA encoding part of an immunoglobulin heavy chain. Nucleic Acids Res. 1979 Jul 25;6(10):3305–3321. doi: 10.1093/nar/6.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Valbuena O., Marcu K. B., Weigert M., Perry R. P. Multiplicity of germline genes specifying a group of related mouse kappa chains with implications for the generation of immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):780–784. doi: 10.1038/276780a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. IgD and B cell differentiation. Immunol Rev. 1977;37:50–88. doi: 10.1111/j.1600-065x.1977.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Sato K., Shimizu A., Kataoka T., Mano Y., Ono M., Kawakami M., Honjo T. Mutual homology of mouse immunoglobulin gamma-chain gene sequences. Biochemistry. 1979 Feb 6;18(3):490–494. doi: 10.1021/bi00570a018. [DOI] [PubMed] [Google Scholar]