Abstract
Chronic exposure to nicotine during the first postnatal week in rats, a developmental period that corresponds to the third trimester of human gestation, results in sexually dimorphic long-term functional defects in the adult hippocampus. One potential cause could be the sex-specific differences in the maturation of GABAA receptor-mediated responses from excitatory to inhibitory, which depends on the expression of the Na2+/K+/Cl−-co-transporter NKCC1 and the K+/Cl− co-transporter KCC2. In the rat hippocampus, this switch occurs during the first and second postnatal week in females and males, respectively, and is regulated by nicotinic receptor activation. Excitatory GABAergic signaling can increase BDNF expression, which might exacerbate sex differences by impacting synaptogenesis. We hypothesized that chronic neonatal nicotine (CNN) exposure differentially regulates the expression of these co-transporters and BDNF in males and females. We use quantitative isotopic in situ hybridization to examine the expression of mRNAs for NKCC1, KCC2, BDNF, and NMDA receptor subunits NR2A and NR2B in the postnatal day (P) 5 and 8 rat hippocampus in both sexes that were either control-treated or with 6 mg/kg/day nicotine in milk formula (CNN) via gastric intubation starting at P1. In line with prolonged GABAergic excitation, we found that at P5 males had significantly higher mRNA expression of NKCC1 and BDNF than females. CNN treatment resulted in a significant increase in KCC2 and BDNF mRNA expression in male but not female hippocampus (p<0.05). Males also had higher expression of NR2A and lower expression of NR2B at P5 compared to females (p<0.05). At P8, there were neither sex nor treatment effects on mRNA expression, indicating the end of a critical period for sensitivity to nicotine. These results suggest that differential maturation of GABAAR-mediated responses result in sex-specific sensitivity to nicotine during early postnatal development, potentially explaining the differential long-term effects of CNN on hippocampal function.
Keywords: nicotinic, nAChR, GABAergic, NMDA, development, in situ hybridization
1. Introduction
Despite warnings about the dangers of cigarette smoke, more than 15% of American women continue to smoke while pregnant (SAMHSA, 2010). This exposes their unborn children to large amounts of nicotine, which binds to nicotinic acetylcholine receptors (nAChRs); pentameric, ligand-gated channels composed of different combinations of α and β subunits in a heteromeric or homomeric fashion. Neuronal nAChRs can be located either presynaptically or postsynaptically and are widely expressed in the mammalian brain, with the most prolific subtypes being the high affinity α4β2 heteromeric and the low affinity α7 homomeric subtypes (Sargent, 1993, Tribollet et al., 2004, Gotti et al., 2006). In the hippocampus, presynaptic modulation of γ-aminobutyric acid (GABA) and glutamate neurotransmitter release occurs via β2-containing heteromeric and α7 homomeric nAChRs on GABAergic interneurons (Alkondon et al., 1999) and α7 nAChRs on glutamatergic terminals (Gray et al., 1996, Radcliffe and Dani, 1998). In addition, postsynaptic α7 nChRs are located on hippocampal interneurons (Jones and Yakel, 1997, Alkondon et al., 1998, Frazier et al., 1998).
nAChRs are expressed in the fetal brain, where they are believed to be involved in a variety of processes, including gene expression, neurite outgrowth, and developmental cell death (Dwyer et al., 2008). nAChRs can regulate both GABAergic and glutamatergic transmission during early postnatal development in rats (Maggi et al., 2001, Maggi et al., 2003, Maggi et al., 2004), a period relatively equivalent to the third trimester of human brain development (Dobbing and Sands, 1979, Winzer-Serhan, 2008). During this developmental stage, nAChRs are significantly upregulated in the hippocampus (Adams et al., 2002, Winzer-Serhan and Leslie, 2005, Son and Winzer-Serhan, 2006), making this a period of increased sensitivity to nicotine. In rats, chronic nicotine exposure during the early postnatal period results in altered hippocampal morphology (Huang et al., 2007a) and physiology (Damborsky et al., 2012), and a long-lasting increase in anxiety-like behavior (Huang et al., 2007b).
During prenatal and early postnatal development GABA serves as an excitatory neurotransmitter due to the relatively high expression of the Na2+/K+/Cl− co-transporter NKCC1 and low expression of the K+/Cl− co-transporter KCC2, resulting in a high internal Cl− concentration (Rivera et al., 1999, Ben-Ari, 2002, Payne et al., 2003). As development proceeds, NKCC1 is downregulated, and KCC2 is upregulated, resulting in an extrusion of Cl− from inside the cell and a switch to inhibitory GABA signaling, a process that occurs in females earlier in postnatal development than in males (Nunez and McCarthy, 2007, Galanopoulou, 2008b, a). nAChR activation increases GABA release during development (Maggi et al., 2001), and can regulate the switch from depolarizing to hyperpolarizing GABAAR-mediated signaling by controlling the levels of NKCC1 and KCC2 (Liu et al., 2006). An increase in excitatory GABAergic signaling caused by nicotine could alter the expression of brain-derived neurotrophic factor (BDNF) expression, which is increased by excitatory but not inhibitory GABA (Berninger et al., 1995, Represa and Ben-Ari, 2005), and has previously been shown to be enhanced by postnatal nicotine exposure (Son and Winzer-Serhan, 2009).
NMDA receptors (NMDARs) are tetrameric ligand-gated channels that contain NR1 subunits with at least one NR2. NR3 subunits can be incorporated into an NR1/NR2 complex, but cannot form receptors independently. NMDAR functional variability is conferred by the combination of the NR2 subunits, of which there are 4 types (NR2A-D) (Ishii et al., 1993, Cull-Candy et al., 2001). The primary NR2 subunits found in the hippocampus are NR2A and NR2B (Goebel and Poosch, 1999). During early postnatal development in the hippocampus, NR2B mRNA is highly expressed, whereas NR2A mRNA is low at birth, but increases significantly over time, especially during the second postnatal week (Monyer et al., 1994, Zhong et al., 1995). Postnatal nicotine exposure has previously been found to enhance NMDAR-mediated excitatory postsynaptic potentials and modulate synaptic development in the auditory cortex (Aramakis et al., 2000), and alter expression of NR2A and 2B mRNA in the auditory cortex and medial geniculate nucleus (Hsieh et al., 2002). Within the hippocampus, gestational nicotine increases NR1, 2A, and 2D protein levels (Wang et al., 2011).
Although it is known that the GABAergic system develops at different speeds in males and females, it has not been determined whether or not the glutamatergic system is similarly sex-specific in its development. Additionally, while there is evidence that developmental nicotine exposure can result in long-lasting sex-specific effects (Vaglenova et al., 2004, Vaglenova et al., 2008, Damborsky et al., 2012), it is not known what changes are occurring during the treatment window that are leading to these long-lasting consequences. It is the aim of this study to determine if there is sex-specific mRNA expression of KCC2, NKCC1, BDNF, NR2A or NR2B within the postnatal rat hippocampus. Additionally, we will use a previously established chronic neonatal nicotine (CNN) treatment model (Huang et al., 2006) to determine how chronic nicotine exposure during the first postnatal week can affect the expression of these mRNAs, and determine if there are any sex-specific differences in response to nicotine. Based on the previously stated evidence that GABAergic signaling remains excitatory for a longer period of time in the male hippocampus (Nunez and McCarthy, 2007, Galanopoulou, 2008a), as well as previous data from our lab showing that males seem to be affected to a greater extent by CNN treatment (Damborsky et al., 2012), we hypothesized that CNN treatment would affect mRNA expression to a greater extent in males compared to females.
2. Experimental Design
2.1 Nicotine treatment
All animal treatment protocols were approved by the Texas A&M University Institutional Animal Care and Use Committee under the guidelines set by the National Institute of Health. Virgin-mated pregnant Sprague-Dawley rats (Harlan, Houston, TX) arrived between gestational day 14 and 16, and were housed in accordance with the rules stipulated by the Texas A&M University Animal Care and Use Committee. The date of birth was termed postnatal day (P) 0, and litters were culled to 8-10 pups on P1. Starting on P1, pups were treated using a previously described treatment model (Huang et al., 2006). Briefly, pups were given milk formula (Enfamil; Mead Johnson & Company, Evansville, IN) at a volume 1/36 their total body weight three times a day via oral gastric intubation. One half of the pups in each litter were given 2 mg/kg/dose nicotine (Sigma Chemical, St. Louis, MO) mixed in milk formula for a total of 6 mg/kg/day nicotine, the other pups were given milk formula only. This nicotine concentration roughly corresponds to what would be experienced by a heavy smoker (Murrin et al., 1987), and what would be experienced in utero given the ease with which nicotine can cross the placental barrier (Luck et al., 1985). Treatment was ended at P5, with only one treatment occurring the morning of P5, or was continued to P8, with pups receiving one treatment the morning of P8.
2.2 Tissue Collection
Three hours following the last treatment, pups were decapitated and their brains removed. Whole brains were immediately submerged in isopentane held at −20°C for 30 s, then covered in dry ice. Brains were kept at −80°C until ready to be cut. 20 μm coronal sections were taken through the hippocampus using a Microtome cryostat (MICROM International GmbH) kept at −20°C and thaw mounted on slides coated in poly-l-lysine (Sigma Chemical, St. Louis, MO). For each probe, slices were collected every 200 μm throughout the entire hippocampus. Slides were then fixed in 6% formaldehyde in 0.1 M Phosphate Buffer solution (PB), then rinsed twice in 0.1 M PB, once in ddH20, dried in a cold air stream and stored at −20°C.
2.3 In situ Hybridization
An 35S-UTP-labeled cRNA probe for BDNF was produced using a pBSks plasmid containing a 750 base region complementary to rat BDNF mRNA (kindly provided by Dr. Carl Cotman, UC Irvine, CA) constructed as previously described (Berchtold et al., 1999). 35S-UTP cRNA probes were synthesized using pBS-SK (+) plasmids containing a 282 base region complementary to NR2A mRNA or a 319 base region complementary to NR2B mRNA (both kindly provided by Dr. Frances Leslie, UC Irvine, CA), constructed as previously described (Hsieh et al., 2002). NKCC1 and KCC2 templates were constructed via RT-PCR using primers describe in Table 1 (Integrated DNA Technologies), subcloned into a pPCR-Script AMP SK (+) plasmid (Agilent Technologies, Santa Clara, CA), and sequence verified (Gene Technology Lab, Texas A&M University). Plasmids were linearized via restriction enzyme digest. cRNA probes were synthesized in antisense orientations with T7 or T3 RNA polymerase (Ambion, Austin, TX) in the presence of 35S-UTP (PerkinElmer, Boston, MA). The newly generated antisense probes for NKCC1 and KCC2 exhibited the expected hybridization pattern (Plotkin et al., 1997, Wang et al., 2002, Stein et al., 2004), and the sense probes showed very low or no hybridization signal indicating negligible non-specific hybridization (Fig. 1) and suggesting specificity for the NKCC1 and KCC2 templates.
Table 1. Primer sequences for the generation of cRNA templates.
| Name | Gene Accession Nuinbei | Piitnet Sequence |
|---|---|---|
| NKCC1 | AF 051561 (1345.1947.e04 bp) |
For: 5′ -TAC AGT GGT GGT TCT TCT GGG CAT3′ Rev: 3′-AGT GCT GAT GAG AGT GTG GCT GAA-5′ |
| KCC2 | U55816 (2693-3258.560 lip) |
For: 5′ -GCG CAG ATG GAT GAC AAC AGC ATT- 3′ Rev: 3′ -CGC ACG TTG GAC TGG TTC AAG TTT- 5* |
Figure 1. NKCC1 and KCC2 expression at postnatal day 8.
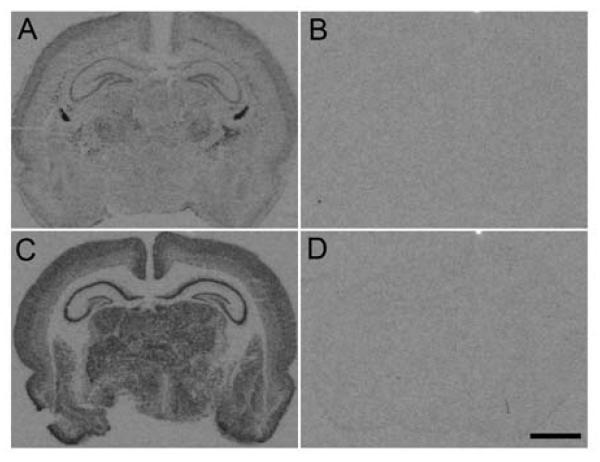
Autoradiographic images show hybridization signal to antisense (A and C) and sense (B and D) probes for NKCC1 (A and B) and KCC2 (C and D) in coronal sections of P8 rat brains. Scale bar: 2 mm.
In situ hybridization was performed as previously described (Winzer-Serhan et al., 1999). Briefly, slides containing coronal brain slices through the hippocampus were pretreated for 10 min with protease K (0.1 μg/mL), acetylated, dehydrated using increasing concentrations of ethanol (50, 70, 95, and 100%) and dried in a cold air stream. Slides were then covered with the 35S-UTP-labeled cRNA probe in a hybridization solution (50% formamide, 10% dextran sulfate, 500 μg/ml tRNA, 10mM dithiothreitol, 0.3M NaCl, 10mMTris, pH 8.0, and 1mM EDTA, pH 8.0) and incubated overnight (16-20 hours) at 60°C. The following day, any unbound RNA was washed away using RNAse A (10 μg/ml, Fisher Scientific, Pittsburgh, PA), and salinity was adjusted using decreasing concentrations of standard sodium citrate buffer (4×-0.1×). Slides were then dehydrated with ethanol, dried in a cold air stream, and apposed to Kodak BioMax-MR film (Fisher Scientific, Pittsburgh, PA) along with [14C]-standards of known radioactivity (Amersham Bioscience, Buckinghamshire, England), where they were exposed at 4°C.
For darkfield images, slides were dipped in Kodak autoradiography NTB emulsion (VWR, West Chester, PA) and exposed at 4°C. After 3-4 weeks, slides were developed in 50% Kodak D-19, fixed in Kodak Professional fixer (Fisher Scientific, Pittsburgh, PA), and counter stained with a Cresyl-Violet solution. Slides were then dehydrated in graded ethanol, coated in xylenes, and cover slipped with DPX mounting medium (Fluka, Ronkonkoma, NY). Visualization of darkfield images was carried out using a DP7-1 digital camera (DP manager, Leeds Insturments, Irving, TX). Brightness and contrast of images were adjusted for publication using Adobe Photoshop 8.0.
2.4 Data Analysis
A computer-based image analysis system (MCID, Imaging Research Inc. St. Catherine, Canada; now InterFocus Imaging Ltd, UK) was used to analyze 35S-hybridization signals from autoradiograms. 14C-standards were used to construct a calibration curve comparing relative optical density (ROD) to radioactivity in nCi/g. Hybridization was analyzed by converting ROD for antisense probes into nCi/g as a measure of expression intensity, which was then adjusted for decay based on the 35S calibration date. All probes were measured in the CA1, CA3, and Dentate gyrus (DG) regions of the hippocampus. Hybridization intensities were measured from at least three slices from each brain and averaged for analysis. A two-way ANOVA was performed for each probe in each region of the hippocampus to look for sex and treatment effects at P5 or P8. Because we hypothesized that CNN treatment would affect mRNA expression in males and not females, we also planned comparisons between Control- and CNN-treated rats in males and female groups using a Student’s t-test. Significance was described as a p value of 0.05 or less.
3. Results
3.1 NKCC1 and KCC2 mRNA expression in male and female control- and CNN-treated pups at P5 and P8
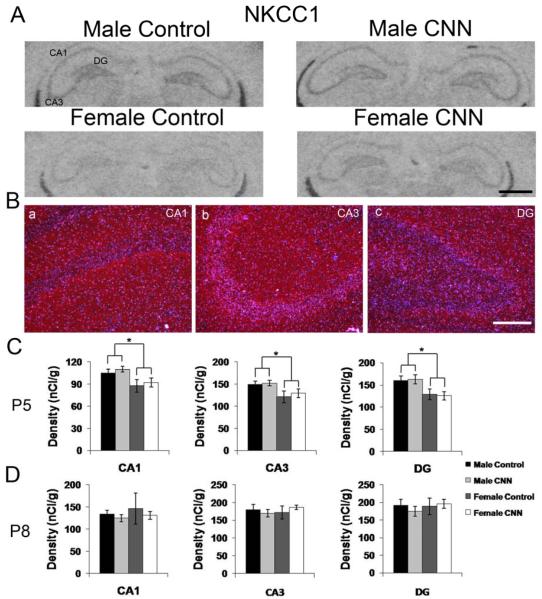
The spatial mRNA expression pattern of NKCC1 was identical to that previously reported for postnatal rat brain including the expression in the hippocampus (Fig. 1) (Plotkin et al., 1997, Wang et al., 2002). Autoradiographic images generated by hybridization with the radiolabeled antisense probe to NKCC1 mRNA in P5 rat hippocampus show identical pattern in both sexes in controls and the expression pattern was not affected by treatment (Fig. 2A). Darkfield images revealed weak hybridization signals in stratum (s). pyramidale of CA1 and CA3 and granule layer of DG with no signals in cells located in s. oriens, or radiatum of either CA1 or CA3, where interneurons are located (Fig. 2B). The expression intensities were slightly higher in CA3 and DG than CA1 (Fig. 2C and D). Although there was no significant treatment effect in either male or female pups, there was a small overall sex effect in all three regions of the hippocampus, with males showing small but significantly greater mRNA expression compared to females (Fig. 2C, p<0.05 for all regions). At P8, there was neither a treatment nor sex effect on NKCC1 expression (Fig. 2D).
Figure 2. NKCC1 mRNA expression in the P5 and P8 rat hippocampus.
(A) Autoradiographic images demonstrate expression of NKCC1 mRNA in the P5 male and female hippocampus following Control or CNN treatment. (B) Darkfield images from a male Control-treated P5 rat showing expression patterns of NKCC1 in CA1 (a) CA3 (b) and DG (c). (C) Quantification of hybridization intensities from P5 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in each group. (D) Quantification of hybridization intensities from P8 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in male Control, female Control and female CNN groups, and 5 in male CNN group. Data shown are ± S.E.M. Scale bars: 1 mm in A, 200 μM in B. * indicates p<0.05.
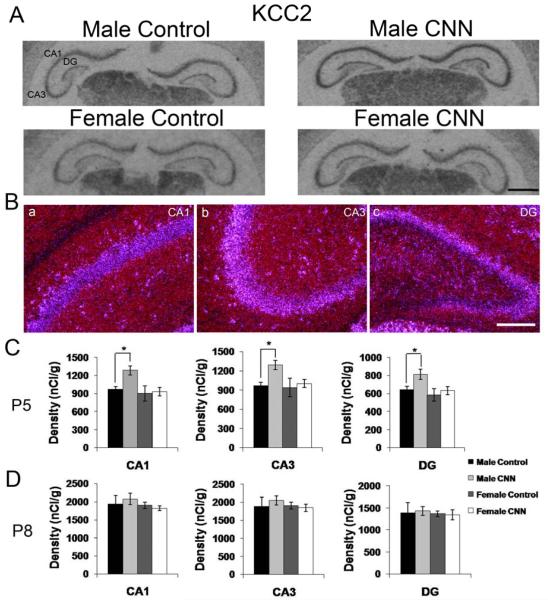
The spatial mRNA expression pattern of KCC2 was identical to that previously reported for postnatal rat brain including the expression in the hippocampus (Fig. 1) (Wang et al., 2002, Stein et al., 2004). Autoradiographic images show strong expression of KCC2 mRNA throughout the hippocampus in male and female control- and CNN-treated P5 pups (Fig. 3A). The hippocampal expression patterns were similar in male and female control and CNN-treated rat pups at P5 and P8. Hybridization signal was detected in CA1 and CA3 pyramidal cells and interneurons located in s. oriens and radiatum. In the DG, KCC2 mRNA hybridization signal was detected in the granule cell layer and interneurons in the molecular layer (Fig. 3B). In P5 control-treated rat pups, there was no sex difference detected in KCC2 mRNA expression intensities in the principal cell layers. Planned comparisons between Control- and CNN-treated pups in males and females revealed that in male but not female pups, CNN treatment resulted in increased expression in the principal layers of CA1 (32%, p<0.05), CA3 (33%, p<0.05) and DG (27%, p<0.05) (Fig. 3C). In animals treated until P8, there were no longer any treatment or sex effects detected in any region (Fig. 3D).
Figure 3. KCC2 mRNA expression in the P5 and P8 rat hippocampus.
(A) Autoradiographic images demonstrate expression of KCC2 mRNA in the P5 male and female hippocampus following Control or CNN treatment. (B) Darkfield images from a male Control-treated P5 rat showing expression patterns of KCC2 in CA1 (a) CA3 (b) and DG (c). (C) Quantification of hybridization intensities from P5 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in each group. (D) Quantification of hybridization intensities from P8 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in male Control, female Control and female CNN groups, and 5 in male CNN group. Data shown are ± S.E.M. Scale bars: 1 mm in A, 200 μM in B. * indicates p<0.05.
Given the roles of NKCC1 and KCC2 in Cl− accumulation and extrusion, respectively (Rivera et al., 1999, Yamada et al., 2004), it is likely that the ratio of expression of these co-transporters determines the actual internal Cl− concentration. The ratio of KCC2 to NKCC1 mRNA expression was therefore compared across both ages using a three-way ANOVA. There was a significant increase in the KCC2/NKCC1 ratio in all areas, in both treatment groups in males and females between P5 and P8 (Fig.4).
Figure 4. KCC2/NKCC1 ratios in P5 and P8 rat hippocampus.
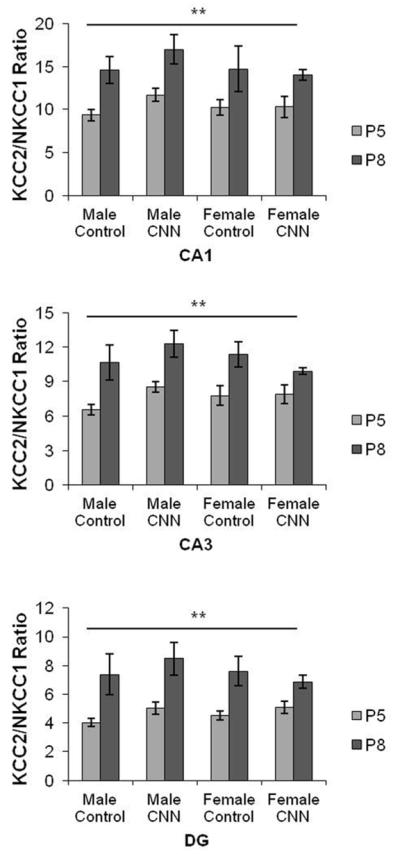
KCC2/NKCC1 mRNA hybridization intensity ratios were plotted for male and female Control- and CNN-treated pups at P5 and P8 in CA1, CA3, and DG. ** indicates significant effect of age across all sex and treatment groups, p<0.01. Data shown are ± S.E.M.
3.2 BDNF mRNA expression in male and female control- and CNN-treated pups at P5 and P8
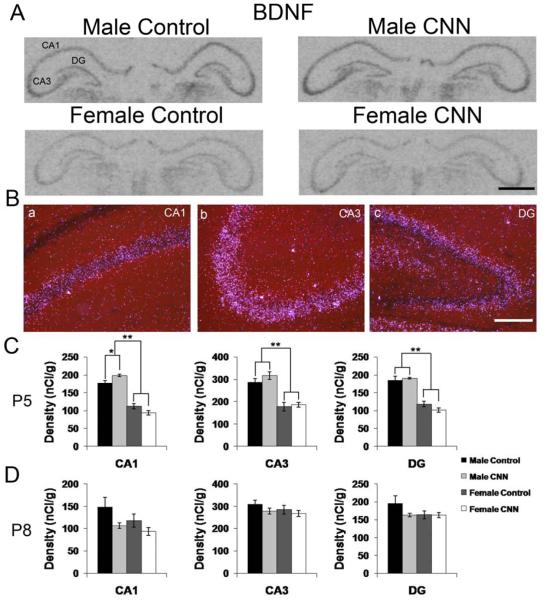
There is evidence that inhibitory and excitatory GABAAR-mediated signaling differentially affects BDNF mRNA expression (Berninger et al., 1995), we therefore determined whether chronic nicotine treatment affected BDNF mRNA expression. Autoradiographic and darkfield images show BDNF mRNA expression in the hippocampus of P5 control- and CNN-treated male and female pups (Fig. 5A and B). The BDNF mRNA expression pattern was identical in males and females, and between treatment groups. BDNF expression was highest in s. pyramidale in CA3, and was primarily confined to the pyramidal cell layers of CA1 and CA3 and the DG granule cell layer (Fig. 5A and B). Quantitative analysis revealed that there was an overall sex effect in the principal cell layers of all three regions of the hippocampus in P5 rat pups, with males having significantly higher BDNF mRNA expression levels (Fig. 5A, p<0.01). In the CA1 hippocampus, male CNN-treated P5 pups had a small but significant 12% increase in BDNF mRNA expression compared to male controls (Fig. 5A, p<0.05). In animals treated until P8, there was no longer any sex effect, and there was no significant treatment effect in any region.
Figure 5. BDNF mRNA expression in the P5 and P8 rat hippocampus.
(A) Autoradiographic images demonstrate expression of BDNF mRNA in the P5 male and female hippocampus following Control or CNN treatment. (B) Darkfield images from a male Control-treated P5 rat showing expression patterns of BDNF in CA1 (a) CA3 (b) and DG (c). (C) Quantification of hybridization intensities from P5 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in each group. (D) Quantification of hybridization intensities from P8 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in male Control, female Control and female CNN groups, and 5 in male CNN group. Data shown are ± S.E.M. Scale bars: 1 mm in A, 200 μM in B. * indicates p<0.05, ** indicates p<0.01.
3.3 NR2A mRNA expression in male and female control- and CNN-treated pups at P5 and P8
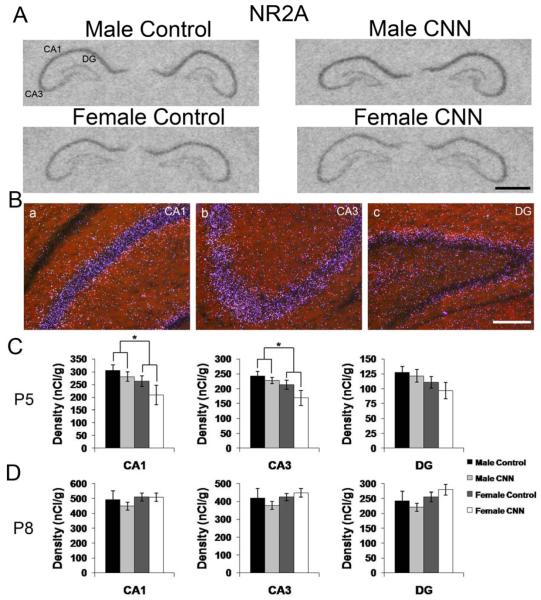
NMDAR subunit expression is also developmentally regulated (Zhong et al., 1995, Cull-Candy et al., 2001), and has been shown to be affected by developmental nicotine treatment (Hsieh et al., 2002). To determine if there are any developmental sex differences in NMDAR subunit expression or if there is a CNN treatment effect, NR2A and 2B subunit mRNA expression was examined in P5 and P8 control- and CNN-treated pups. NR2A mRNA expression pattern was identical in male and female pups, and the distribution was not affected by CNN-treatment. In all groups, expression was mostly restricted to the principal layers, and was higher in s. pyramidale in CA1 and CA3, compared to the granule cell layer in the DG (Fig. 6A and B). Only a scarce population of neurons in the s. oriens and radiatum in exhibited hybridization signals. At P5, there was a significant sex effect observed in CA1 and CA3 hippocampus, with males exhibiting slightly higher expression of NR2A mRNA compared to females (Fig. 6C; CA1: p<0.05, CA3: p<0.05). In animals treated until P8, there were no detectable sex or treatment effects in any region of the hippocampus (Fig. 6D).
Figure 6. NR2A mRNA expression in the P5 and P8 rat hippocampus.
(A) Autoradiographic images demonstrate expression of NR2A mRNA in the P5 male and female hippocampus following Control or CNN treatment. (B) Darkfield images from a male Control-treated P5 rat showing expression patterns of NR2A in CA1 (a) CA3 (b) and DG (c). (C) Quantification of hybridization intensities from P5 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in each group. (D) Quantification of hybridization intensities from P8 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in male Control, female Control and female CNN groups, and 5 in male CNN group. Data shown are ± S.E.M. Scale bars: 1 mm in A, 200 μM in B. * indicates p<0.05.
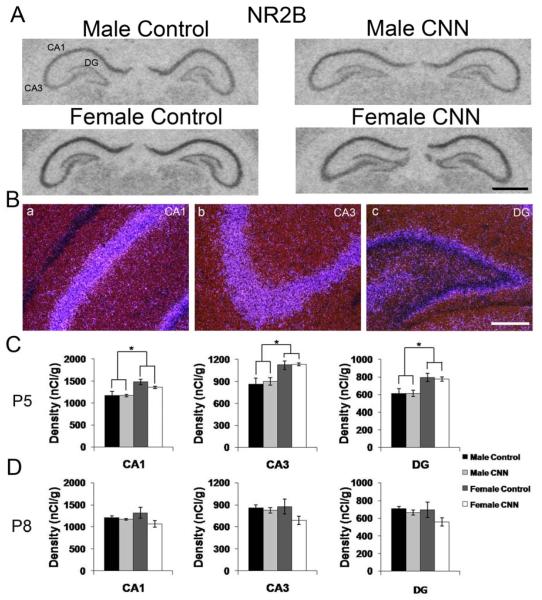
The NR2B mRNA expression pattern was identical in male and female postnatal rat pups, and the distribution was not affected by CNN treatment. Autoradiographic images of NR2B subunit mRNA expression at P5 show strong expression in principal layers in CA1 and CA3 and in the DG (Fig 7A). Darkfield images confirm the primary localization of NR2B subunit mRNA in CA1 and CA3 pyramidal cells and granule cells in the DG, and expression in interneurons in CA1, CA3 and DG regions of the hippocampus (Fig. 7B). Quantitative analysis of autoradiograms revealed that there was a significant sex effect at P5, with females showing higher hybridization signals in principal cell layers of the CA1, CA3 and DG compared to males in all three regions of the hippocampus (Fig. 7C, p<0.01 for all regions). There was no treatment effect for either sex at P5 (Fig. 7C). In pups treated until P8, there were no sex or treatment effects observed in any region of the hippocampus (Fig. 7D).
Figure 7. NR2B mRNA expression in the P5 and P8 rat hippocampus.
(A) Autoradiographic images demonstrate expression of NR2B mRNA in the P5 male and female hippocampus following Control or CNN treatment. (B) Darkfield images from a male Control-treated P5 rat showing expression patterns of NR2B in CA1 (a) CA3 (b) and DG (c). (C) Quantification of hybridization intensities from P5 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in each group. (D) Quantification of hybridization intensities from P8 male and female Control- and CNN-treated pups in CA1, CA3 and DG. n=4 in male Control, female Control and female CNN groups, and 5 in male CNN group. Data shown are ± S.E.M. Scale bars: 1 mm in A, 200 μM in B. * indicates p<0.05.
4. Discussion
In this study, we evaluated expression levels of the cation chloride co-transporters KCC2 and NKCC1, the neurotrophic factor BDNF, and the NMDAR subunits NR2A and NR2B in male and female rat pups treated with nicotine during the first postnatal week. This is the first study that demonstrates differential effects of neonatal nicotine treatment in male and female pups for KCC2 and BDNF mRNA expression and sex differences in the expression levels of NR2A and NR2B. Although the changes reported here only correspond to transient increases in mRNA expression, and might not reflect changes in protein expression, it is possible that even small differences in mRNA expression could translate into changes in protein levels with consequences for hippocampal synapse and circuit formation. In turn, this could lead to an imbalance of excitatory and inhibitory transmission in the hippocampus and account for the differential response to nicotine in males and females (Damborsky et al., 2012).
4.1 Effects of sex and CNN treatment on NKCC1 and KCC2 expression
In CA1 hippocampal pyramidal neurons, hyperpolarizing GABAAR postsynaptic currents appear during the first postnatal week in female, but during the second postnatal week in male rats (Galanopoulou, 2008a). This sex specific delay in maturation of the GABAergic system has been attributed to differences in the relative abundance of the cation chloride co-transporters KCC2 and NKCC1; the protein level of phosphorylated NKCC1 is higher in P0 and P7 male hippocampus compared to age matched females, while KCC2 protein expression is higher in P7 and P10 female hippocampus (Nunez and McCarthy, 2007, Galanopoulou, 2008b, a). It has previously been shown that endogenous nicotinic signaling is able to regulate NKCC1 and KCC2 protein expression, and is important for the appropriate timing of the switch from excitatory to inhibitory GABAAR-mediated responses (Liu et al., 2006). Given the role of nicotinic signaling in the regulation of the maturational switch from depolarizing to hyperpolarizing GABAAR-mediated currents, neonatal nicotine exposure may accelerate this transition in a sex-dependent manner. We therefore expected to detect sexually dimorphic differences in the expression of the co-transporters NKCC1 and KCC2 at P5 and P8, and their differential regulation after chronic nicotine treatment during the first postnatal week, when sex differences in GABAAR reversal potential are most pronounced. Our results show that there was significantly higher hybridization signal for NKCC1 but not KCC2 in P5 male compared to female hippocampus. In contrast, no sex differences were detected at P8. In all sex and treatment groups, there was a significant increase in the KCC2/NKCC1 ratio between P5 and P8, suggesting a move towards full maturation of the GABAergic system (Rivera et al., 1999, Yamada et al., 2004). Expression levels of NKCC1 and KCC2, or their ratio, are essential determinants of the chloride gradient across membranes (Yamada et al., 2004), and the increased expression of NKCC1 mRNA in P5 males seen here is in line with longer depolarizing actions of GABA in neonatal male hippocampus (Pfeffer et al., 2009). Although mRNA expression levels do not always correlate with protein levels, and posttranslational modifications greatly affect the function of the cotransporter NKCC1 (Nunez and McCarthy, 2007), these results support, in part, our expectation of sex specific regulation of co-transporter expression.
Chronic treatment with nicotine significantly increased KCC2 mRNA expression in males but not females at P5, but did not affect expression of NKCC1 in either male or female hippocampus. This is in line with findings by others that KCC2 expression is regulated in response to changing conditions; in male neonates, KCC2 but not NKCC1 immunoreactivity, is regulated in response to kainate-induced seizures or maternal separation (Galanopoulou, 2008a), and GABA itself can increase KCC2 mRNA expression levels in immature neurons (Ganguly et al., 2001). These findings support the idea that KCC2 is the driving force behind the switch from excitatory to inhibitory GABAAR-mediated responses (Lee et al., 2005), and by regulating KCC2 mRNA expression, neurons seem to be able to regulate intracellular chloride concentrations in response to changing environmental conditions. It remains to be seen if a CNN treatment-induced increase in KCC2 mRNA expression affects the chloride gradient in hippocampal neurons. However, several studies have shown that increases in KCC2 correlate with modifications of GABAergic transmission (Lu et al., 1999, Rivera et al., 1999, Ganguly et al., 2001, Galanopoulou, 2008a). Thus, a nicotine-induced increase in KCC2 mRNA expression, as described in this study, is likely to have functional consequences; perhaps changing the developmental timing of the transition to inhibitory GABAergic signaling in males. Therefore, our results support data that endogenous nicotinic signaling is critical for the appropriate expression of KCC2 (Liu et al., 2006), and suggest that endogenously or exogenously induced nicotinic activity may play an important role in the maturation of the GABAergic system. However, additional studies are needed to determine if the sex-specific differential effect of CNN treatment on KCC2 mRNA expression translates into changes in intracellular chloride concentration and Cl− reversal potentials in male but not female hippocampus. In that case, immature male pups would be more likely to develop long-term effects after developmental nicotine exposure, because a premature change in reversal potential of GABAAR currents could result in altered morphological neuronal maturation in males but not females (Cancedda et al., 2007).
The mechanism by which developmental nicotine exposure alters the expression of KCC2 is not known. However, it is known that activation of presynaptic nAChRs by nicotine can increase GABA release (Alkondon et al., 1999, Radcliffe et al., 1999, Maggi et al., 2001), which may lead to an increase in Ca2+ influx via L-type voltage gated Ca2+ channels following GABAAR-mediated depolarization in immature neurons (Obrietan and van den Pol, 1995, Ganguly et al., 2001). This increase in Ca2+ may then lead to an increase in KCC2 mRNA expression (Ganguly et al., 2001, Bray and Mynlieff, 2009).
4.2 Effects of sex and CNN treatment on BDNF expression
In the hippocampus, GABAAR-mediated depolarization can lead to an increase in BDNF expression in immature neurons; an effect that is not seen when GABA becomes hyperpolarizing (Berninger et al., 1995, Obrietan et al., 2002, Represa and Ben-Ari, 2005). Given the relationship between excitatory GABA actions and increased BDNF expression, we anticipated sexually dimorphic differences in the expression of BDNF at P5 and P8, and that CNN-treatment would raise levels of BDNF mRNA in male but not in female pups. We observed increased BDNF expression in males compared to females at P5, when GABA is depolarizing in males but not in females (Galanopoulou, 2008a), and a further increase in BDNF expression in CA1 of P5 CNN-treated males but not females. These results support our hypothesis that there is sex and treatment specific regulation of BDNF expression in the neonatal hippocampus, an effect that we believe is linked to the excitatory action of GABA in neonates. Increased levels of BDNF correlate with increased synaptogenesis during development, in particularly of the GABAergic system (Yamada et al., 2002, Aguado et al., 2003, Wardle and Poo, 2003). Thus, even a brief change in BDNF expression levels could have a dramatic impact on the development of the hippocampus, potentially leading to some of the long-term, sex-specific functional changes we previously described (Damborsky et al., 2012).
4.3 Differential expression of NR2A and NR2B mRNA in the postnatal male and female hippocampus
In neonates, glutamatergic synaptic connections are very immature and gradually develop during the first 2 weeks of life (Fiala et al., 1998, Hsia et al., 1998, Tyzio et al., 1999). Furthermore, during the first postnatal week, the majority of glutamatergic Schaeffer collateral–CA1 synapses are functionally silent (Isaac et al., 1995, Durand and Konnerth, 1996). In neonates, synaptic activity can drive NR2A subunits into hippocampal synapses, and switch synaptic NMDARs from NR2B to NR2A containing ones (Barria and Malinow, 2002, Bellone and Nicoll, 2007), and neonatal nicotine can activate presynaptically silent excitatory synapses (Maggi et al., 2003). Thus, in neonates, nicotine-stimulated glutamate and GABA release could result in increased excitatory activity, raising intracellular Ca2+ concentrations with subsequent effects on NMDAR subunit expression. This idea is supported by reports that prenatal exposure to nicotine transiently increases immunoreactivity for NR2A but not NR2B in one day old rat pups (Wang et al., 2011), and that postnatal nicotine exposure increases cortical mRNA expression of NR2A (Hsieh et al., 2002). Therefore, we examined whether CNN treatment altered mRNA expression levels of NR2A and NR2B in the postnatal hippocampus at P5 and P8. Our results show that nicotine treatment had no effect on NR2A or NR2B mRNA expression in either males or females, suggesting that the first postnatal week, when excitatory NMDAR-mediated transmission is low, is not a critical period for nicotine-induced changes in NMDAR subunit expression in the hippocampus. This is in line with evidence that the postnatal age is critical for nicotine’s effects on NMDAR function and expression; nicotine exposure during the second but not first postnatal week alters NMDAR function and NR2A mRNA expression in the auditory cortex (Aramakis et al., 2000, Hsieh et al., 2002).
Compared to the well-established sex differences in the maturation of the GABAergic system, such differences have not been described for the developmental expression of NMDAR subunits. NMDAR subunit composition is strongly regulated during development, with NR2B being expressed during embryonic and postnatal development followed by an activity-dependent upregulation of NR2A, especially during the second postnatal week (Monyer et al., 1994, Zhong et al., 1995, Barria and Malinow, 2002, Bellone and Nicoll, 2007, Matta et al., 2011). The data here show that males have slightly higher levels of NR2A mRNA and lower levels of NR2B mRNA compared to females at P5. The switch to NR2A subunits depends on activation of NMDARs, and requires a rise in postsynaptic calcium concentrations (Matta et al., 2011), conditions which might occur more often in males than in females during the first postnatal week. Additionally, because the subunit composition of NMDARs confers different functionality (Ishii et al., 1993, Cull-Candy et al., 2001), sex-dependent expression of NMDAR subunits could mean that male and female NMDAR-mediated responses are functionally different during early postnatal development. Additional experiments would need to be done to determine if the differences in mRNA expression described here translate into differences in protein expression, and results in any functional differences.
4.4 Increased sensitivity to CNN treatment in male pups
The baseline sex differences in mRNA expression for NKCC1, BDNF, NR2A and NR2B described here, together with previously published work on GABAAR maturation during early postnatal development, strongly suggest that males and females have very different routes to maturity, and may be differentially susceptible to nicotine at different times during development. Previous studies have indeed shown that developmental nicotine exposure may lead to sex-specific outcomes in humans (Weissman et al., 1999) and rodents (Vaglenova et al., 2004, Vaglenova et al., 2008). Recently, our lab demonstrated that CNN treatment results in long-lasting changes in hippocampal function, with males affected to a greater degree than females (Damborsky et al., 2012). In the present study, we show that there are sexually dimorphic responses to nicotine in neonatal rat pups with increases in KCC2 and BDNF mRNA expression in P5 male but not female hippocampus, which could potentially speed up the switch to hyperpolarizing GABAAR signaling in males and alter the course of hippocampal development
It is interesting to note that no sex- or treatment-related changes were found for BDNF or KCC2 at P8, although, in males GABAAR-mediated transmission should still be depolarizing at this age. This might reflect a gradual transition from depolarization to hyperpolarization and/or age-related reduced ability of depolarizing GABA actions to increase intracellular Ca2+ concentrations (Ganguly et al., 2001). This transient nature of CNN-induced changes in male pups suggests that as development proceeds, nicotine’s effects are diminishing. In the case of the GABAergic system, it could be that as the change to hyperpolarizing GABA approaches, nicotine no longer has an effect on KCC2 and BDNF expression. Thus, there seems to be a narrow window of vulnerability to nicotine exposure during the first postnatal week in males that might not be there, or might occur at a different developmental time, in females. Altered nicotinic cholinergic activity during this time, either by increased activation due to maternal smoking or by decreased activation due to nAChR dysfunction of blockage, could result in altered excitatory/inhibitory ratio in hippocampal and cortical structures in a sex-specific manner.
4.5 Conclusions
Together, our data suggest that key mRNAs involved in GABAergic and glutamatergic signaling during early postnatal development are differentially expressed in male and female rat pups, and that CNN treatment during the first postnatal week affects males to a greater extent than females. Although our results strongly indicate that male rats may be more sensitive to nicotine during early postnatal development, this does not mean that females are insensitive to the effects of nicotine. We previously demonstrated that both CNN-treated males and females exhibit increased anxiety-like behaviors later in life (Huang et al., 2007b), and long-term changes in hippocampal activity were still recorded in CNN-treated adult females (Damborsky et al., 2012). However, with regards to the GABAergic system, which matures earlier in females than males, sensitive periods to nicotine could be shifted to earlier developmental timepoints in females. Sex-specific susceptibility could be drastically different depending on target genes and brain regions being studied, and more studies will be required to determine critical periods of nicotine sensitivity in various brain regions and for different gene targets in males and females.
The baseline sex differences and sex-specific effects of CNN described here suggest that sex needs to be taken into account when evaluating the effects of nicotine even in neonates. Additionally, it is unlikely that the differences in the maturation of the GABAergic system result in sex-specific responses to only nicotine. Rather, it is probable that a variety of environmental conditions which impact GABAergic transmission during development may also result in sex-specific outcomes and differentially derail normal development in males and females. Such differences might explain the fact that males are disproportionally affected by neurodevelopmental disorders such as schizophrenia, autism and ADHD (Bale et al., 2010).
Highlights.
mRNA expression patterns were examined in male and female rat hippocampus at postnatal day 5 and 8
The effects of chronic neonatal nicotine exposure on mRNA expression was compared in males and females
Males have higher expression of NKCC1, BDNF and NR2A and lower expression of NR2B at postnatal day 5
Postnatal nicotine exposure increase KCC2 and BDNF expression in male but not female hippocampus
No sex or treatment effect on mRNA expression were observed at postnatal day 8
Acknowledgements
The authors would like thank Dr. Jong-Hyun Son and Simona Slaton for their excellent technical assistance.
Role of the Funding Source:
J.C.D. received funding from:
Texas Consortium in Behavioral Neuroscience Predoctoral Fellowship (NIH #2-T32-MH065728)
Texas Brain and Spine Institute Graduate Fellowship
U. W.S. received funding from the Texas A&M Health Science Center Bridge Grant.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CNN
chronic neonatal nicotine
- DG
dentate gyrus
- GABA
γ-aminobutyric acid
- GABAAR
GABAA receptor
- KCC2
K+/Cl− co-transporter 2
- nAChR
nicotinic acetylcholine receptor
- NKCC1
Na2+/K+/Cl− co-transporter 1
- NMDAR
NMDA receptor
- NR2A
NMDA receptor subunit 2A
- NR2B
NMDA receptor subunit 2B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–6116. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Oliff HS, Isackson P, Cotman CW. Hippocampal BDNF mRNA shows a diurnal regulation, primarily in the exon III transcript. Brain Res Mol Brain Res. 1999;71:11–22. doi: 10.1016/s0169-328x(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- Bray JG, Mynlieff M. Influx of calcium through L-type calcium channels in early postnatal regulation of chloride transporters in the rat hippocampus. Dev Neurobiol. 2009;69:885–896. doi: 10.1002/dneu.20749. erratum 897-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Damborsky JC, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine exposure increases excitation in the young adult rat hippocampus in a sex-dependent manner. Brain Res. 2012;1430:8–17. doi: 10.1016/j.brainres.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Durand GM, Konnerth A. Long-term potentiation as a mechanism of functional synapse induction in the developing hippocampus. J Physiol Paris. 1996;90:313–315. doi: 10.1016/s0928-4257(97)87905-3. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008a;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008b;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Goebel DJ, Poosch MS. NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Brain Res Mol Brain Res. 1999;69:164–170. doi: 10.1016/s0169-328x(99)00100-x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Leslie FM, Metherate R. Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain. Brain Res Dev Brain Res. 2002;133:19–25. doi: 10.1016/s0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Abbott LC, Winzer-Serhan UH. Effects of chronic neonatal nicotine exposure on nicotinic acetylcholine receptor binding, cell death and morphology in hippocampus and cerebellum. Neuroscience. 2007a;146:1854–1868. doi: 10.1016/j.neuroscience.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Hsiao SH, Trzeciakowski J, Frye GD, Winzer-Serhan UH. Chronic nicotine induces growth retardation in neonatal rat pups. Life Sci. 2006;78:1483–1493. doi: 10.1016/j.lfs.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Liu X, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine increases anxiety but does not impair cognition in adult rats. Behav Neurosci. 2007b;121:1342–1352. doi: 10.1037/0735-7044.121.6.1342. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, et al. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504(Pt 3):603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen CX, Liu YJ, Aizenman E, Kandler K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur J Neurosci. 2005;21:2593–2599. doi: 10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature “silent” connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux JP, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. J Physiol. 2004;559:863–874. doi: 10.1113/jphysiol.2004.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JT. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron. 2011;70:339–351. doi: 10.1016/j.neuron.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Ferrer JR, Zeng WY, Haley NJ. Nicotine administration to rats: methodological considerations. Life Sci. 1987;40:1699–1708. doi: 10.1016/0024-3205(87)90020-8. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88:1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK, Stein V, Keating DJ, Maier H, Rinke I, Rudhard Y, Hentschke M, Rune GM, Jentsch TJ, Hubner CA. NKCC1-dependent GABAergic excitation drives synaptic network maturation during early hippocampal development. J Neurosci. 2009;29:3419–3430. doi: 10.1523/JNEUROSCI.1377-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann N Y Acad Sci. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- SAMHSA . In: Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Office of Applied Studies, N. S. H.-A., editor. Rockville, MD: 2010. HHS Publication No. SMA 10-4856Findings. [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Postnatal expression of alpha2 nicotinic acetylcholine receptor subunit mRNA in developing cortex and hippocampus. J Chem Neuroanat. 2006;32:179–190. doi: 10.1016/j.jchemneu.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Chronic neonatal nicotine exposure increases mRNA expression of neurotrophic factors in the postnatal rat hippocampus. Brain Res. 2009 doi: 10.1016/j.brainres.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Mem. 2008;90:527–536. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Davila-Garcia MI, Yarl W, Gondre-Lewis MC. Gestational nicotine exposure regulates expression of AMPA and NMDA receptors and their signaling apparatus in developing and adult rat hippocampus. Neuroscience. 2011;188:168–181. doi: 10.1016/j.neuroscience.2011.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Broide RS, Chen Y, Leslie FM. Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect alpha 2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res Brain Res Protoc. 1999;3:229–241. doi: 10.1016/s1385-299x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. J Comp Neurol. 2005;481:19–30. doi: 10.1002/cne.20357. [DOI] [PubMed] [Google Scholar]
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol. 2004;557:829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]