Abstract
Background & Aims
Bmi1 is a member of the Polycomb protein family and represses transcription by modifying chromatin organization at specific promoters. Bmi1 is implicated in the control of stem cell self-renewal and has been shown to regulate cell proliferation, tissue homeostasis, and differentiation. Bmi1 is present in a subpopulation of self-renewing pancreatic acinar cells and is expressed in response to pancreatic damage. We investigated the role of Bmi1 in exocrine pancreas regeneration.
Methods
Acute pancreatitis was induced in Bmi1−/− mice with caerulein; pancreatic cell regeneration, differentiation, and apoptosis were assessed. Cultured Bmi1−/− and wild-type primary acini were analyzed in vitro, to determine acinar-specific consequences of Bmi1 deletion. To investigate cell-autonomous vs non–cell-autonomous roles for Bmi1 in vivo, pancreatitis was induced in Bmi1−/− mice reconstituted with a wild-type hematopoietic system.
Results
Bmi1 expression was upregulated in the exocrine pancreas during regeneration after caerulein-induced pancreatitis. Exocrine regeneration was impaired following caerulein administration to Bmi1−/− mice. Pancreata of Bmi1−/− mice were hypoplastic, and the exocrine pancreas was replaced with ductal metaplasia that had increased apoptosis and decreased cell proliferation, compared to that of wild-type mice. Expression of Cdkn2a and p53-dependent apoptotic genes were markedly upregulated in Bmi1−/− pancreas, compared to wild-type mice, after injury. Furthermore, after transplantation of bone marrow from wild-type to Bmi1−/− mice, the chimeric mice had intermediate levels of pancreatic hypoplasia and significant, but incomplete, rescue of impaired exocrine regeneration after caerulein injury.
Conclusions
Bmi1 contributes to regeneration of the exocrine pancreas after caerulein-induced injury through cell-autonomous mechanisms—in part by regulating Cdkn2a expression—and non-cell-autonomous mechanisms.
Keywords: pancreatitis, polycomb proteins, tissue regeneration, mouse model
Bmi1, first identified as a collaborating oncogene through its ability to cooperate with cMYC in the induction of lymphoma1, 2, is a member of the Polycomb group proteins (PCG)3. PCG proteins combine in Polycomb repressive complexes (PRC) to epigenetically regulate gene expression by modifying chromatin organization at specific promoters4. Two major Polycomb repressive complexes (PRC1 and PRC2) have been described. PRC2, which contains EZH2, catalyses the trimethylation of histone H3 at lysine K27 (H3-K27)4. The lysine H3-K27 tag serves as a docking site for recruitment of PRC1, resulting in transcriptional repression via PRC1 mediated ubiquitylation of histone H2A on lysine 119 or protein sumoyation4. Bmi1 is indispensible for the function of the PRC1 complex, making it a key component of PCG mediated gene regulation.
Bmi1 is essential for the self-renewal of several types of adult stem cells including those within the hematopoietic system5, nervous system6, mammary gland7 and prostate8. A similar role has been noted within cancer stem cell populations found in leukemia9, breast7 and lung cancers10. These functions are in part attributed to the ability of the PRC1 complex to bind to and repress the Cdkn2a locus, which encodes both p16 (Ink4a) and p19 (Arf) 4, 11. p16 functions upstream of the tumor suppressor Rb and inhibits cell cycle progression by specifically blocking the cyclin-D/CDK4/6 complex, whereas p19 functions upstream of p53 by stabilizing p53 through inhibition of Mdm2 function11. Indeed, Bmi1 dependent regulation of these pathways has been shown to be important for tissue homeostasis, as evidenced by proliferative arrest and p53-dependent cell death of hematopoietic stem cells in the context of de-regulated p16Ink4a and p19Arf in Bmi1 knock out (KO) mice5.
Mouse models of acute pancreatitis have revealed that the exocrine pancreas possesses remarkable regenerative capacity after inflammatory tissue damage. In response to acute pancreatitis induced by the cholecystokinin analogue caerulein, acini can transiently de-differentiate into proliferative, duct-like structures that express markers characteristic of pancreatic embryonic progenitors, such as Pdx112. Developmental signaling pathways, including the Hedgehog, Notch, and Wnt pathways are also reactivated during exocrine regeneration, and have been shown to be critical for exocrine recovery following caerulein treatment in mice13–16. In the absence of further damage, re-differentiation into functional acinar cells occurs rapidly within one week after cessation of caerulein treatment12–15, 17.
Recently, cell lineage tracing experiments performed in Bmi1-CreER mice have revealed a subpopulation of differentiated pancreatic acinar cells with proliferative capacity18. Long-term Bmi1 lineage tracing experiments in the pancreas demonstrate that Bmi1 lineage-labeled cells remain consistent over a year, demonstrating that Bmi1 is present in a subpopulation of self-renewing pancreatic acinar cells18. In addition, Bmi1 expression is induced in pancreatic acinar cells after acute injury during regeneration18, 19 and is critical for beta cell regeneration through regulation of the Ink4a/Arf locus20. However, the functional role of Bmi1 in exocrine pancreas regeneration remains unknown.
In the present study, we show impaired exocrine pancreas regeneration resulting in organ hypoplasia in Bmi1 KO mice after caerulein-induced injury. The decrease in regenerative capacity was associated with markedly increased apoptosis and decreased cell proliferation. Furthermore, Bmi1 target genes, p16, p19 and downstream p53 pro-apoptotic genes were markedly upregulated in Bmi1 KO pancreas after injury. Finally, using in vivo bone marrow transplantation and in vitro acinar culture approaches, we show that pancreatic hypoplasia and impaired exocrine regeneration is mediated through a combination of cell autonomous and non-cell autonomous mechanisms in Bmi1 KO mice after caerulein injury.
Materials and Methods
Detailed methods are described in the Supplementary Materials and Methods.
Mice
Bmi1GFP/+ knock-in mice have been described previously21. Transgenic mice were on a mixed, background. Littermate Bmi1GFP/+ or wild-type mice were used as controls. All mouse experiments were performed under the approval of the UCSF Institutional Care and Use of Animals Committee (IACUC).
Results
Bmi1 expression is up-regulated during exocrine regeneration following acute caerulein pancreatitis
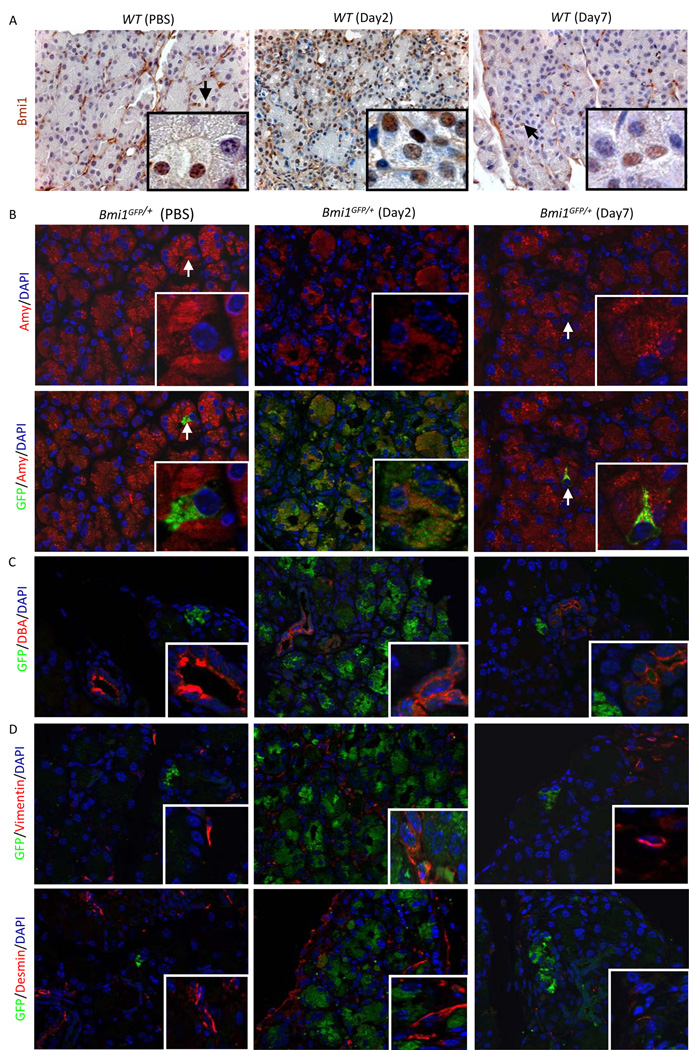
To explore a possible role for Bmi1 during exocrine regeneration, we first ascertained the Bmi1 expression pattern following acute caerulein pancreatitis. As shown previously18, Bmi1 is present in a small subset (~1.5%) of adult acini in wild-type (WT ) mice at the age of 5–8 weeks (Figure 1A). In contrast, we observed a significant expansion of Bmi1 expression in acinar cells (~97%) in WT mice two days after acute caerulein-induced injury (Figure 1A). Seven days after caerulein treatment, following considerable regeneration, exocrine Bmi1 expression was again restricted to rare acini (Figure 1A). This pattern was also confirmed using GFP as a reporter for Bmi1 expression in Bmi1GFP/+ mice, in which the endogenous Bmi1 gene has been replaced through homologous recombination with GFP21. A small subset (~0.9%) of acinar cells were positive for GFP in Bmi1GFP/+ mice before caerulein injury (Figure 1B) compared to nearly ubiquitous GFP expression in acinar cells (~98%) at day 2 post-caerulein treatment (Figure 1B). We also noted GFP expression in damaged acini at 4, 8, and 24 hours after the last injection of caerulein (Supplementary Figure 1). GFP expression did not colocalize with the mature duct marker DBA lectin, or the pancreatic stellate cell markers vimentin and desmin in PBS-treated mice, at day 2 and day 7 post-caerulein treatment (Figure 1C, 1D). These data indicate dynamic Bmi1 expression following caerulein pancreatitis that is mainly limited to acinar cells during exocrine pancreas regeneration.
Figure 1. Bmi1 expression is upregulated in the exocrine pancreas after caerulein injury.
(A) Immunohistochemistry for Bmi1 in the pancreas in adult wild-type mice, 2 days post-PBS injection or 2 and 7 days post-caerulein injection. Inset, arrows indicate Bmi1-positive acinar cells.
(B) Immunofluorescence for amylase, GFP, and DAPI in the pancreas of adult Bmi1GFP/+ mice after PBS injection or 2 and 7 days post-caerulein injection. Arrows indicate GFP-positive acinar cells.
(C) Immunofluorescence for DBA lectin, GFP, and DAPI in the pancreas of adult Bmi1GFP/+ mice after PBS injection or 2 and 7 days post-caerulein injection.
(D) Immunofluorescence for vimentin or desmin, GFP, and DAPI in the pancreas in adult Bmi1GFP/+ mice after PBS injection or 2 and 7 days post-caerulein injection.
Bmi1 KO mice display pancreatic hypoplasia and impaired exocrine pancreas regeneration after acute caerulein and CDE diet induced pancreatitis
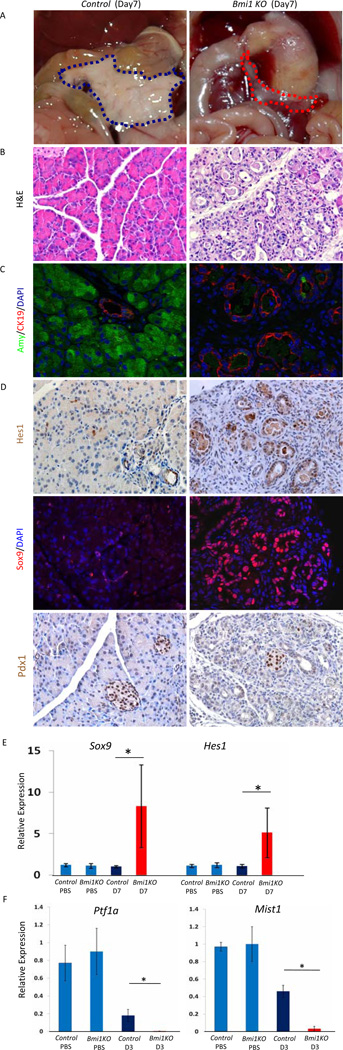
In order to determine if Bmi1 plays a functional role in exocrine pancreas regeneration, we subjected homozygous Bmi1GFP/GFP mice (Bmi1 KO ) to acute caerulein pancreatitis at 6 weeks of age21. In the absence of damage, pancreatic architecture and exocrine histology was indistinguishable between Bmi1 KO and control mice (data not shown), indicating that Bmi1 is dispensable for exocrine pancreas development. Seven days after caerulein treatment in WT controls, exocrine regeneration was nearly complete and acinar morphology appeared nearly indistinguishable from untreated mice (Figure 2B). Strikingly, Bmi1 KO mice displayed gross pancreatic hypoplasia (Figure 2A). H&E staining and immunostaining for amylase (acini) and cytokeratin19 (CK19, ducts) revealed a dramatic reduction in acinar cells accompanied by a profound increase in duct-like epithelial cells (Figure 2B & 2C). Notably, pancreatic hypoplasia, ductal metaplasia, and loss of acinar cells persisted 3 weeks after caerulein treatment, suggesting that acinar regeneration is indeed blocked, and not merely delayed, in the absence of Bmi1 (Supplementary Figure 2A–2C).
Figure 2. Impaired exocrine pancreas regeneration in Bmi1 KO mice after caerulein pancreatitis.
Control or Bmi1 KO mice were injected with caerulein and sacrificed 7 days post-injection.
(A) Macroscopic views of pancreas (outlined with dashed lines), showing hypoplastic pancreas in Bmi1 KO mice (red) compared to control mice (blue).
(B) H&E staining showing impaired exocrine pancreas regeneration with increased duct-like structures and reduced acinar area in Bmi1 KO pancreas compared to control mice.
(C) Co-staining for amylase/CK19/DAPI reveals reduced number of amylase-positive cells and increased number of CK19-positive duct-like epithelial cells in Bmi1 KO pancreas.
(D) Immunostaining for Hes1, Sox9, and Pdx1. Immunohistochemistry shows Hes1 expression in the duct-like epithelial cells in Bmi1 KO pancreas 7 days post-injection. Co-staining for Sox9/DAPI demonstrates Sox9 expression in the duct-like epithelial cells in Bmi1 KO pancreas 7 days post-injection. Immunohistochemistry shows absence of Pdx1 expression in the duct-like epithelial cells in Bmi1 KO pancreas 7 days post-injection. Pdx1 remains expressed in control and mutant islets.
(E) Relative expression levels of Sox9 and Hes1 in control and Bmi1 KO pancreata 7 days post-injection by q-PCR. N = 3 mice.
(F) Relative expression levels of Ptf1a and Mist1 in control and Bmi1 KO pancreata 3 days post-injection. N = 3 mice. Means ± SD. * p< 0.05.
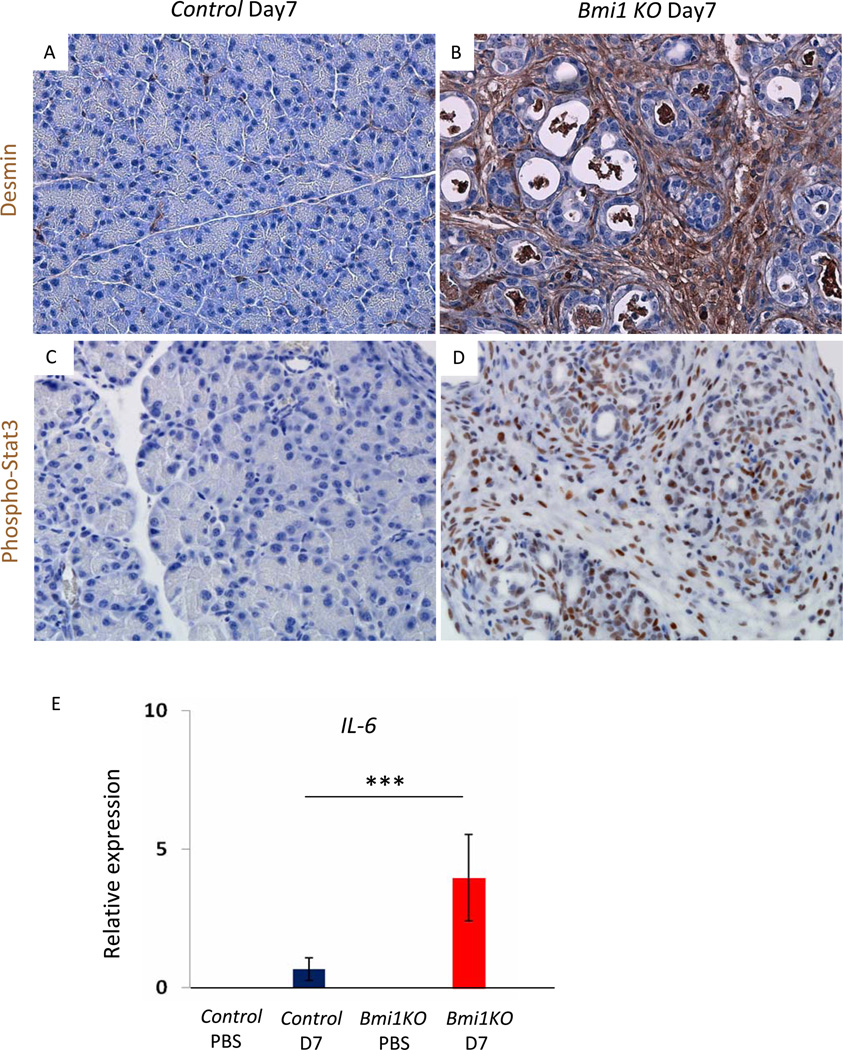
During regeneration, acinar cells temporarily express embryonic progenitor markers, including Pdx1, Hes1, and Sox912, 13. Immunostaining and quantitative RT-PCR (q-PCR) analyses revealed persistent expression of Sox9 and Hes1 in Bmi1KO epithelium at day 7 after injury (Figure 2D, 2E). In contrast, epithelial cells remained negative for Pdx1 (Figure 2D). This suggests that damaged acinar cells were either eliminated and replaced by ductal cells, or remained duct-like and failed to reactivate the complement of differentiation pathways characteristic of acinar regeneration. Indeed, the expression of transcription factors Ptf1a and Mist1, which are expressed in adult acini and crucial for exocrine pancreas differentiation22, 23,24, were dramatically down-regulated in Bmi1 KO pancreas compared to control pancreas 3 days after injury (Figure 2F). Furthermore, H&E and desmin staining showed that the remaining duct structures were embedded in an expanded stromal compartment, absent in regenerating control mice day 7 after injury (Figure 2B, 3A, 3B). The persistent stromal reaction occurred in parallel with prolonged inflammation in Bmi1 KO pancreas at day 7 after injury, as evidenced by sustained upregulation of IL6 expression and active, phospho-Stat3 (Figure 3C, 3D, 3E).
Figure 3. Stromal reaction and persistent inflammation in Bmi1 KO pancreas after caerulein pancreatitis.
(A–B) Immunohistochemistry for desmin in control (A) and Bmi1 KO pancreata (B) 7 days after caerulein treatment.
(C–D) Immunohistochemistry for phospho-Stat3 in control (C) and Bmi1 KO pancreata (D) 7 days after caerulein treatment.
(E) Relative expression levels of IL-6 in control and Bmi1 KO pancreata by q-PCR analysis. N = 3 mice. Means ± SD. ***p< 0.001.
To determine if the requirement for Bmi1 is exclusive to exocrine regeneration following careulein pancreatitis, we also challenged female WT and Bmi1 KO mice with choline deficient ethionine-supplemented (CDE) diet induced pancreatitis25. H&E staining revealed a similar degree of exocrine disorganization and mild edema at day 4 after the initiation of the CDE diet in both control and Bmi1KO female mice (Supplementary Figure 3A). Immunohistochemistory showed that Bmi1 and Sox9 were expressed in acinar cells in Bmi1GFP/+ mice at that time point (Supplementary Figure 3B). Furthermore, we observed coexpression of GFP and clusterin, a marker of immature, regenerating acini13, 14, in acinar cells in Bmi1GFP/+ mice, suggesting that Bmi1 is expressed in damaged acinar cells following CDE diet induced pancreatitis in a similar fashion as what we have observed after caerulein pancreatitis (Fig. 1; Supplementary Figure 3B). At day 8 after initiation of the CDE diet, acinar regeneration was completed in control mice, whereas acinar regeneration remained impaired in Bmi1 KO mice (Supplementary Figure 3C). Thus, Bmi1 is critical for acinar regeneration following both caerulein and CDE induced pancreatitis.
Characterization of the initial stages of pancreatitis in Bmi1 KO mice
To determine if compromised acinar regeneration correlated with a more severe initial response to caerulein pancreatitis, we analyzed Bmi1 KO mice at day 1 and day 2 post-caerulein injection. H&E staining showed duct-like epithelial cells in both control and Bmi1 KO pancreata at the first two days post-caerulein injection (Supplementary Figure 4A). At day 1, duct-like epithelial cells frequently co-expressed amylase and CK19. At day 2 we detected widespread co-expression of clusterin and Sox9 in both control and Bmi1 KO mice (Supplementary Figure 4C), Furthermore, serum amylase, another parameter for the severity of acute pancreatitis, was indistinguishable between control and Bmi1 KO mice before caerulein injection and at day 1 post-caerulein injection (Supplementary Figure 5A). Thus, Bmi1 null acini appear to respond to acute pancreatitis in a manner comparable to WT acini. However, while double amylase, CK19 positive cells persisted at day 2 in WT mice, the level of amylase expression appeared relatively reduced at the expense of CK19 in duct-like epithelial cells in Bmi1 KO mice (Supplementary Figure 4B). These data indicate that acinar regeneration starts to be compromised at day 2 following caerulein in Bmi1 KO mice.
Prior work from our lab and others has demonstrated that the early stages of caerulein pancreatitis are associated with pancreatic inflammation26–28. To determine whether Bmi1 loss affects inflammatory cell infiltration at the onset of caerulein pancreatitis, we performed staining for markers of hematopoietic cells and macrophages, respectively. Staining for CD45 and F4/80 showed a similar degree of inflammatory infiltrates and macrophages at day 1 and day 2 post-caerulein injection, suggesting that the initial inflammation in the pancreas is comparable between Bmi1 KO and control mice after caerulein injury (Supplementary Figure 6). In addition, to assess another established parameter for the systemic severity of pancreatitis, we analyzed lung histology after caerulein injury. H&E staining showed that the degree of alveolar membrane thickening and infiltration of inflammatory cells were comparable between Bmi1 KO and control mice both at day 1 and day 2 post-caerulein injection (Supplementary Figure 5B). In addition, immunostaining for CD45 and myeloperoxidase (MPO) in the lung revealed that the degree of inflammatory and neutrophil infiltration was comparable between Bmi1 KO and control mice at day 2 post-caerulein injection (Supplementary Figure 5C). Therefore, Bmi1 loss does not appear to significantly alter acute inflammation during caerulein induced pancreatitis.
Increased apoptosis and decreased cell proliferation in Bmi1 KO mice after caerulein injury
Next we tested whether the pancreatic hypoplasia in Bmi1 KO mice after injury was associated with changes in apoptosis or cell proliferation. Immunostaining and quantification of cleaved-Caspase3/E-cadherin double-positive cells revealed a dramatic increase in cleaved-Caspase3 positive pancreatic epithelial cells in Bmi1 KO mice compared to control mice at both day 3 and 7 after caerulein-induced injury (Figure 4A and 4C and data not shown). Increased apoptosis was also confirmed by a similar increase in the number of TUNEL/E-cadherin double-positive cells in the Bmi1 KO pancreatic epithelium when compared to controls (Figure 4B). In addition, pancreatitis associated apoptosis was also increased in Bmi1 KO mice compared to WT mice 8 days after the initial CDE treatment (Supplementary Figure 3C).
Figure 4. Increased apoptosis and decreased cell proliferation in Bmi1 KO pancreas after caerulein pancreatitis.
(A) Co-staining for cleaved Caspase 3 (c-Caspase 3)/E-cadherin/DAPI in control and Bmi1 KO pancreata 3 days post caerulein injection.
(B) Co-staining for TUNEL/E-cadherin/DAPI in contro l and Bmi1 KO pancreata 3 days post caerulein injection.
(C) Quantification of the number of Cleaved Caspase 3/E-cadherin double positive cells per field in control and Bmi1 KO pancreata 3 days and 7 days post-injection. N = 3 mice.
(D) Co-staining for phospho-histone H3 (PHH3)/E-cadherin/DAPI in control and Bmi1 KO pancreata 3 days post caerulein injection.
(E) Quantification of the number of PHH3/E-cadherin double positive cells per field in control and Bmi1 KO pancreata 3 days and 7 days post caerulein injection. N = 3 mice. Means ± SD. ** p < 0.01.
We next determined whether cell proliferation is affected in Bmi1 KO pancreas after caerulein injury. Immunostaining and quantification demonstrated that the number of cells double positive for the mitosis marker phospho-histone H3 (pHH3) and E-cadherin was significantly decreased in the Bmi1 KO pancreatic epithelium compared to control mice at both day three and day seven after caerulein treatment (Figure 4D and 4E and data not shown). Therefore, both markedly increased apoptosis and decreased cell proliferation contribute to the hypoplastic pancreas observed in Bmi1 KO mice folowing acute caerulein pancreatitis.
p16, p19, and p53 dependent apoptotic genes are upregulated in Bmi1 KO pancreas after injury
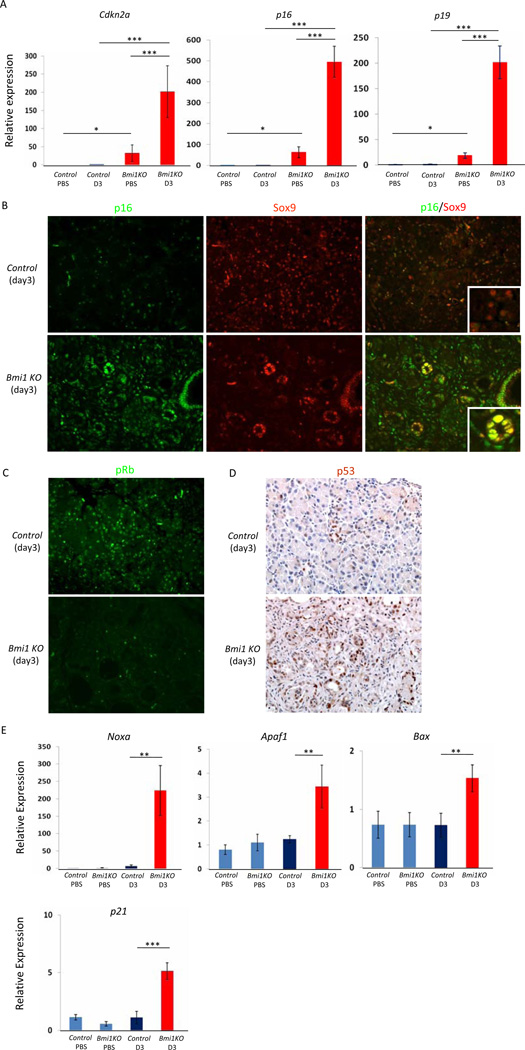
Bmi1 has been shown to control cell proliferation during self-renewal in part through controlling transcription of the Cdkn2a locus, encoding the tumor suppressors p16 and p19 4, 11. We found that Bmi1 constrained Cdkn2a, p16, and p19 expression in the pancreas, as PBS-treated Bmi1 KO mice displayed increased expression compared to PBS-treated wild-type controls (Figure 5A). Furthermore, we observed a dramatic increase of both p16 (Ink4a ) and p19 (Arf ) mRNA transcripts in Bmi1 KO pancreas three days after caerulein-injury compared to control pancreas (Figure 5A). Co-immunostaining of p16 and Sox9 confirmed increased p16 expression in pancreatic epithelium undergoing metaplasia in Bmi1 KO mice compared to control mice three days after injury (Figure 5B). p16 impairs cell cycle progression by inhibiting the phosphorylation and subsequent inactivation of Rb. Correlating with increased p16 expression levels, we observed a significant reduction in phospho-Rb levels in Bmi1 KO pancreas compared to control pancreas after injury (Figure 5C). In parallel, p19 expression leads to stabilization of the tumor suppressor p53 via inhibition of its repressor Mdm211. Immunofluorescence revealed increased p53 expression in Bmi1 KO mice compared to control mice 3 days after injury (Figure 5D). In addition, q-PCR analyses revealed that p53 target genes involved in apoptosis, including Noxa, Apaf1, and Bax were significantly upregulated in Bmi1 KO pancreas (Figure 5E). Furthermore, the expression of the cell cycle inhibitor p21, regulated in part through p53, was also significantly elevated in Bmi1 KO pancreas (Figure 5E). Taken together, loss of Bmi1 function during exocrine regeneration results in dramatic upregulation of cell cycle inhibitory mechanisms downstream of p16 and p19, as well as p53 dependent apoptotic genes.
Figure 5. p16, p19 and p53 apoptotic pathways are upregulated in Bmi1 KO pancreata after caerulein injury.
(A) Relative expression levels of Cdkn2a, p16 and p19 in the pancreas by q-PCR analyses. N = 3 mice. Means ± SD
(B) Co-staining for p16/Sox9 in control and Bmi1 KO pancreata 3 days post-injection. The merged image shows increased p16 expression in Sox9-positive duct-like epithelial cells in Bmi1 KO mice 3 days post caerulein injection.
(C) Co-staining for phospho-Rb/DAPI in control and Bmi1 KO pancreata 3 days post caerulein injection.
(D) Immunohistochemistry for p53 in control and Bmi1 KO pancreata 3 days post caerulein injection.
(E) Relative expression levels of Noxa, Apaf1, Bax and p21 in the pancreas by q-PCR analyses. N = 3 mice. Means ± SD. * p < 0.05,** p < 0.01, and ***p< 0.001.
Pancreatic hypoplasia and impaired exocrine regeneration are mediated through combination of cell-autonomous and non-cell-autonomous mechanisms in Bmi1 KO mice
Previous reports have shown that Bmi1 is essential for maintenance of adult hematopoietic stem cells and that Bmi1 KO mice have defective hematopoietic systems with markedly reduced hematopoietic stem cells5, 29. Hematopoietic cell numbers are reduced in Bmi1 KO mice beginning in early development and the absolute numbers of both B lymphoid and myeloid cell populations are significantly reduced in adult Bmi1 KO mice compared to WT mice29. Therefore, it is possible that the profound systemic hematopoietic defects in Bmi1 KO mice and the prolonged inflammation observed in Bmi1 KO mice might contribute to non-cell-autonomous mechanisms that affect exocrine regeneration after caerulein injury.
To address this question, we transplanted WT bone marrow into irradiated WTh and Bmi1 KO mice at 6–9 weeks of age (Figure 6A). Eight weeks after bone marrow transplantation, we confirmed hematopoietic reconstitution in Bmi1 KO chimeric mice by assessing the presence and relative abundance of GFP-negative, CD45-positive cells by flow cytometry (Supplementary Figure 7A) and the presence of the Bmi1 WT allele by PCR from splenic DNA (Supplementary Figure 7B). Transplanted chimeric animals were subjected to caerulein induced pancreatitis and analyzed for pancreatic defects seven days after caerulein treatment. Interestingly, we observed an intermediate level of pancreatic hypoplasia in Bmi1KO chimeric mice that was more severe than what we found in chimeric WT control mice, but less pronounced than the defects observed in non-chimeric Bmi1 KO mice (Figure 6B, 6C). Histologic, morphometric, and immunofluorescence analysis of regenerated acinar area confirmed this observation (Figure 6B, 6D). Immunostaining for E-cadherin/cleaved Caspase 3 revealed increased apoptosis in the pancreas of the reconstituted Bmi1 KO chimeras (Figure 6E) compared to WT reconstituted controls, findings that were supported by increased expression of Cdkn2a, p16 and p19 as well as the p53 dependent pro-apoptotic genes Noxa and Apaf1 in the reconstituted Bmi1 KO chimeric mice (Figure 6F). Although there was a trend towards partially reduced expression of p16, p19, Noxa, and Apaf1 in WT reconstituted Bmi1KO mice compared to Bmi1 KO mice 7 days after caerulein, only Apaf1 expression was significantly decreased (Figure 6F). Therefore, while epithelial Bmi1 may play a major role in suppressing genes that could drive exocrine cell death, Bmi1 appears to function both in pancreatic epithelial cells as well as in hematopoietic cells to regulate acinar cell regeneration after caerulein-induced injury.
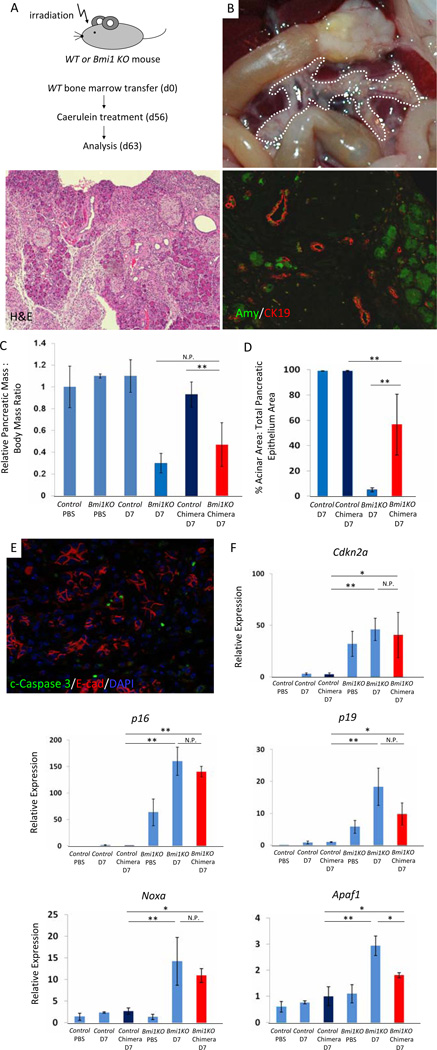
Figure 6. Hypoplastic pancreas and impaired exocrine pancreas regeneration are mediated through a combination of cell autonomous and non-cell autonomous mechanisms in Bmi1 KO mice after caerulein pancreatitis.
(A) Experimental outline. Wild-type bone marrow was transplanted into irradiated wild-type or Bmi1 KO mice at 6–8 weeks. 8 weeks after bone marrow transplantation, reconstituted Bmi1 KO chimera and wild type mice were subjected to caerulein pancreatitis and were sacrificed 7 days after treatment.
(B) Macroscopic view of the pancreas reveals moderate pancreatic hypoplasia in reconstituted Bmi1 KO chimera mice 7 days after caerulein treatment. H&E stain and Co-staining for amylase/CK19 show exocrine pancreas regeneration is partially impaired in reconstituted Bmi1 KO chimeric mice 7 days after caerulein treatment.
(C) Relative pancreatic weight normalized to body weight. N = 3 to 6 mice. Means ± SD. Quantification of acinar regeneration. N = 3 or 4 mice. Means ± SD. Co-staining for cleaved Caspase 3/E-cadherin/DAPI in reconstituted Bmi1 KO chimeric mice reveals presence of apoptotic cells 7 days after caerulein treatment.
(D) Relative expression levels of Cdkn2a, Noxa, and Apaf1 in the pancreas by q-PCR analysis. N = 3 or 4 mice. Means ± SD. *p < 0.05, ** p < 0.01. N.P. = not significant p value.
To further define the cell autonomous consequences of Bmi1 depletion, we examined changes in potential Bmi1 targets in cultured primary acini treated with 10 nM caerulein for 2 days. Immunostaining confirmed the lack of Bmi1 protein in mutant, amylase positive acini in vitro (Figure 7A–7D). We found that Bmi1 deletion results in significant upregulation of the Cdkn2a locus and its splice variants p16 and p19 even in the absence of caerulein treatment. p16 expression remained high and Cdkna2, p19, and Noxa expression were further increased two days after caerulein treatment (Figure 7E). These data support a cell autonomous role for Bmi1 in preventing expression of anti-proliferative or pro-apoptotic genes. Taken together with the in vivo result showing considerable recovery of exocrine parenchyma in WT reconstituted Bmi1 KO mice, Bmi1 appears to regulate pancreatic exocrine regeneration through a combination of cell-autonomous and non-cell-autonomous mechanisms.
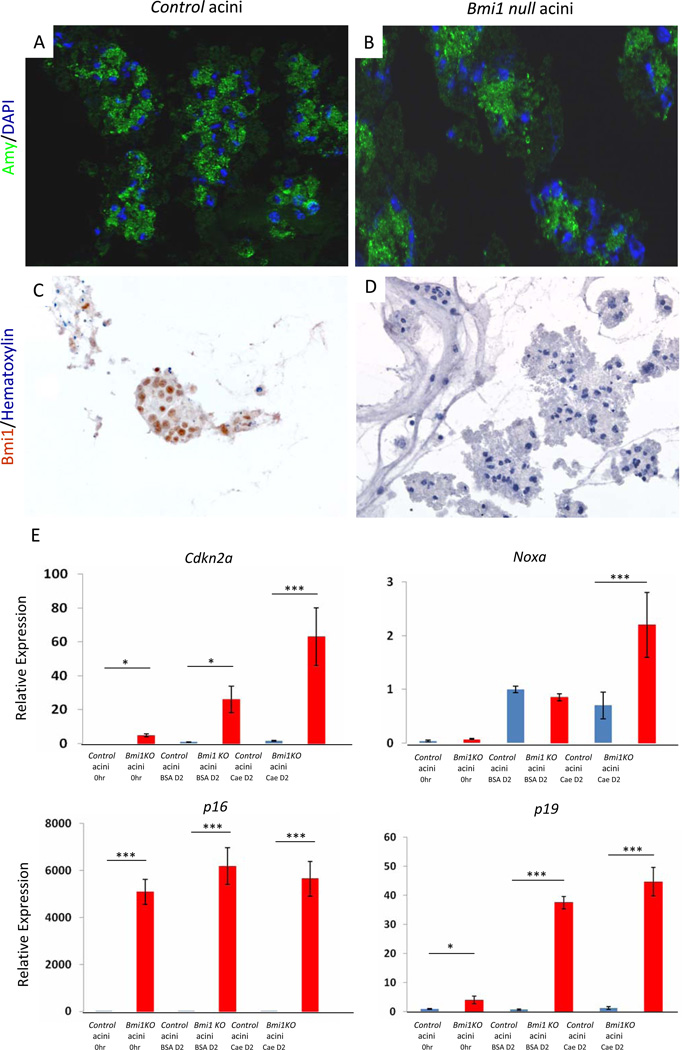
Figure 7. Cdkn2a and Noxa expression is upregulated in a cell-autonomous manner in Bmi1 null cultured acini treated with caerulein in vitro.
(A–B) Immunohistochemistry for amylase and DAPI in control (A) and Bmi1 KO cultured acini (B) in vitro.
(C–D) Immunohistochemistry for Bmi1 in control (C) and Bmi1 KO cultured acini (D) in vitro.
(E) Relative expression levels of Cdkn2a and Noxa in control or Bmi1 null cultured acini treated with BSA or caerulein in vitro by q-PCR analyses. N = 3. Means ± SD. *p < 0.05 and ***p < 0.001.
Discussion
Recent reports have revealed that when damaged, adult acinar cells undergo de-differentiation, regeneration, and in some cases trans-differentiation, depending on the severity of damage and the genetic context in which it takes place13, 30, 31. Here, we show that Bmi1 functions to inhibit apoptosis and permit proliferation during injury-induced acinar regeneration. Bmi1 has been shown to be critical for the self-renewal of several types of adult stem cells. Although a number of recent studies have aimed to define adult pancreatic cells with a “stem-like” capacity to recapitulate multiple cell compartments32–35, acinar cells appear to predominantly regenerate from pre-existing acinar cells that undergo de-differentiation to assume a facultative progenitor state following caerulein pancreatitis12, 15. Interestingly, we observe no gross defects in Bmi1 null pancreata unless challenged with acute chemical or diet induced pancreatitis. Therefore, our data suggests that Bmi1 predominately functions to acutely inhibit apoptosis and support proliferation of regenerating exocrine cells that have transiently changed their differentiation state, which may reflect a general role for Bmi1 in cells undergoing self renewal.
In Bmi1 KO mice, de-regulated expression of p16Ink4a and p19Arf in hematopoietic stem cells results in proliferative arrest and p53-dependent cell death, respectively5. Also, Bmi1 inhibits apoptosis in neurons through repression of p53 dependent apoptosis36. In this study, we observed dramatic upregulation of p16, p19, and p53 dependent apoptotic genes in Bmi1KO pancreata after caerulein injury. Therefore, regulation of Cdkn2a expression appears to be a general mechanism by which Bmi1 contributes to tissue regeneration. However, specific removal of Cdkn2a in acinar cells in the context of Bmi1 mutant mice would need to be performed to determine if other growth inhibitory pathways might depend on pancreatic Bmi1 expression for appropriate regulation during regeneration.
Recently, Mallen-St Clair and colleagues reported that EZH2, an indispensible component of the Polycomb repressive complex 2 (PRC2), plays a critical role in exocrine pancreas regeneration37. They observed similarly compromised acinar regeneration in mice with pancreas specific EZH2 deletion after caerulein-induced pancreatitis as observed in Bmi1 KO mice in this study37. Furthermore, they showed that deleting Ink4a in the absence of EZH2 rescued acinar regeneration, demonstrating a critical involvement of the EZH2/Ink4a axis in this process. Given the fact that the lysine H3-K2 tag catalyzed by PRC2 facilitates PRC1 mediated transcriptional repression of the Ink4a locus4, their work supports Bmi1 dependent control of Ink4a expression as an important component of exocrine regeneration. Notably, deregulated Ink4a expression is also a critical downstream target of Bmi1 and EZH2 in endocrine cells as Bmi1 KO mice and mice in which EZH2 has specifically been deleted in beta cells display similar diabetic phenotypes and deficient beta cell regeneration38.
Our data showing that exocrine regeneration is significantly restored in Bmi1 KO mice reconstituted with WT bone marrow demonstrate that non-cell autonomous effects of Bmi1, possibly due to defects in hematopoietic cells, contribute to the impaired exocrine regeneration in Bmi1 KO mice. While some inflammatory cell types, such as CD4+ T-cells and dendritic cells26 39, have been shown to play roles in both the development of acute pancreatitis and subsequent tissue regeneration, hematopoietic cell types specifically dependent on Bmi1 (such as myeloid cells and B-Cells) have not been investigated in this process. Further investigation is required to determine what roles these cell types might play in maintaining viability, proliferation, and re-establishing tissue architecture during pancreatic regeneration. Interestingly, we show that Bmi1 KO mice display persistent inflammation following caerulein pancreatitis, as evidenced by chronic cytokine expression and Stat3 signaling. Therefore, Bmi1 dependent inflammatory cells might be involved in constraining the inflammatory response during pancreatic regeneration.
Taken together, our findings that Bmi1 is required for normal acinar regeneration have possible implications for pancreatic disease in humans. Bmi1 appears to play a critical role in permitting a proliferative state that is not only required for pancreatic homeostasis, but one that shares similarities with pancreatic cells undergoing both persistent damage, such as in chronic pancreatitis, as well as transformation and malignant progression.
Supplementary Material
Acknowledgements
We thank Irving L. Weissman for providing Bmi1GFP/+ mice. We indebted to Cecilia Austin for tissue processing and preparation, Renee Vanderlaan and Ruth Taniguchi for technical help, Limor Landsman for flow cytometry analyses, and all Hebrok lab members for helpful discussion. Work in M.H.’s laboratory was supported by a grant from the NIH (CA112537). A. Fukuda was supported by a post-doctoral Research Fellowship of the Japan Society for the Promotion of Science, a Fellowship from the U.S. National Pancreas Foundation, and the Klein Family Foundation Fellowship. Image acquisition was supported by the imaging core of the UCSF Diabetes and Endocrinology Research Center (DERC) NIH grant P30DK63720. Flow cytometry analysis was supported by the DERC FACS Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ involvement
A.F.: study concept and design, acquisition and analysis of data, drafting of the manuscript J.P.M.IV: technical support, interpretation of data, critical revision of the manuscript M.H.: study supervision, including study design, analysis and interpretation of data, critical revision of the manuscript.
The authors declare no conflicts of interest.
REFERENCES
- 1.van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991;65:737–752. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]
- 2.Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991;65:753–763. doi: 10.1016/0092-8674(91)90383-a. [DOI] [PubMed] [Google Scholar]
- 3.van Lohuizen M, Frasch M, Wientjens E, Berns A. Sequence similarity between the mammalian bmi-1 proto-oncogene and the Drosophila regulatory genes Psc and Su(z)2. Nature. 1991;353:353–355. doi: 10.1038/353353a0. [DOI] [PubMed] [Google Scholar]
- 4.Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer. 2009;9:773–784. doi: 10.1038/nrc2736. [DOI] [PubMed] [Google Scholar]
- 5.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 6.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7:682–693. doi: 10.1016/j.stem.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 10.Dovey JS, Zacharek SJ, Kim CF, Lees JA. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci U S A. 2008;105:11857–11862. doi: 10.1073/pnas.0803574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Morris JPt, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siveke JT, Lubeseder-Martellato C, Lee M, Mazur PK, Nakhai H, Radtke F, Schmid RM. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, Dor Y, Stoffers D, Jensen J, Leach SD, Maitra A. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keefe MD, Wang H, De La OJ, Khan A, Firpo MA, Murtaugh LC. beta-catenin is selectively required for the expansion and regeneration of mature pancreatic acinar cells in mice. Dis Model Mech. 2012 doi: 10.1242/dmm.007799. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De La OJ, Murtaugh LC. Notch and Kras in pancreatic cancer: at the crossroads of mutation, differentiation and signaling. Cell Cycle. 2009;8:1860–1864. doi: 10.4161/cc.8.12.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci U S A. 2009;106:7101–7106. doi: 10.1073/pnas.0902508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Romero C, Rooman I, Skoudy A, Guerra C, Molero X, Gonzalez A, Iglesias M, Lobato T, Bosch A, Barbacid M, Real FX, Hernandez-Munoz I. The epigenetic regulators Bmi1 and Ring1B are differentially regulated in pancreatitis and pancreatic ductal adenocarcinoma. J Pathol. 2009;219:205–213. doi: 10.1002/path.2585. [DOI] [PubMed] [Google Scholar]
- 20.Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev. 2009;23:906–911. doi: 10.1101/gad.1742609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosen N, Yamane T, Muijtjens M, Pham K, Clarke MF, Weissman IL. Bmi-1-green fluorescent protein-knock-in mice reveal the dynamic regulation of bmi-1 expression in normal and leukemic hematopoietic cells. Stem Cells. 2007;25:1635–1644. doi: 10.1634/stemcells.2006-0229. [DOI] [PubMed] [Google Scholar]
- 22.Krapp A, Knofler M, Ledermann B, Burki K, Berney C, Zoerkler N, Hagenbuchle O, Wellauer PK. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–3763. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 24.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lombardi B, Estes LW, Longnecker DS. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975;79:465–480. [PMC free article] [PubMed] [Google Scholar]
- 26.Demols A, Le Moine O, Desalle F, Quertinmont E, Van Laethem JL, Deviere J. CD4(+ )T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;118:582–590. doi: 10.1016/s0016-5085(00)70265-4. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeki K, Kanai T, Nakano M, Nakamura Y, Miyata N, Sujino T, Yamagishi Y, Ebinuma H, Takaishi H, Ono Y, Takeda K, Hozawa S, Yoshimura A, Hibi T. CCL2-Induced Migration and SOCS3-Mediated Activation of Macrophages Are Involved in Cerulein-Induced Pancreatitis in Mice. Gastroenterology. 2012 doi: 10.1053/j.gastro.2011.12.054. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 30.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2010;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 33.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatoo W, Abdouh M, David J, Champagne MP, Ferreira J, Rodier F, Bernier G. The polycomb group gene Bmi1 regulates antioxidant defenses in neurons by repressing p53 pro-oxidant activity. J Neurosci. 2009;29:529–542. doi: 10.1523/JNEUROSCI.5303-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallen-St Clair J, Soydaner-Azeloglu R, Lee KE, Taylor L, Livanos A, Pylayeva-Gupta Y, Miller G, Margueron R, Reinberg D, Bar-Sagi D. EZH2 couples pancreatic regeneration to neoplastic progression. Genes Dev. 2012;26:439–444. doi: 10.1101/gad.181800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, Kim SK. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bedrosian AS, Nguyen AH, Hackman M, Connolly MK, Malhotra A, Ibrahim J, Cieza-Rubio NE, Henning JR, Barilla R, Rehman A, Pachter HL, Medina-Zea MV, Cohen SM, Frey AB, Acehan D, Miller G. Dendritic Cells Promote Pancreatic Viability in Mice with Acute Pancreatitis. Gastroenterology. 2011;141:1915–1926. doi: 10.1053/j.gastro.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.