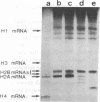
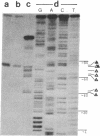
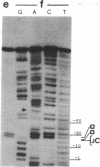
Abstract
Conserved DNA sequence elements of putative regulatory functions were deleted from the prelude region of a sea urchin H2A histone gene. For this, the wild-type H2A gene of the 6-kilobase histone DNA repeat unit was replaced by various mutant H2A genes by cloning. The effects of the manipulation on H2A mRNA synthesis were studied by injection of the mutant DNAs into centrifuged Xenopus oocytes. The unmanipulated H2B gene residing within the same repeat unit provided a suitable internal control for these studies. Deletion of the T-A-T-A-A-A-T-A motif, once thought to be the functional equivalent of the bacterial Pribnow box, did not abolish transcription of the gene; instead, a number of novel mRNA 5' termini were generated. We argue that the T-A-T-A-A-A-T-A motif is a specificity element, a selector of eukaryotic gene transcription. Deletion of the "cap-sequence," 5' pyrimidine-C-A-T-T-C-purine 3' and most of the mRNA leader sequence did not abolish transcription but created yet another mRNA 5' terminus. In contrast to these deletions, which are both down-mutations, deletion of H2A gene-specific conserved DNA sequences upstream from the T-A-T-A-A-A-T-A motif enhanced mRNA synthesis. A hypothesis for the function of these DNA sequences as eukaryotic promoter elements is discussed.
Full text
PDF

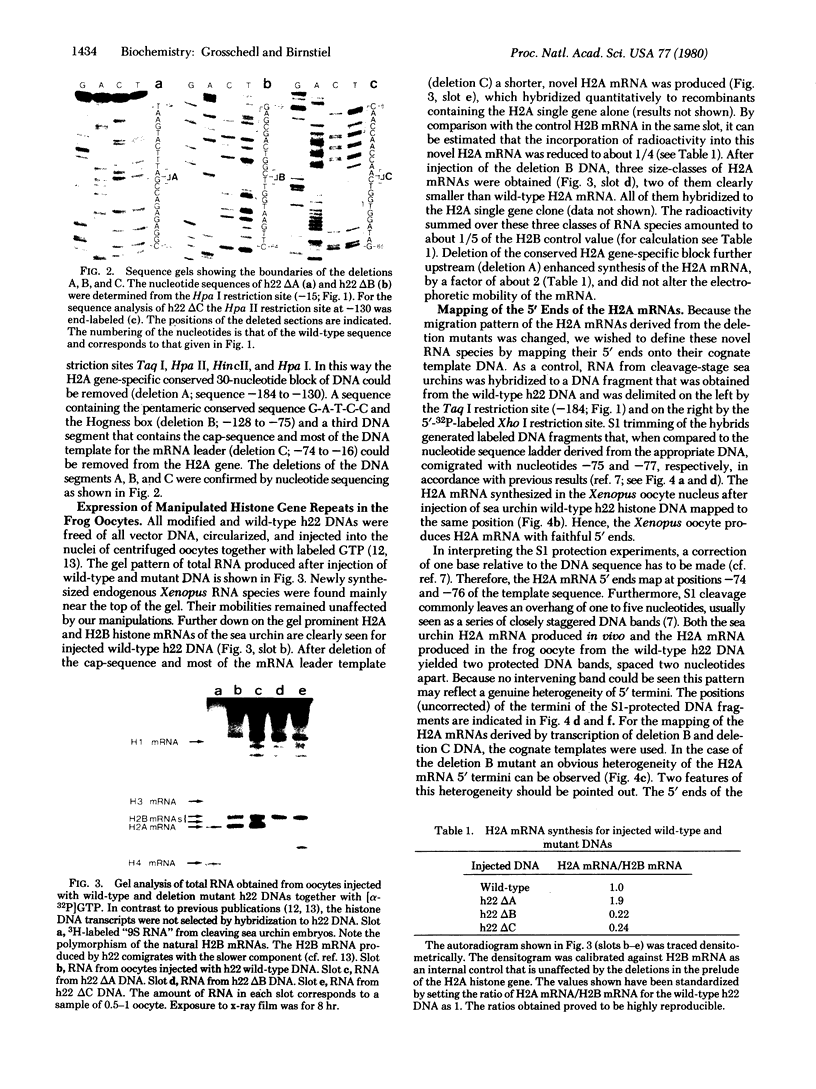


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Herisse J., Courtois G., Galibert F., Ziff E. Messenger RNA for the Ad2 DNA binding protein: DNA sequences encoding the first leader and heterogenity at the mRNA 5' end. Cell. 1979 Oct;18(2):569–580. doi: 10.1016/0092-8674(79)90073-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Faust M., Millward S., Duchastel A., Fromson D. Methylated constituents of poly(A)- and poly(A)+ polyribosomal RNA of sea urchin embryos. Cell. 1976 Dec;9(4 Pt 1):597–604. doi: 10.1016/0092-8674(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K., Probst E., Schaffner W., Birnstiel M. Molecular analysis of the histone gene cluster of Psammechinus miliaris: I. Fractionation and identification of five individual histone mRNAs. Cell. 1976 Aug;8(4):455–469. doi: 10.1016/0092-8674(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Gross K., Schaffner W., Telford J., Birnstiel M. Molecular analysis of the histone gene cluster of Psammechinus miliaris: III. Polarity and asymmetry of the histone-coding sequences. Cell. 1976 Aug;8(4):479–484. doi: 10.1016/0092-8674(76)90215-4. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Traub P., Gallwitz D. The histone genes in HeLa cells are on individual transcriptional units. J Mol Biol. 1978 Dec 25;126(4):619–635. doi: 10.1016/0022-2836(78)90012-8. [DOI] [PubMed] [Google Scholar]
- Haegeman G., van Heuverswyn H., Gheysen D., Fiers W. Heterogeneity of the 5' terminus of late mRNA induced by a viable simian virus 40 deletion mutant. J Virol. 1979 Aug;31(2):484–493. doi: 10.1128/jvi.31.2.484-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn L. J., Brown D. D. Nucleotide sequence of Xenopus borealis oocyte 5S DNA: comparison of sequences that flank several related eucaryotic genes. Cell. 1978 Dec;15(4):1145–1156. doi: 10.1016/0092-8674(78)90042-9. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Clarkson S. G., Telford J. L., Birnstiel M. L. Transcription of xenopus tDNAmet1 and sea urchin histone DNA injected into the Xenopus oocyte nucleus. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1077–1082. doi: 10.1101/sqb.1978.042.01.108. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Hofstetter H., Di Capua E., Grosschedl R., Birnstiel M. L. A tRNA gene of Xenopus laevis contains at least two sites promoting transcription. Nucleic Acids Res. 1979 Dec 11;7(7):1749–1763. doi: 10.1093/nar/7.7.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifton R. P., Goldberg M. L., Karp R. W., Hogness D. S. The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1047–1051. doi: 10.1101/sqb.1978.042.01.105. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Clarkson S. G. Nucleotide sequence of genes coding for tRNAPhe and tRNATyr from a repeating unit of X. laevis DNA. Cell. 1980 Feb;19(2):345–353. doi: 10.1016/0092-8674(80)90509-7. [DOI] [PubMed] [Google Scholar]
- Probst E., Kressmann A., Birnstiel M. L. Expression of sea urchin histone genes in the oocyte of Xenopus laevis. J Mol Biol. 1979 Dec 15;135(3):709–732. doi: 10.1016/0022-2836(79)90173-6. [DOI] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Gross K., Telford J., Birnstiel M. Molecular analysis of the histone gene cluster of psammechinus miliaris: II. The arrangement of the five histone-coding and spacer sequences. Cell. 1976 Aug;8(4):471–478. doi: 10.1016/0092-8674(76)90214-2. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Kunz G., Daetwyler H., Telford J., Smith H. O., Birnstiel M. L. Genes and spacers of cloned sea urchin histone DNA analyzed by sequencing. Cell. 1978 Jul;14(3):655–671. doi: 10.1016/0092-8674(78)90249-0. [DOI] [PubMed] [Google Scholar]
- Surrey S., Nemer M. Methylated blocked 5' terminal sequences of sea urchin embryo messenger RNA classes containing and lacking poly(A). Cell. 1976 Dec;9(4 Pt 1):589–595. doi: 10.1016/0092-8674(76)90041-6. [DOI] [PubMed] [Google Scholar]
- Telford J. L., Kressmann A., Koski R. A., Grosschedl R., Müller F., Clarkson S. G., Birnstiel M. L. Delimitation of a promoter for RNA polymerase III by means of a functional test. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2590–2594. doi: 10.1073/pnas.76.6.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff E. B., Evans R. M. Coincidence of the promoter and capped 5' terminus of RNA from the adenovirus 2 major late transcription unit. Cell. 1978 Dec;15(4):1463–1475. doi: 10.1016/0092-8674(78)90070-3. [DOI] [PubMed] [Google Scholar]